Abstract
Sunflower seeds are a rare source of allergy, but several cases of occupational allergies to sunflowers have been described. Sunflower allergens on the whole, however, still await precise and systematic description. We present an interesting case of a 40-year-old male patient, admitted to hospital due to shortness of breath and urticaria, both of which appeared shortly after the patient ingested sunflower seeds. Our laryngological examination revealed swelling of the pharynx with retention of saliva and swelling of the mouth and tongue. During diagnostics, 2 months later, we found that skin prick tests were positive to mugwort pollen (12/9 mm), oranges (6/6 mm), egg protein (3/3 mm), and hazelnuts (3/3 mm). A native prick by prick test with sunflower seeds was strongly positive (8/5 mm). Elevated concentrations of specific IgE against weed mix (inc. lenscale, mugwort, ragweed) allergens (1.04 IU/mL), Artemisia vulgaris (1.36 IU/mL), and Artemisia absinthium (0.49 IU/mL) were found. An ImmunoCap ISAC test found an average level of specific IgE against mugwort pollen allergen component Art v 1 - 5,7 ISU-E, indicating an allergy to mugwort pollen and low to medium levels of specific IgE against lipid transfer proteins (LTP) found in walnuts, peanuts, mugwort pollen, and hazelnuts. Through the ISAC inhibition test we proved that sunflower seed allergen extracts contain proteins cross-reactive with patients’ IgE specific to Art v 1, Art v 3, and Jug r 3. Based on our results and the clinical pattern of the disease we confirmed that the patient is allergic to mugwort pollen and that he had an anaphylactic reaction as a result of ingesting sunflower seeds. We suspected that hypersensitivity to sunflower LTP and defensin-like proteins, both cross-reactive with mugwort pollen allergens, were the main cause of the patient’s anaphylactic reaction.
Keywords: allergen components, IgE test, ISAC inhibition, lipid transfer protein (LTP), skin prick test, sunflower
Introduction
Sunflower seed allergies are rare, and only several cases of patients with symptoms of anaphylactic reactions to ingested sunflower seeds have been heretofore described. Bird breeders are among professions especially predisposed to sunflower allergy.
The aim of this study is to present an unusual case of a patient who experienced an allergic reaction to sunflower seeds in the absence of occupational or bird-related allergies.
Sunflower seed allergy
The sunflower (Helianthus annuus) is a genus of plants in the Asteraceae (Compositae) family. It is an annual plant, with a thick, rigid stem growing up to 3 m in height. Characteristically for sunflowers, the flower head consists of 1000–2000 disc florets, and spreads up to 60 cm in diameter (30 cm on average). The outer part of the head is surrounded by large, yellow petals. The seeds, surrounded by a hard-shelled pericarp, ripen between September and October. A characteristic feature of sunflowers is their ability to turn the heads in the direction of the sun (heliotropism). Sunflowers are native to Central America but they grow easily in any temperate climate region. They are harvested for sunflower seeds, sunflower oil, and their leaves and hulls, which can be used to produce fertilizer.1,2 Sunflower seeds are also a food source for domestic birds, especially parrots, and can be added to cattle fodder.3
People may experience allergic reactions after eating whole grain bread because sunflower allergens are highly heat resistant and do not disintegrate, even in temperatures as high as 200°C, not even after 1 h of baking. Additionally, even though sunflower oil is considered to be safe for patients with food allergies because it does not contain proteins, numerous exceptions to this have been confirmed.3
In 1986, Halsey et al. studied two patients allergic to sunflower seeds, their allergies objectively confirmed with skin prick tests and a RAST test. The researchers also tested samples of cold-pressed sunflower oil and found traces of protein, but during an open-label sunflower oil provocation patients did not report any clinical symptoms.4
Sunflower allergens have so far been described relatively poorly. Up until now the following have been described:
Hel a 1 – a major allergen, 34 kDa which belongs to inhalatory allergens, cross-reactive with other members of the Asteraceae.5,6 The sequence is unavailable, but we can speculate that Hel a 1 is a homologous molecule of the defensin-like protein family. Gruber et al. investigated cross-reactive behaviors of Art v 1 and identified cross-reactive structures in sunflower pollen.7
Hel a 2 – profilin, 4.7 kDa, which belongs to inhalatory allergens.
Hel a 3 – a lipid transfer protein (called non-specific lipid transfer protein type 1 [nsLTP1]), non-specific, 9 kDa, a food allergen.6
Sunflower seeds also contain 2S albumin, rich in methionine and storage protein 12 kDa.
Sunflower pollen contains also other allergens, weighing 32, 24, 42.8, and 55 kDa.3,8
Sunflower allergens cross-react with other members of the Asteraceae family: wormwood, goldenrod, dandelion, chrysanthemum, and ambrosia. The strongest cross-reactivity occurs between sunflower pollen allergens and sagebrush. Cross-allergy with plants other than Asteraceae may appear due to the presence of a plant panallergen, profilin (Hel a 2). Hel a 2 reacts with profilins of ragweed, olive tree, mugwort, and Mercurialis perennis. Sunflower pollen can also cross-react with sunflower seeds.5,9
One of the most important members of the Asteraceae family is mugwort (Artemisia vulgaris). Mugwort pollen is one of the main causes of allergic reactions in Europe. Researchers estimate that above 95% of mugwort pollen-allergic patients are sensitized to Art v 1, a major mugwort pollen allergen.7 Art v 1 is a modular glycoprotein with a defensin-like and hydroxyproline-rich domain.10
Lipid transfer proteins (LTP) are a widespread family of plant panallergens. One of the described sunflower allergens, Hel a 3, is a lipid transfer protein. Allergies to LTP are particularly significant and frequent in the Mediterranean. Usually, peach LTP is the primary sensitizing allergen, with the highest concentration of allergen in the peel of the fruit. LTPs are, in general, resistant to cooking and digestion in the gastrointestinal tract.11
Symptoms of an allergic reaction to sunflower include oral allergy syndrome, bronchial asthma, allergic rhinitis, conjunctivitis, angioedema, contact dermatitis, and skin lesions of an acute urticaria.12
The first case of sunflower seed allergy was described in 1979. Authors discussed the cases of three patients who, after ingesting sunflower seeds, suffered from anaphylactic reactions. All patients underwent skin prick tests, which confirmed IgE-mediated reactions. RAST inhibition tests were positive in two patients.13
The case was different with the third patient, occupationally exposed to sunflower pollen. The patient, 24 years old, within 5 years of exposure to sunflower pollen developed symptoms of rhinitis, conjunctivitis, and asthma. Symptoms withdrew after the cessation of exposure to the allergen. The patient was diagnosed with an occupational allergy, based on his medical history, skin prick tests, and the RAST test.14
In 1994, another case of four patients allergic to sunflower seed was described. These patients displayed symptoms after ingesting sunflower seeds, all of them were also bird breeders. Three patients had an anaphylactic reaction and one patient had a bronchial asthma attack. The authors wanted to determine the frequency of sunflower seed allergy. They studied 84 patients with atopy. Among them, only three tested positive in a RAST inhibition test with sunflower allergens. It was thus inferred that allergy to sunflower is rare. It was noted, however, that among bird breeders positive skin tests with sunflower allergens were observed among 79% of patients, while 21% of the tested patients got positive results in a RAST inhibition test. These patients had no allergic symptoms.15
In 2002, Atis et al. carried out a study of sunflower seed allergic reactions in a sunflower processing factory in Turkey. Researchers studied 102 employees who had direct contact with sunflower pollen, with a control group of employees with no contact with the allergen. It was found that a high percentage of workers in the study group (23.5%) were allergic to sunflower. In comparison to the control group, the patients suffered from rhinitis and conjunctivitis significantly more often.16
An unusual case of oral allergy syndrome caused by sunflower was described in 2005 by Palma-Carlos et al. The allergen exposure occurred through inhalation during feeding birds. The prick by prick native test was positive with fresh sunflower seeds. A test with commercial extracts of sunflower was negative. Significantly elevated levels of antigen specific IgE against sunflower allergens were found in the patients’ blood serum.17
The first case of anaphylaxis associated with sunflower LTP sensitization was described in 2010. After eating five sunflower seeds, a 22-year-old female patient treated for atopic dermatitis experienced an anaphylactic reaction in the form of dyspnea and wheezing, rash all over the body, and a swelling of the larynx. She was treated with steroids and adrenaline with a good therapeutic effect. A concentration of antigen specific IgE against sunflower allergens − 34.1 UA/mL (class 4) was found in her system. The authors of the study identified several IgE-binding protein bands through an immunoblot assay using sunflower seed extract − 13, 14, and 37 kDa. After the inhibition immunoblot assay the IgE-binding signal of one band (13 kDa) disappeared completely. The 13 kDa protein closely matched LTPs from sunflower seeds.18
A case study
In what follows, we wish to present the case of a 40-year-old man admitted to the Department of Allergology, Immunology, and Internal Medicine due to dyspnea, wheezing, and skin lesions in the form of red wheals, which appeared after he ingested sunflower seeds. The lesions were located on the upper limbs, lower limbs, and the chest. Before being admitted, this patient had not been treated for allergic diseases. Family history of allergic diseases was negative. The patient is an office worker and neither in the workplace nor at home has any contact with sunflower allergens. He does not have a pet at home. A detailed medical interview revealed that in August the patient experienced allergic symptoms in the form of nasal obstruction and rhino-rhea.
Upon admission to the hospital, a physical examination revealed the following abnormalities: multiple multiform lesions on the skin of the lower limbs, arms, and chest; and wheezing scattered over both lung fields. Moreover, through a laryngological examination we found swelling of the hypopharynx with retention of saliva, and swelling of the floor of the mouth and tongue. The implemented symptomatic treatment (steroids and antihistamines intravenously) was very effective. Furthered diagnostics was postponed.
Eight weeks after the anaphylactic reaction described above, the patient visited the clinic again, for fathered diagnostics. We determined the level of allergen specific IgE against common inhalatory allergens, using the Hycor Biomedical Technique EIA (in accordance with the WHO standards the results were indicated in kU/L). The patient underwent skin prick tests with food allergens (hen’s egg, cow’s milk, cocoa, tomatoes, carp, apples, bananas, strawberries, flour, rye flour, wheat flour, peanuts, nuts, celery, pork, poultry, citrus) and inhalatory allergens (birch, alder, hazel, ragweed, mugwort, grasses, house dust mites, mold, animal dander) with the use of an Allergopharma set. A skin prick test with standardized allergen extracts of sunflower was not performed because it was unavailable at the time.
We performed the native prick by prick test with sunflower seeds. Microarray ImmunoCap ISAC test was also performed to determine the level of the allergen specific IgE against a wide set of allergen components.
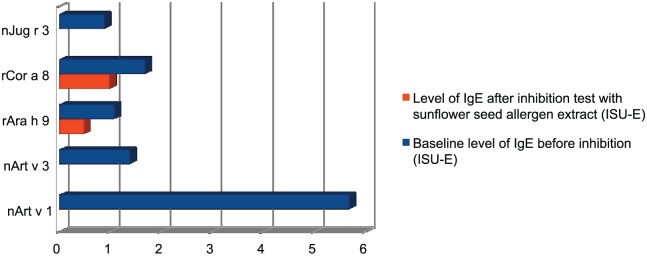
Our findings were as follows. Skin tests were positive to mugwort pollen (12/9 mm, histamine 3/4 mm, negative control 0/0 mm), oranges (6/6 mm), egg protein (3/3 mm), and hazelnuts (3/3 mm). The native prick by prick test with sunflower seeds was strongly positive (8/5 mm). We also found an elevated concentration of specific IgE directed against weed mix allergens (including lenscale, mugwort, ragweed; 1.04 IU/mL), mugwort pollen (1.36 IU/mL), and mugwort wormwood pollen (0.49 IU/mL). Through the ImmunoCap ISAC test, we found medium levels of specific IgE against mugwort pollen allergens component Art v 1-5,7 ISU-E, indicating an allergy to mugwort pollen, and low and medium levels of specific IgE against LTPs, which can be found in walnuts (Jug r 3-0.9 ISU-E), peanuts (Ara h 9-1.1 ISU-E), mugwort (Art v 3-1.4 ISU-E), and hazelnuts (Cor a 8-1.7 ISU -E). The levels of IgE directed against other allergen components available in ImmunoCap ISAC were not elevated. The results of the Components Resolved Diagnosis (CRD) did not help to clarify if it was Art v 1 or Art v 3, which was the molecule responsible for the described sunflower seed allergic reaction. In order to determine this, we decided to perform an ISAC inhibition test using sunflower seed extracts for the inhibition process. The test was modeled on the SPHIA (Single Point Highest Inhibition Achievable assay) test by Bernardi et al.19 The sunflower seeds, purchased in a local eco shop, were frozen in liquid nitrogen, then crushed with a pestle in a mortar until a smooth powder was obtained. Seed proteins were extracted at 0.5 M NaCl, at 4°C in a time span of 2 h. After the protein extraction we centrifuged the seed samples at 12,500 × g for 60 min and collected the supernatants. We then estimated the protein concentration in the extracts and used the samples at a 1 mg/mL concentration, allowing for their total protein content. Finally, as a part of this IgE inhibition experiment 20 μL of patients’ sera was incubated overnight with 20 μL of the sunflower seed allergen solution. After the incubation, we evaluated the IgE binding inhibition by running the ISAC microarray. The results of the ISAC microarray before and after the inhibition are compared in Table 1 and Figure 1.
Table 1.
Results of ISAC microarray before and after inhibition, only positive results acknowledged.
| Allergen component/allergen source/protein family | Baseline level of IgE before inhibition (ISU-E) | Level of IgE after inhibition test with sunflower seed allergen extract (ISU-E) |
|---|---|---|
| nArt v 1 / mugwort / defensing | 5.7 | <0.3 |
| nArt v 3 / mugwort / nsLTP | 1.4 | <0.3 |
| rAra h 9 / peanut / nsLTP | 1.1 | 0.5 |
| rCor a 8 / hazelnut / nsLTP | 1.7 | 1 |
| nJug r 3 / walnut / nsLTP | 0.9 | <0.3 |
Figure 1.
Graphical presentation of the results of ISAC inhibition test with sunflower seed allergen extract.
In the inhibition test Art v 1, Art v 3, and Jug r 3 were inhibited by the protein present in the sunflower seed allergen extract. Lower concentration of specific IgE directed against Ara h 9 and Cor a 8 can be explained either by SPHIA (the serum is diluted by the allergen extract) or by the partial homology of the sunflower seed allergen extract with the nsLTPs of hazelnuts and peanuts. The results prove that the patient was allergic to sunflower seeds because of a cross-allergy with mugwort pollen allergens, defensin Art v 1 and nsLTP Art v 3 together. Moreover, proteins (probably sunflower seed LTPs) cross-reactive with the walnut LTP Jug r 3 were found in the sunflower seed allergen extract. In the microarray test the elevated levels of IgE directed against nsLTP of peanuts and hazelnuts were clearly noticeable, suggesting possible cross-reactions with various fruits and vegetables in this patient’s system.
The patient displayed symptoms of a seasonal allergic rhinitis due to allergy to mugwort pollen.
We may suspect that hypersensitivity to sunflower LTP and defensin-like proteins was the main cause of the anaphylactic reaction of this patient. ImmunoCap ISAC, unfortunately, has no sunflower LTPs allergens.
Discussion
The described case is interesting due to the fact that the allergy to sunflower seeds is relatively rare, in most cases displayed by workers occupationally exposed to sunflower allergens and bird breeders,14–16 whereas in this case the allergy occurred without such exposure and outside of workplace conditions.
The first-line treatment for anaphylaxis is an immediate intramuscular injection of adrenaline,20 but in the case described here this procedure was not performed. Luckily, however, the implemented symptomatic treatment that included steroids and antihistamines was very effective and the patient avoided anaphylactic shock. This case is consistent with data from several cohort studies which state that in the treatment of anaphylaxis adrenaline is, in fact, used much less often than antihistamines and corticosteroids, despite their delayed onset of action. The relatively rare use of adrenaline in treating anaphylaxis may be caused by a lack of in-depth knowledge about this treatment among health professionals.21
Due to the lack of availability of a standardized allergen extract of the sunflower allergen we used fresh seeds for the diagnostic prick by prick tests. Other studies, however, suggest that native prick by prick tests with, for instance, both the peel and the pulp of a kiwi fruit, have an advantage over skin prick tests with standardized allergenic extracts.22
The described patient had a strongly positive native test result, consistent with data cited elsewhere, that prick by prick tests with allergens technologically unmodified exhibit high specificity.23
The patient’s diagnostics were expanded to include allergen component testing with the use of the microarray method: ImmunoCap ISAC. This method is based on native and recombinant allergen molecules and is used to determine the allergic profile of the patient. While using this method, it is extremely important to identify the allergens causing a high risk of severe, life-threatening anaphylactic reactions and to determine the likelihood of a cross-allergy.
The occurrence of a specific IgE directed against LTP may suggest cross-reactivity with a variety of vegetables and fruits, because LTP is a plant panallergen. A related, peculiar disease, the so-called LTP syndrome, is characterized by symptoms that are heterogeneous with an unstable clinical pattern of the disease. Typically for the LTP syndrome, allergic reactions occur after exposure to the allergen combined with a co-factor (the overlap of stimuli syndrome). The co-factors of an allergic reaction may include alcohol, stress, physical exertion, menstruation, or the use of NSAIDs. The exact concentration and amount of allergenic products provoking anaphylaxis may differ for the same patient. We now know that there are different recognition patterns of LTPs – some patients react only to peaches and apples (Rosaceae fruit, where sensitization to peach LTP Pru p 3 can be a marker), while others react to a wide range of unrelated kinds of food, like hazelnuts or walnuts. The second instance is related to a sensitization to mugwort LTP – Art v 3.24,25 The insightful findings of Gao et al. provide convincing evidence for the theory that there are patients with a peach LTP allergy whose primary sensitization to pollen LTP (Art v 3) is the cause of a given food allergy.26
We found elevated levels of IgE specific to Art v 3 (ns LTP, mugwort) and also other nsLTPs (peanuts: Ara h 9, hazelnuts: Cor a 8, walnuts: Jug r 3). The levels of IgE directed against Art v 1, the main allergen of mugwort pollen, was high. Through the ISAC inhibition test, we proved that the sunflower seed allergen extract contains proteins cross-reactive with patient’s IgE specific to Art v 1, Art v 3, and Jug r 3.
The patient was recommended to eliminate sunflower seeds, hazelnuts, walnuts, and peanuts from his diet, and to begin immunotherapy with mugwort pollen allergens. He was also recommended to carry a rescue kit containing adrenaline in an autoinjector, an oral steroid, and an antihistamine with him at all times.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Panero JL, Funk VA. (2002) Toward a phylogenetic subfamilial classification for the Asteraceae (Asteraceae). Proceedings of the Biological Society of Washington 115: 909–922. [Google Scholar]
- 2. de la, Hoz F, Melero JA, Gonzalez R, et al. (1994) Isolation and partial characterization of allergens from Helianthus annuus (sunflower) pollen. Allergy 49: 848–854. [DOI] [PubMed] [Google Scholar]
- 3. Rudzki E. (2009) Alergeny. Kraków: Medycyna Praktyczna, 2009. [Google Scholar]
- 4. Halsey AB, Martin ME, Ruff ME, et al. (1986) Sunflower oil is not allergenic to sunflower seed-sensitive patients. Journal of Allergy and Clinical Immunology 78: 408–410. [DOI] [PubMed] [Google Scholar]
- 5. Weber RW. (2005) Cross-reactivity of pollen allergens: Recommendations for immunotherapy vaccines. Current Opinion in Allergy and Clinical Immunology 5: 563–569. [DOI] [PubMed] [Google Scholar]
- 6. The WHO/IUIS Allergen Nomenclature Sub-committee (2015) Available at: http://www.allergen.org.
- 7. Gruber P, Gadermaier G, Bauer R, et al. (2009) Role of the polypeptide backbone and post-translational modifications in cross-reactivity of Art v 1, the major mugwort pollen allergen. Biological Chemistry 390: 445–451. [DOI] [PubMed] [Google Scholar]
- 8. de la, Hoz F, Melero JA, Gonzalez R, et al. (1994) Isolation and partial characterization of allergens from Helianthus annuus (sunflower) pollen. Allergy 49: 848–854. [DOI] [PubMed] [Google Scholar]
- 9. Vallverdu A, Asturias JA, Arilla MC, et al. (1998) Characterization of recombinant Mercurialis annua major allergen Mer a 1 (profilin). Journal of Allergy and Clinical Immunology 101: 363–370 [DOI] [PubMed] [Google Scholar]
- 10. Himly M, Jahn-Schmid B, Dedic A, et al. (2003) Art v 1, the major allergen of mugwort pollen, is a modular glycoprotein with a defensin-like and a hydroxyproline-rich domain. FASEB Journal 17: 106–108. [DOI] [PubMed] [Google Scholar]
- 11. Pascal M, Muñoz-Cano R, Reina Z, et al. (2012) Lipid transfer protein syndrome: Clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clinical and Experimental Allergy 42(10):1529–39 [DOI] [PubMed] [Google Scholar]
- 12. Machet L, Vaillant L, Callens A, et al. (1993) Allergic contact dermatitis from sunflower (Helianthus annuus) with cross-sensitivity to arnica. Contact Dermatitis 28: 184–185. [DOI] [PubMed] [Google Scholar]
- 13. Noyes JH, Boyd GK, Settipane GA. (1979) Anaphylaxis to sunflower seed. Journal of Allergy and Clinical Immunology 63: 242–244. [DOI] [PubMed] [Google Scholar]
- 14. Bousquet J, Dhivert H, Clauzel AM, et al. (1985) Occupational allergy to sunflower pollen. Journal of Allergy and Clinical Immunology 75: 70–74. [DOI] [PubMed] [Google Scholar]
- 15. Axelsson IG, Ihre E, Zetterström O. (1994) Anaphylactic reactions to sunflower seed. Allergy 49: 517–520. [DOI] [PubMed] [Google Scholar]
- 16. Atis S, Tutluoglu B, Sahin K, et al. (2002) Sensitization to sunflower pollen and lung functions in sunflower processing workers. Allergy 57: 35–39. [PubMed] [Google Scholar]
- 17. Palma-Carlos AG, Palma-Carlos ML, Tengarrinha F. (2005) Allergy to sunflower seeds. European Annals of Allergy and Clinical Immunology 37: 183–186. [PubMed] [Google Scholar]
- 18. Yagami A. (2010) Anaphylaxis to lipid transfer protein from sunflower seeds. Allergy 65: 1340–1341 [DOI] [PubMed] [Google Scholar]
- 19. Bernardi ML, Giangrieco I, Camardella L, et al. (2011) Allergenic lipid transfer proteins from plant-derived foods do not immunologically and clinically behave homogeneously: The kiwifruit LTP as a model. PLoS One 6: e27856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simons FE, Ardusso LR, Bilo MB, et al. (2011) World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organization Journal 4: 13– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song TT, Worm M, Lieberman P. (2014) Anaphylaxis treatment: Current barriers to adrenaline auto-injector use. Allergy 69: 983–991. [DOI] [PubMed] [Google Scholar]
- 22. Novembre E, Bernardini R, Bertini G, et al. (1995) Skin-prick-test-induced anaphylaxis. Allergy 50: 511–513. [DOI] [PubMed] [Google Scholar]
- 23. Castillo R, Delgado J, Quiralte J, et al. (1996) Food hypersensitivity among adult patients: Epidemiological and clinical aspects. Allergologia and Immunopathologia 24: 93–97. [PubMed] [Google Scholar]
- 24. Garcia-Selles FJ, Diaz-Perales A, Sanchez-Monge R, et al. (2002) Patterns of reactivity to lipid transfer proteins of plant foods and Artemisia pollen: an in vivo study. International Archives of Allergy and Immunology 128: 115–122. [DOI] [PubMed] [Google Scholar]
- 25. Fernández-Rivas M, Benito C, González-Mancebo E, et al. (2008) Allergies to fruits and vegetables. Pediatric Allergy and Immunology 19: 675–681. [DOI] [PubMed] [Google Scholar]
- 26. Gao ZS, Yang ZW, Wu SD, et al. (2013) Peach allergy in China: A dominant role for mugwort pollen lipid transfer protein as a primary sensitizer. Journal of Allergy and Clinical Immunology 131: 224–226. [DOI] [PubMed] [Google Scholar]