Abstract
Sjögren’s syndrome (SS) is an autoimmune epithelitis characterized by mononuclear cell (MNC) infiltration of the lacrimal and salivary glands (SG), as well as the presence of serum autoantibodies. This condition is a growing public health concern in Algeria. Herein, we sought to determine if the levels of interleukin (IL)-6, IL-17A, and nitric oxide (NO), were correlated with the extent of MNC infiltration. The expression of inducible NO synthase (NOS2) and CD68 was measured in the SG of all patients, but not in those of the normal controls (NCs). We included 44 primary Sjögren’s syndrome (pSS) patients and 15 NCs in this study; we found that the expression of NOS2 and CD68 was elevated in all of the SG of SS patients. Additionally, the serum and saliva levels of IL-6, IL-17A, and NO were higher in the pSS patients, compared with the NCs. Furthermore, the NOS2-induced excess NO was associated with the extent of the MNC infiltration, and thereby with tissue injury. It is also important to note that there were correlations between the levels of IL-6, IL-17A, and NO. Such findings indicate that through the effects of NO, IL-17A participates in the pathophysiology of the disease. With the purpose of improving both the diagnosis and prognosis, IL-6, IL-17A, and NO should be assayed in the serum and saliva of patients suspected of SS.
Keywords: inducible nitric oxide synthase, interleukin-6, interleukin-17A, nitric oxide, primary Sjögren’s syndrome
Introduction
Sjögren’s syndrome (SS) is a type of autoimmune epithelitis characterized by mononuclear cell (MNC) infiltration of the exocrine tissues.1 The ensuing dryness results in the lacrimal keratoconjunctivitis sicca and xerostomia of the salivary glands (SG). This syndrome may occur alone as primary (pSS) or develop out of a connective tissue disease, known as secondary (sSS) (e.g. scleroderma, rheumatoid arthritis, and systemic lupus erythematosus). Moreover, pSS may be complicated by a variety of extraglandular disorders, including peripheral neuropathy or lung disease. Genetic, environmental, and hormonal features interact to breach tolerance by activating T and B lymphocytes, and the affected SGs are characterized by lymphocytic foci surrounding the acini and salivary ducts. Given their local predominance over B cells, T cells have long been suggested to play a leading role in the development of this autoimmune condition. In this respect, the reciprocal development pathways2 for the generation of pathogenic T helper (Th)-17 and regulatory T cells (Treg) are aberrant, endowing T cells with a pivotal role in the pathophysiology of the disease.3 The consequence is that a myriad of self-reactive antibodies (Ab) are produced. However, in contrast to this early paradigm, recent evidence suggests that B cells contribute to the development of SS.4–6
In an attempt to unravel this complex network, a number of untested hypotheses have been proposed.7 At the forefront remains rheumatoid factor (RF), which reflects the disease activity, as well as antinuclear Ab (ANA), involving those directed to SSA or SSB antigens (Ag). These are the only serological markers promoted as criteria for the classification of pSS by the American–European Consensus Group.8 The balance of pro-inflammatory relative to anti-inflammatory cytokines is also disturbed.9 By definition, the first group of cytokines favors inflammation, including tumor-necrosis factor (TNF)-α, produced by CD4+ T cells, as well as myeloid or epithelial cells (EC), and exerts costimulatory effects on various cell types. Interleukin (IL)-6 is another primary cytokine, promoting the synthesis of auto-antibodies through secondary cytokine production by local B lymphocytes.10 Alternatively, IL-6 can synergize with IL-1β and transforming growth factor (TGF) β to skew the polarization of Th cells towards Th17 at the expense of Tregs.2,3 The production of IL-17A exhibits pro-inflammatory properties, and is derived from T cells, as well as natural killer and dendritic cells (DC). IL-17A production also influences multiple types of leukocytes (i.e. B cells and Ag-presenting cells), encouraging the release of supplemental cytokines, chemokines, and metalloproteases.3,11,12 Conversely, IL-10 is produced by macrophages (MØ), DCs, Tregs, and B lymphocytes, and functions to suppress the immune response. However, IL-10 has recently been shown to play a paradoxical role, in that excess secretion can worsen several diseases, leading to the destruction of ECs.13 One of the plausible mediators is nitric oxide (NO), a free radical synthesized from L-arginine14 by inducible NO synthase (NOS2). NO has an immunoregulatory effect and, as a result, governs the function of the SG.15–17 Interestingly, NO overproduction has also been seen in hydatidosis,18 Behçet’s disease,19 periodontitis,20 and inflammatory bowel diseases.21 In MØ, NO is synthesized by NOS222,23 following TLR stimulation (e.g. lipopolysaccharide exposure), as well as the presence of TNF-α and interferon (IFN)-γ, inferring that the NOS2 gene is differentially regulated by IL-4, IL-6, IL-10, TNF-α, and IFN-γ.
Since pSS is a growing public health concern in our country as in Morocco,24 we explored the cytokines and NO levels in both the serum and saliva in Algerian pSS patients. These levels were then compared to the extent of the infiltration of SG and the ensuing tissue damage. Next, the local NOS2 expression in the salivary gland was evaluated relative to the serial serum and salivary levels of IL-6, IL-10, TNF-α, and NO. The results below establish that these mediators function in concert in the development of SS.
Material and methods
Patients and controls
The Department of Internal Medicine of Maillot Hospital recruited 44 patients fulfilling the 2002 revised American–European classification criteria for pSS.8 For the purpose of uniformity, patients with sSS were excluded. There were 40 women and four men, with an average age of 43.9 ± 13.1 years, and a disease duration of 6.9 ± 5.4 years. The most frequent extraglandular disorders consisted of joint involvement in 25 cases and Raynaud’s phenomenon in 20 cases. None of the patients were administered genuine immunosuppressive drugs, although they all received steroids at a dose of 5 mg/d for an average of 5.7 ± 3.2 years. Written, informed consent was signed by the patients as required by our ethical committee. The 15 NCs consisted of healthy volunteers age and sex-matched to the patients. There were 12 women and three men aged 41.4 ± 15.9 years. None of the subjects had a history of lymphoma, infection with human immunodeficiency virus, or hepatitis B/C virus infection. All patients (Table 1) were examined for extraglandular complications.
Table 1.
Characteristics of primary Sjögren’s syndrome patients.
| Age (years) | 43.9 ± 13.1 |
| Sex: women/men | 40/4 |
| Disease duration (years) | 6.9 ± 5.4 |
| Xerostomia and/or xerophthalmia | 43/44 (97.7) |
| Cutaneous vasculitis | 14/44 (31.8) |
| Raynaud’s phenomenon | 20/44 (45.4) |
| Articular manifestation | 25/44 (56.8) |
| Peripheral neuropathy | 13/44 (29.5) |
Values are expressed as the mean ± standard deviation. Numbers of positive cells/total, with the percentages of positivity in parentheses.
Examination of the serum and saliva
Nephelometry was used to detect gammaglobulin and RF, and indirect immunofluorescence was used to assess ANA, anticentromere (ACA), anti-SSA, and anti-SSB Abs. A serum aliquot was stored at −45°C until cytokine and NO2− analysis was performed. The serum and saliva were collected from patients and NCs after an overnight fast. Before supplying their saliva, all rinsed their mouth with water for 1 min. Mastication was stimulated by chewing parafilm for 5 min. Saliva was then submitted to a 5 min 1000 × g centrifugation at 4°C. Supernatants were kept frozen until they were analyzed.
Labial salivary gland biopsies
SG biopsy specimens were fixed for 24 h in phosphate-buffered saline containing 10% formaldehyde at room temperature, embedded in paraffin, and 2-µm tissue sections were stained with hematoxylin and eosin. The samples were blindly analyzed by two histopathologists. All specimens revealed at least one focus of ~50 MNC per 4 mm2.25,26 Finally, the lesions were categorized into: (1) early SS with 1–2 foci per lobule scored 1 as described in Tarpley et al.;27 (2) intermediate SS with 2–3 foci per lobule scored 2; and (3) severe SS with diffuse infiltration of the acini were scored 3 when their destruction was partial or 4 when it was total.
Cytokine assays
The levels of serum and salivary IL-6, IL-10, IL-17A, and TNF-α (sensitivity: 2, 0.2, 0.1, and 1 pg/mL, respectively) were determined using commercial enzyme-linked immunosorbent assays (ELISA) according to the manufacturers’ instructions (Invitrogen for IL-6, IL-10 and 1L-17A, and IBL International for TNF-α). The concentrations were calculated using standard curves.
Measurement of nitric oxide production
The modified Griess method was used to determine the NO2− levels in the saliva and serum samples. NO2− is a stable downstream product of NO, as described by our group.18,19 Briefly, a 100 μL sample was mixed with 50 μL of 5% sulfanilamide, 0.5% naphthyl ethylenediamine dihydrochloride, plus 20% HCl. The preparations were then incubated for 20 min and optical densities read using a spectrophotometer at 543 nm. The nitrite concentration was determined relative to the standard curve constructed using sodium nitrite.
Immunohistochemistry of the salivary gland samples
Paraffin-embedded SG sections were deparaffinized, rehydrated, subjected to Ag retrieval in the appropriate buffer, and placed in a blocking solution containing 5% skimmed milk. These specimens were incubated with anti-CD4 or anti-CD68 Abs (CellMarque) for 1 h, or with 10 µg/mL anti-NOS2 Ab (Santa Cruz) overnight in a humid chamber at 4°C. Its binding was revealed using a horseradish-peroxidase-conjugated anti-mouse IgG Ab followed by DAB (ThermoScientific). The DAB solution was freshly prepared, counter-stained with Mayer’s hematoxylin, and mounted with Eukitt (both from Sigma). As a negative control, the first-layer Ab was omitted. Slides were examined using a standard light microscope, and photos were taken using a digital camera at a ×100 and ×400 magnification. To quantify CD4, CD68, and NOS2 expression, electronic images of 30 optical fields per biopsy under high-power field microscopy (×400) were chosen using the Powershot A640 digital camera (Canon). Stained cells were manually enumerated using the cell counter plugin of the ImageJ software by two independent observers. The markers were expressed as the numbers of positive cells per mm2. Moreover, as a control, the first-layer Ab was omitted.
Statistical analysis
Data were expressed as the means ± standard deviation. Differences between groups were evaluated using a Mann–Whitney U test, and correlations were calculated using the Spearman test. Results were considered significant when the P value was <0.05.
Results
Serum markers parallel mononuclear cell infiltration
Anti-SSA Abs were found in 35 patients and anti-SSB in 21 pSS patients (Table 2). The relationships between these Abs and the levels of RF and ACA, as well as the grade of MNC infiltration, are depicted in Figure 1. The grade elevation was non-significant in the presence anti-SSA Abs, but was significant for anti-SSB Abs. However, this was not the case for RF and ACA.
Table 2.
Serological abnormalities in 44 primary Sjögren’s syndrome patients.
| Hypergammaglobulinemia | 40 (90.9) |
| Rheumatoid factor | 17 (38.6) |
| Antinuclear antibody | 32 (72.7) |
| Anti-SSA positive | 35 (79.5) |
| Anti-SSB positive | 21 (47.7) |
| Anti-ACA positive | 11 (25.0) |
Numbers of positive individuals, with the percentages of positivity in parentheses.
Figure 1.
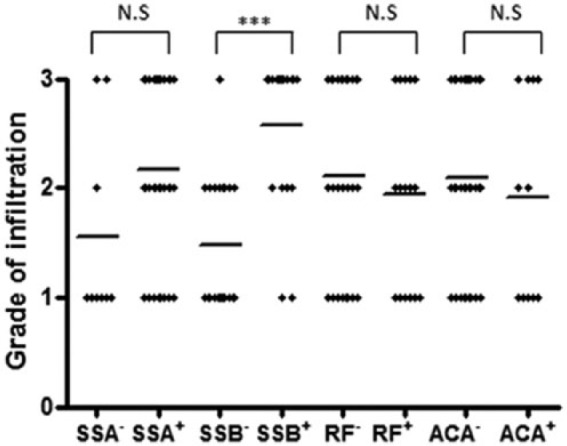
Infiltrated salivary glands of primary Sjögren’s syndrome patients, were classified according to serological markers. Patients were positive or negative for anti-SSA antibodies (Ab), anti-SSB Ab, rheumatoid factor (RF), and anti-centromere Ab (ACA). Grade differences between the positive and negative patients were: ***P <0.001 between SSB+ and SSB–, non-significant (NS) for SSA+ vs. SSA-, RF+ and RF–, and ACA+ vs. ACA– patients.
IL-6, IL-10, IL-17A, and TNF-α in the saliva and serum
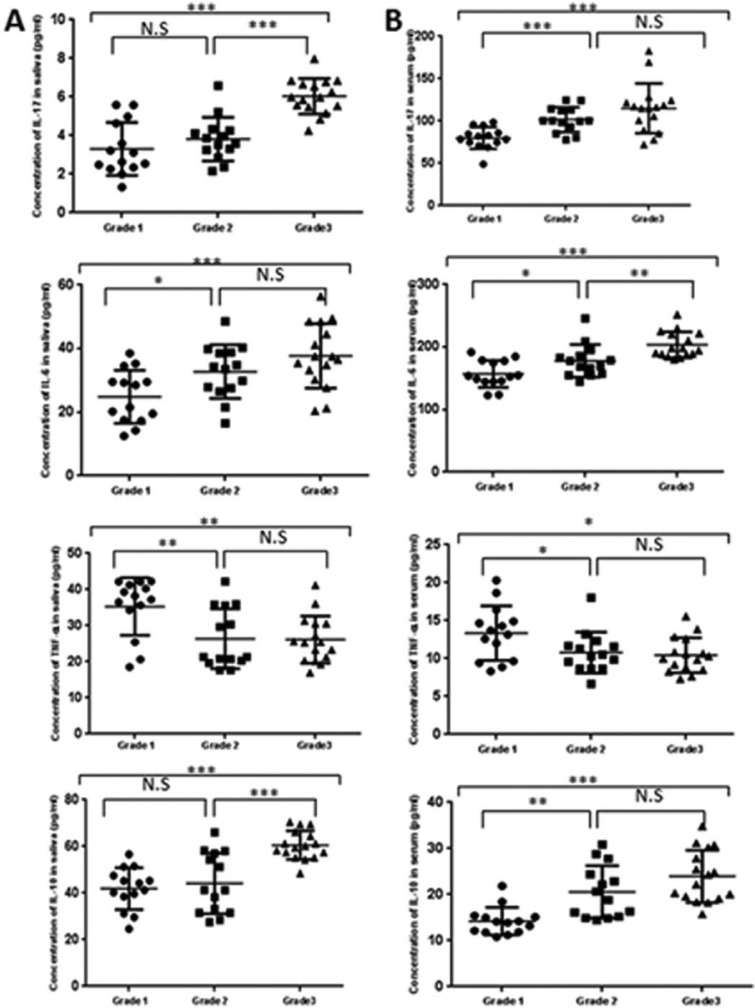
The levels of IL-6, IL-10, IL-17A, and TNF-α in the serum and saliva (Table 3) were elevated in the pSS patients compared with the NCs. Importantly, none had cytokine levels below the limit of sensitivity for any of the tests. Additionally, there was no relationship between these levels and the length of time the patients had been taking steroids. However, it is worth noting that the overproduction of IL-6, IL-10, and IL-17A in the saliva (Figure 2a) and serum (Figure 2b) correlated with the grade of SG infiltration. In contrast, this was not observed for TNF-α.
Table 3.
Interleukin (IL)-17A, 6, and 10, as well as tumor necrosis factor (TNF)-α (pg/mL) in 44 patients with primary Sjögren’s syndrome and 15 normal controls (NC). A –Saliva.
| IL-17A | IL-6 | TNF-α | IL-10 | |
|---|---|---|---|---|
| Saliva | ||||
| NC | 0.3 ± 0.2 | 9.8 ± 2.9 | 6.4 ± 1.6 | 1.2 ± 0.5 |
| pSS | 4.4 ± 1.6 | 32.0 ± 10.4 | 29.1 ± 8.5 | 49.4 ± 12.7 |
| Serum | ||||
| NC | 21.0 ± 3.3 | 41.3 ± 6.6 | 2.5 ± 1.4 | 4.3 ± 1.0 |
| Pss | 99.6 ± 25.1 | 180.7 ± 29.6 | 11.5 ± 3.1 | 19.8 ± 6.3 |
Means ± standard deviation. All differences between NC and patients are <0.001.
Figure 2.
Cytokine levels in the saliva (a) and serum (b) relative to the grade of infiltration. Grades 1, 2, and 3 display mild, intermediate, and advanced lesions, respectively. NS, non-significant. P values are *<0.05, **<0.01, and ***<0.001).
IL-6 and IL-17A levels correlate with nitric oxide production
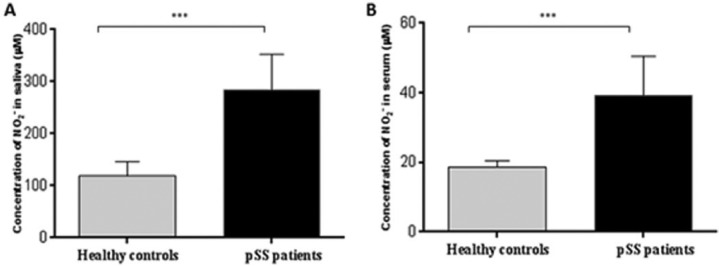
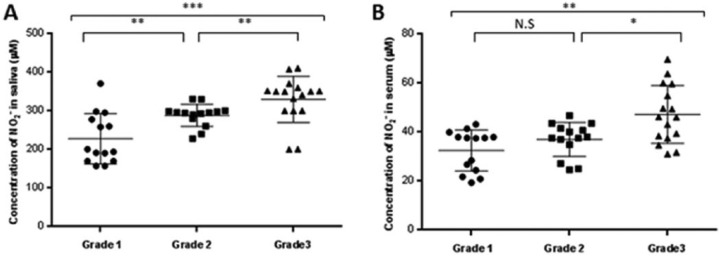
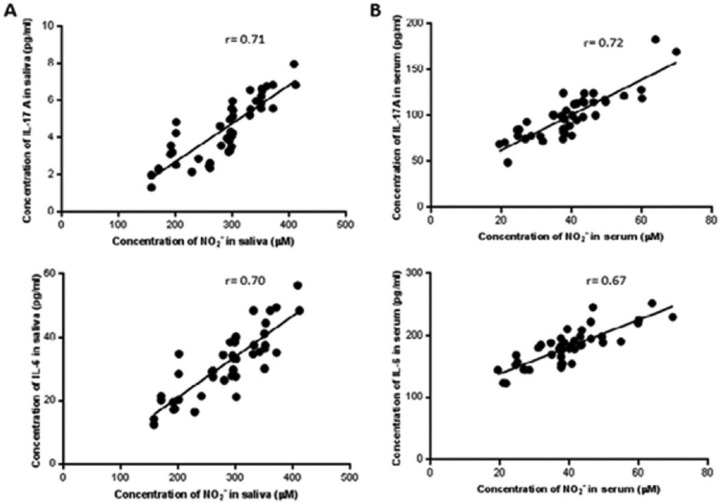
Compared with the NCs, the level of NO was higher in the pSS patients’ serum (Figure 3a: 284.4 ± 67.2 vs. 118.3 ± 36.7 µM; P <0.001) and the saliva (Figure 3b: 39.5 ± 11.5 vs. 18.6 ± 1.8 μM;P <0.001). Similar to that described above, the NO levels of the pSS patients varied according to their infiltration grade. Furthermore, variations in the salivary NO levels over time paralleled with the infiltration grade progression (Figure 4a). There was also a similar trend of these relationships observed in the serum (Figure 4b). In contrast, there was a striking correlation between the levels of NO and that of IL-6 and IL-17A in the saliva (Figure 5a, P <0.001 for both cytokines) and serum (Figure 5b, P <0.001 for both cytokines).
Figure 3.
Salivary and serum nitric oxide production in patients with primary Sjögren’s syndrome compared with normal controls.
Figure 4.
Salivary (a) and serum (b) nitric oxide relative to the grade of the salivary gland lesions. For grades 1–3, see the legend in Figure 2. NS, non-significant. P values are *<0.05, **<0.01, and ***<0.001.
Figure 5.
Correlation between IL-17A, IL-6, and nitric oxide (NO) in saliva (a) and serum (b). The production of NO correlated with salivary IL-17A and IL-6 levels (r = 0.71, P <0.001, and r = 0.70, P <0.001), serum IL-17A and IL-6 (r = 0.72, P <0.001, and r = 0.67, P <0.01).
Histopathology of the salivary glands
Histopathology of the pSS patients’ SGs (NCs were not biopsied) revealed inflammatory infiltrates and focal sialadenitis close to the acini and the ducts. The degree of MNC infiltration increased, together with moderate acinar atrophic changes in early and intermediate pSS (from the left to the right in Figure 6a). As predicted, patients with severe SS exhibited such an extensive infiltration of MNC, widespread destruction of the ECs, and general dilatation of the acini and ducts.
Figure 6.
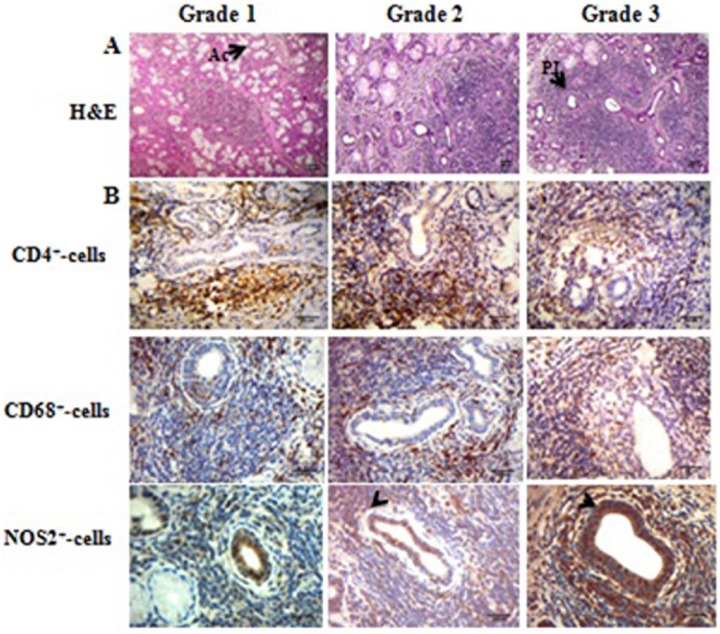
(a) A representative example of hematoxylin and eosin-stained diseased salivary glands. For grades 1–3, refer to the legend in Figure 2. Note the extensive mononuclear cell infiltration and tissue disruption. Ac, acini; PII, periductal inflammatory infiltrate. Original magnification ×100. (b) A representative example of CD4, CD68, and NOS2 expression. Patients from the three groups are presented. The arrow points to a single-positive cell for NOS staining. Original magnification ×400.
Detection of CD4, CD68, and NOS2 in PSS patients’ salivary glands
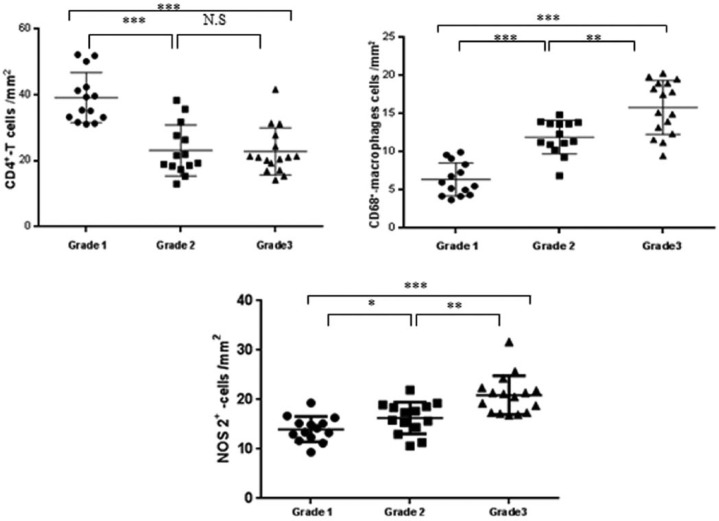
An in-depth histochemical analysis revealed that there were CD4+, CD68+, and NOS2+ expressing cells in the inflammatory areas (from the top to the bottom in Figure 6b). Moreover, all of the SG samples produced NOS2. Intriguingly, NOS2 was predominant in the acinar and ductal ECs (arrowheads in Figure 6b). On the other hand, CD4+ T lymphocytes and CD68+ MØ were confined to the inflammatory infiltrate. Moreover, the numbers of NOS2+ and CD68+ cells increased. Conversely, the number of CD4+ T lymphocytes decreased relative to the increase in the grade of inflammation (Figure 7).
Figure 7.
CD4+, CD68+, and NOS2+ cells according to the grade. For grades 1–3, refer to the legend in Figure 2. CD4+ T cells decreased with the grade, whereas CD68+ and NOS2+ cells increased. NS, non-significant. P values are *<0.05, **<0.01, and ***<0.001.
Discussion
Primary SS is associated with a plethora of autoAbs1,4,7,28 and the Algerian pSS patients presented with both SSA and SSB Abs. Not only are these Abs acknowledged as part of the criteria for the classification of pSS, but they are also suspected of being pathogenic.29 In this respect, some reports,7,26,30 but not all authors,1,31,32 allege that serological markers correlate with the various histological abnormalities observed in pSS. Compelling support for this viewpoint is derived from our findings that severe infiltration is associated with anti-SSA and anti-SSB Abs. Therefore, patients positive for both Abs or only for anti-SSB Abs, scored a higher histopathology grade than those positive only for anti-SSA Abs.33
In line with previous reports,29,30,34,35 systemic and salivary NO were elevated in SS patients. Clearly, NO maintains the function of the SGs, and thereby regulates the salivary flux. Peroxynitrite, which is derived from NO reacting with superoxide, is believed to refine cell signaling, and participates in the tissue pathogenesis.36 The higher the serum levels of NO; the more severe the apoptosis-mediated lesions. Therefore, every likelihood asserts that NO damages the SGs, as confirmed by our findings that NOS2 accompanies the presence of numerous MØ as well as extensive tissue damage. In contrast, evidence that CD4+ cells do not increase according to the infiltration grade may simply mean that the response is localized. Furthermore, the presence of MØ teaches implies that even non-specific inflammation is be pathogenic in pSS, as described in a report of MØ related to cryoglobulinemia, ECs involvement, and lymphoma.37 We are the first to demonstrate a relationship between NO, NOS2, histopathology, and tissue lesions in pSS. Thus, an excess of NOS and prominent cellular infiltrate depends on the presence of inflammatory cytokines. Moreover, excessive NO nitrosylates and functional proteins promote apoptosis.34 Thus, the inducible enzyme may participate in tissue damage through the excess production of NO. Indeed, some degree of inflammation is required for NOS2 to be produced in patients with periodontitis.20 Given the presence of NOS2 in the histological samples, both acinar and ductal ECs produce NO. This could be a result of the local cytokines as reflected by the expression of NOS2 and the subsequent synthesis of NO synthesized in ECs cultured with IL-1β.38 Therefore, activated ECs likely initiate the autoimmune cascade. Supporting this view, our results assign a key role to the ECs of the acini and ducts.
The Algerian SS patients in this study were found to have elevated serum and salivary levels of IL-10 as observed in other populations.39–41 Moreover, the serum levels of IL-10 were correlated with the number of MNCs found in the SG infiltrate. Similarly, correlations have also been found between the serum levels of IL-10, and the focus scores of pSS,40 as well as the severity of systemic lupus erythematosus.41 It has also been postulated that high serum IL-10 levels increase the susceptibility to pSS.39 Although IL-10 synergizes with IL-6 to promote the maturation of plasma cells, it does not equilibrate the system. Furthermore, resistance to apoptosis would be defective in pSS.17,42 Our results suggest that IL-10 is not sufficient to resist the level of IL-17A, TNF-α, and IL-6-induced inflammation. Therefore, the balance between pro- and anti-inflammatory cytokines is unbalanced. There is a need for larger samples of SS patients to identify the mechanisms through which IL-10 is regulated in pSS.
The levels of IL-6, IL-17A, and TNF-α follow the inflammatory status.38–43 However, it is unclear if the increases in IL-610,43,44 and IL-17A3,12 are involved in NOS2 upregulation. The assumption is endorsed by the correlation between the in situ production of these cytokines, the over-expression of NOS2, and the subsequent progression of the disease. Supporting this concept, Alunno et al.45 have reported that IL-17A serum levels are correlated with the SS disease duration. Surprisingly, in contrast to NO, TNF-α diminished as the infiltration evolved, confirming the previously reported46 lack of correlation between the serum levels of TNF-α and the focus score. This negative result does not negate a role for the cytokine, since there was a higher concentration of serum and salivary TNF-α in the SS patients than in the NCs.45 It is also important to note that the serum and salivary IL-6 and IL-17A correlated with the amount of infiltration, implicating these cytokines in its progression. Their expression also correlates with the degree of SG inflammation.10,44,46 Moreover, IL-6 influences the immune response by inhibiting Tregs, and favoring IL-17-producing Th17 cells which are key in the physiopathology of SS.3,11,12 This is confirmed by our finding that IL-6, IL-17A, and NO evolve together with the amount of cellular infiltration. Given that Toll-like receptor (TLR)-2,47 TLR-4,48 and TLR-949 have an effect on IL-6 production and B cell maturation, anti-TLRs,50 competitors of the IL-6 receptor,51 warrant further attention for the treatment of non-organ-specific autoimmune diseases. In addition, several studies conducted in inflammatory and experimental autoimmune models indicate a beneficial immunomodulatory effect of TLR2/4 inhibitors, such as VGX-1027.52–54 In this context, it was reported that the use of TLR2 and TLR4 inhibitors have exhibited preclinical efficacy in human immuno-inflammatory diseases, such as rheumatoid arthritis and colitis.52–54 The VGX-1027 molecule may be a potential drug candidate for pSS treatment. In addition, IL-6 synergizes with other cytokines to polarize Th cells towards an Th17 phenotype. These Th17 cells function to orchestrate autoreactive B cell germinal centers in autoimmune conditions.55 This effect may be targeted by rituximab (RTX), directed towards the B cell CD20 marker, as reported elsewhere.56 Although IL-17-producing pathogenic T cells bear CD20,57 and RTX modulates IL-17 expression in the SG of patients,58 B lymphocytes may be the primary culprits of the auto-immune process. Among other evidence, this mechanism relies on: (1) the B cell dependence of T cell activation in the rheumatoid synovium;59 (2) the contribution of IL-6 on the expression of recombination-activating gene enzymes in B lymphocytes;60 and (3) the need for RTX-induced Il-6-producing B cell depletion for the treatment of T cell-mediated autoimmune diseases (e.g. multiple sclerosis).61 Finally, the impact of epigenetics on immune tolerance should also be elucidated.62
Taken together, our results suggest that the IL-6-IL-17A axis operates in connection with NOS2 overexpression. Furthermore, IL-6, IL-17A, and NO provide novel targets to improve management of SS. Therefore, there is a need to pursue future studies aimed at elucidating the mechanisms of the NOS2 upregulation via the Th17 pathway in pSS.
Acknowledgments
The authors thank the patients and the controls. They also express their gratitude to the surgical staff of the Department of Internal medicine of Maillot Hospital in Algiers.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the Ethical Committee of the National Agency of Research Development in Health.
References
- 1. Moutsopoulos HM. (2014) Sjögren’s syndrome: A forty-year scientific journey. Journal of Autoimmunity 51: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. Betteli E, Carrier Y, Gao W, et al. (2006) Reciprocal development pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 3. Alunno A, Carubbi F, Bistoni O, et al. (2015) T regulatory and T helper 17 cells in primary Sjögren’s syndrome: Facts and perspectives. Mediators of Inflammation 2015: 243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youinou P, Devauchelle V, Pers JO. (2010) Significance of B cells and B cell clonality in Sjögren’s syndrome. Arthritis and Rheumatism 62: 2605–2610. [DOI] [PubMed] [Google Scholar]
- 5. Youinou P, Saraux A, Pers JO. (2012) B lymphocytes govern the pathogenesis of Sjögren’s syndrome. Current Pharmaceutical Biotechnology 3: 2071–2077. [DOI] [PubMed] [Google Scholar]
- 6. Pers JO, Youinou P. (2016) B lymphocytes in primary Sjögren’s syndrome. In: Gerli R. (ed.) Sjögren’s syndrome. Amsterdam: Elsevier. [Google Scholar]
- 7. Youinou P, Pers JO. (2015) Primary Sjögren’s syndrome at a glance today. Joint Bone Spine 82: 75–76. [DOI] [PubMed] [Google Scholar]
- 8. Vitali C, Bombardieri S, Jonsson R, et al. (2002) Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American–European Consensus Group. Annals of the Rheumatic Diseases 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Youinou P, Pers JO. (2011) Disturbance of the cytokine network in Sjögren’s syndrome. Arthritis Research & Therapy 13: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Youinou P, Jamin C. (2009) The weight of IL-6 in B cell-related autoimmune diseases. Journal of Autoimmunity 32: 206–210. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen CQ, Yin H, Lee BH, et al. (2010) Pathogenic effect of IL-17A in induction of Sjögren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Research & Therapy 12: R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Onishi RM, Gaffen SL. (2010) Interleukin-17 and its target genes: Mechanisms of IL-17 function in disease. Immunology 129: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mocellin S, Panelli MC, Wang E, et al. (2003) The dual role of IL-10. Trends in Immunology 4: 36–43. [DOI] [PubMed] [Google Scholar]
- 14. Griffith OW, Stuehr DJ. (1995) NO synthases: Properties and catalytic mechanism. Annual Review of Physiology 57: 707–736. [DOI] [PubMed] [Google Scholar]
- 15. Rettori V, Lomniczi A, Elverdin JC, et al. (2000) Control of salivary secretion by NO and its role in neuroimmunomodulation. Annals of the New York Academy of Sciences 917: 258–267. [DOI] [PubMed] [Google Scholar]
- 16. Looms D, Tritsaris K, Pedersen AM, et al. (2002) NO signaling in salivary glands. Journal of Oral Pathology & Medicine 31: 569–584. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Jamal SA, Torres-Romero LF, et al. (2014) NF-κB controls resistance of human salivary gland cells to apoptosis in an in vitro model of Sjögren’s syndrome. Open Journal of Rheumatology and Autoimmune Diseases 4: 178–191. [Google Scholar]
- 18. Touil-Boukoffa C, Bauvois B, Sanceau J, et al. (1998) Production of NO in human hydatidosis. Relationship between nitrite and IFN-γ levels. Biochimie 80:739–744. [DOI] [PubMed] [Google Scholar]
- 19. Belguendouz H, Messaoudene D, Lahmar K, et al. (2011) IFN-γ and NO production during Behçet uveitis: Immunomodulatory effect of IL-10. Journal of Interferon & Cytokine Research 31: 643–651. [DOI] [PubMed] [Google Scholar]
- 20. Lappin DF, Kjeldsen M, Sander L, et al. (2000) Inducible NO synthase expression in periodontitis. Journal of Periodontal Research 35: 369–373. [DOI] [PubMed] [Google Scholar]
- 21. Rafa H, Saoula H, Belkhelfa M, et al. IL-23/IL-17A axis correlates with the NO pathway in inflammatory bowel diseases: Immunomodulatory effect of retinoic acid. Journal of Interferon & Cytokine Research 37: 355–368. [DOI] [PubMed] [Google Scholar]
- 22. Coleman JW. (2001) NO in immunity and inflammation. International Immunopharmacology 1: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 23. Pacher P, Beckman JS, Liaudet L. (2007) NO and peroxynitrite in health and disease. Physiological Reviews 87: 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ibn Yacoub Y, Rostom S, Laatiris A, et al. (2012) Primary Sjögren’s syndrome in Moroccan patients: Characteristics, fatigue and quality of life. Rheumatology International 32: 2637–2643. [DOI] [PubMed] [Google Scholar]
- 25. Daniels TE. (1984) Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diag-nostic criterion in 362 suspected cases. Arthritis and Rheumatism 27: 147–156. [DOI] [PubMed] [Google Scholar]
- 26. Daniels TE, Whitcher JP. (1974) Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjögren’s syndrome. Arthritis and Rheumatism 37: 869–877. [DOI] [PubMed] [Google Scholar]
- 27. Tarpley TM, Jr, Anderson LG, White CL. (1974) Minor salivary gland involvement in Sjögren’s syndrome. Oral Surgery, Oral Medicine, and Oral Pathology 37: 64–74. [DOI] [PubMed] [Google Scholar]
- 28. Peri Y, Agmon-Levin N, Theodor E, et al. (2012) Sjögren’s syndrome, the old and the new. Best Practie & Research. Clinical Rheumatology 26: 105–117. [DOI] [PubMed] [Google Scholar]
- 29. Kurien BT, Dsouza A, Igoe A, et al. (2013) Immunization with 60 kD Ro peptide produces different stages of preclinical autoimmunity in a Sjögren’s syndrome model among multiple strains of inbred mice. Clinical and Experimental Immunology 173: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brennan MT, Sankar V, Leakan RA, et al. (2002) Risk factors for positive minor salivary gland biopsy findings in Sjögren’s syndrome. Arthritis and Rheumatism 47: 189–195. [DOI] [PubMed] [Google Scholar]
- 31. Radfar L, Kleiner DE, Fox PC, et al. (2002) Clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis and Rheumatism 47: 520–524. [DOI] [PubMed] [Google Scholar]
- 32. Langerman AJ, Blair EA, Sweiss NJ, et al. (2007) Utility of lip biopsy in the diagnosis and treatment of Sjögren’s syndrome. Laryngoscope 117: 1004–1008 [DOI] [PubMed] [Google Scholar]
- 33. Gerli R, Muscat C, Giansanti M, et al. (1997) Quantitative assessment of salivary gland inflamma-tory infiltration in primary Sjögren’s syndrome: Its relationship to different demographic, clinical and serological features of the disorder. British Journal of Rheumatology 36: 969–975. [DOI] [PubMed] [Google Scholar]
- 34. Konttinen YT, Platts LA, Tuominen S, et al. (1997) Role of NO in Sjögren’s syndrome. Arthritis and Rheumatism 40: 875–883. [DOI] [PubMed] [Google Scholar]
- 35. Wanchu A, Khullar M, Sud A, et al. (2000) Elevated nitric oxide production in patients with primary Sjögren’s syndrome. Clinical Rheumatology 19:360–364. [DOI] [PubMed] [Google Scholar]
- 36. Rosignoli F, Rocca V, Meiss R, et al. (2004) Inhibition of calcium-calmodulin kinase restores NO production and signaling in submandibular glands of a mouse model of salivary dysfunction. British Journal of Pharmacology 143: 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manoussakis MN, Boiu S, Korkolopoulou P, et al. (2007) Rates of infiltration by macrophages and dendritic cells and expression of IL-18 and -12 in the chronic inflammatory lesions of Sjögren’s syndrome. Arthritis and Rheumatism 56: 3977–3988. [DOI] [PubMed] [Google Scholar]
- 38. Beauregard C, Brandt PC, Chiou GC. (2007) Induction of NO synthase and overproduction of NO by IL-1β in cultured lacrimal gland acinar cells. Experimental Eye Research 77: 109–114. [DOI] [PubMed] [Google Scholar]
- 39. Anaya JM, Correa PA, Herrera M, et al. (2002) IL-10 influences autoimmune response in primary Sjögren’s syndrome and is linked to IL-10 gene polymorphism. Journal of Rheumatology 29: 1874–1876. [PubMed] [Google Scholar]
- 40. Bertorello R, Cordone MP, Contini P, et al. (2004) Increased levels of IL-10 in saliva of Sjögren’s syndrome patients. Correlation with disease activity. Clinical and Experimental Medicine 4: 148–151. [DOI] [PubMed] [Google Scholar]
- 41. Park YB, Lee SK, Kim DS, et al. (1998) Elevated IL-10 levels correlated with disease activity lupus. Clinical and Experimental Rheumatology 16:283–288. [PubMed] [Google Scholar]
- 42. Manganelli P, Fietta P. (2003) Apoptosis and Sjögren syndrome. Seminars in Arthritis and Rheumatism 33:49–65. [DOI] [PubMed] [Google Scholar]
- 43. Grisius MM, Bermudez DK, Fox PC. (1997) Salivary and serum IL-6 in primary Sjögren’s syndrome. Journal of Rheumatology 24: 1089–1091. [PubMed] [Google Scholar]
- 44. Nibali L, Fedele S, D’Aiuto F, et al. (2012) IL-6 in oral diseases: A review. Oral Diseases 18: 236–243. [DOI] [PubMed] [Google Scholar]
- 45. Alunno A, Bistoni O, Caterbi S, et al. (2015) Serum IL-17 in primary Sjögren’s syndrome: Association with disease duration and parotid gland swelling. Clinical and Experimental Rheumatology 33: 129. [PubMed] [Google Scholar]
- 46. Katsifis GE, Rekka S, Moutsopoulos NM, et al. (2009) Systemic and local IL-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. American Journal of Pathology 175: 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kwok SK, Cho ML, Her YM, et al. (2012) TLR-2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-κB pathway in patients with primary Sjögren’s syndrome. Arthritis Research & Therapy 14: R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Y, Yin H, Zhao M, et al. (2014) TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clinical Reviews in Allergy & Immunology 47:136–147. [DOI] [PubMed] [Google Scholar]
- 49. Guerrier T, Le Pottier L, Devauchelle V, et al. (2012) Role of TLR in primary Sjögren’s syndrome with special emphasis on B cell marturation within exocrine tissues. Journal of Autoimmunity 39: 69–76. [DOI] [PubMed] [Google Scholar]
- 50. Sada PR. (2015) Biological treatment of Sjögren’s syndrome. Rheumatology (Oxford) 54: 219–230. [DOI] [PubMed] [Google Scholar]
- 51. Maini RN, Taylor PC, Szechinski J, et al. (2006) Double-blind randomized controlled clinical trial of the IL-6 receptor antogonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis and Rheumatism 5: 2817–2829. [DOI] [PubMed] [Google Scholar]
- 52. Fagone P, Muthumani K, Mangano K, et al. (2014) VGX-1027 modulates genes involved in LPS-induced TLR-4 activation in a murine model of lupus Immunology 142: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mangano K, Sardesai NY, Quattrocchi C, et al. (2008) Effects of the immunomodulator, VGX-1027, in endotoxin-induced uveitis in Lewis rats. British Journal of Pharmacology 155: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stojanovic I, Cuzzocrea S, Mangano K, et al. (2007) In vitro, ex vivo and in vivo immunopharmacological activities of the isoxazoline compound VGX-1027: Modulation of cytokine synthesis and prevention of both organ-specific and systemic autoimmune diseases in murine models. Clinical Immunology 123: 311–323. [DOI] [PubMed] [Google Scholar]
- 55. Hsu HC, Yang PA, Wang J, et al. (2008) Interleukin 17-producing T helper cells and IL-17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunology 9: 166–175. [DOI] [PubMed] [Google Scholar]
- 56. Devauchelle V, Mariette X, Jousse S, et al. Treatment of primary Sjögren’s syndrome with ritximab, a randomised trial. Annals of Internal Medicine 60: 243–242. [Google Scholar]
- 57. Alunno A, Carubbi F, Bistoni O, et al. (2016) IL-17-producing T lymphocytes express CD20, and are depleted by rituximab in primary Sjögren’s syndrome: A pilot study. Clinical and Experimental Immunology. DOI: 10.1111/cei.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ciccia F, Guggino G, Rizzo A, et al. (2014) Rituximab modulates IL-17 expression in the salivary glands of patients with Sjögren’s syndrome. Rheumatology (Oxford) 53: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 59. Takemura S, Klimiuk PA, Braun A, et al. (2001) T cell activation in rheumatoid synovium is B cell-dependent. Journal of Immunology 167: 4710–4718. [DOI] [PubMed] [Google Scholar]
- 60. Hillion S, Dueymes M, Youinou P, et al. (2007) Il-6 contributes to the expression of Rags in human mature B cells. Journal of Immunology 179: 6790–6798. [DOI] [PubMed] [Google Scholar]
- 61. Barr TA, Shen P, Brown S, et al. (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of Il-6-producing B cells. Journal of Experimental Medicine 209: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Le Dantec C, Varin MM, Brooks WH, et al. (2012) Epigenetics and Sjögren’s syndrome. Current Pharmaceutical Biotechnology 13: 2046–2053. [DOI] [PubMed] [Google Scholar]