Abstract
Renal Doppler ultrasound is increasingly used in nephrology for the evaluation of renovascular disease, allograft dysfunction, and chronic nephropathies. We compared intrarenal hemodynamic parameters to biopsy findings of glomerular sclerosis, tubular atrophy, interstitial fibrosis, crescents, arteriolosclerosis, and clinical variables in 100 patients. A positive correlation exists between renal function and percentage of glomerular sclerosis (P <0.01, r = 0.26), conversely a negative correlation exists between glomerular filtrate rate and percentage of glomerular sclerosis(P <0.0001, r = −0.35). The percentage of glomerular sclerosis correlate positively with pulsatile index (PI) (P <0.05, r = 0.21) and renal resistive index (RI) (P <0.05, r = 0.20). The percentage of crescents correlates positively with PI(P <0.05, r = 0.21) and RI (P <0.05, r = 0.20). Classifying arteriolosclerosis in four groups according to a severity scale, from absence to severe, PI (P <0.05) and RI (P <0.01) were significantly different. In the post hoc analysis, the median values of PI and RI are significantly different in patients with severe arteriolosclerosis than others. Ultrasound examination is a non-invasive diagnostic technique used on patients with suspected or established renal disease. Our study shows a close correlation between kidney function, ultrasound parameters, and histological findings. Measurement of renal parenchymal resistance by ultrasound could be used in association with biopsy and glomerular function for the evaluation of renal damage in patients with glomerulonephritis.
Keywords: arteriolosclerosis, Doppler ultrasound, glomerulosclerosis, renal pathology, renal resistive index
Introduction
Renal Doppler ultrasound (RDU) may be helpful in the evaluation of different nephropathies. The changes in renal parenchymal resistances often may be detected in course of nephropathies. Among the RDU parameters, the renal resistive index (RI) is the most studied and represents a measure of renal parenchymal resistance. Its use has been proposed in the differential diagnosis of several nephropathies, including the therapeutic management of renal artery stenosis,1 kidney transplantation,2 in long-term outcome chronic nephropathies,3 lupus nephritis evaluation,4 monitoring in renal scleroderma crisis, and systemic sclerosis.5–7 Moreover, some studies report a correlation between RI and renal function in patients with renal diseases.8 The aim of this study is to evaluate the relationship between renal parenchymal resistance and histopathologic parameters of renal damage, particularly vascular features.
Materials and methods
From October 2011 to May 2014, 165 patients underwent renal biopsy in our department. We excluded the following from the study: pediatric patients; transplanted kidney biopsies; patients with known renal artery stenosis; urinary tract obstruction; heart failure; and patients treated with immunosuppressive and non-steroidal anti-inflammatory drugs at the time of biopsy. Therefore, the study was conducted on 100 patients. RDU was performed in each patient at the moment of renal biopsy.
The study was conducted in accordance with the protocol, good clinical practice principles, and the Declaration of Helsinki statements. All patients signed an informed consent and the study was approved by the local ethics committee.
Clinical and laboratory data were collected at time of biopsy. Clinical presentation, 24 h proteinuria, urinalysis, serum creatinine level (sCr), and blood urea nitrogen (BUN) were recorded. The study protocol included a complete physical examination, blood drawing, ultrasonographic assessment, and kidney biopsy. Glomerular filtration rate was estimated (eGFR, mL/min) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.9
Renal Doppler ultrasound
RDU was performed immediately before renal biopsy at the Department of Clinical Medicine, Nephrology Unit, Sapienza University of Rome, by a single expert investigator, blinded to the patients’ clinical data (Aplio Ultrasound System SSA-790 with convex 3.5-MHz probe, Toshiba, Tokyo, Japan).
Renal Doppler evaluation of the arcuate arteries in the region of the corticomedullary junction and the interlobar arteries along the border of medullary pyramids was performed by placing the probe at three different positions (mesorenal, superior, and inferior pole) over both kidneys, guided by color flow mapping. The gain was set so that background echoes were barely visible. Doppler gate width was kept small, and the angle of insonation was corrected. An anterior approach was used to detect renal artery origin and an oblique, lateral approach was used for the intermediate tract and intrarenal vessels. No aliasing was allowed in the interlobar arteries while the following parameters were measured: peak systolic velocity (PSV), end diastolic velocity (EDV), resistive index (RI), pulsatile index (PI), and systolic/diastolic ratio (S/D). RI was calculated as (peak systolic frequency shift–minimum diastolic frequency shift)/peak systolic frequency shift and the PI was calculated as (peak systolic frequency shift–minimum diastolic frequency shift)/mean frequency shift. The average of three measurements for each Doppler parameter of arcuate and interlobar arteries in both kidneys was calculated. RI >0.7 was considered pathologic.3 Weighted kappa was used to evaluate the intra-rater re-liability by the same observer. The kappa values for RI and S/D were 0.975 and 0.965, respectively. The intra-patient coefficient of variation for RI and S/D measurement was 1.2% and 1.3%, respectively
Renal biopsy and histology
Renal tissue was obtained by percutaneous needle biopsy. Tissue cores were received within 10 min of biopsy and divided in three portions for immunofluorescence and light and electron microscopy. Multiple paraffin sections were stained with periodic acid–Schiff (PAS), hematoxylin and eosin, and PAS–silver methenamine. All biopsy samples had at least 10 glomeruli. Immunofluorescence was performed on 5-mm cryostat sections using polyclonal fluorescein isothiocyanate-conjugated antibodies to IgG, IgM, IgA, C3, C1q, kappa, lambda, and fibrinogen (Dako, Carpinteria, CA, USA). The severity of glomerular lesions was graded semi-quantitatively on a scale of 0 to 3 according to the percentage of glomerular involvement: 0, absence of lesions; 1, involvement of 1–30% of specimen/total number of glomeruli; 2, involvement of 31–60% of specimen/total number of glomeruli; and 3, involvement of 61–100% of specimen/total number of glomeruli.10 The following glomerular lesions were evaluated: karyorrexis/fibrinoid necrosis, leukocyte exudation, cellular and fibrous crescents, and segmental/global sclerosis. Tubular lesions evaluated were tubulitis and tubular atrophy; interstitial lesions evaluated were inflammatory infiltration and fibrosis. For arteriolosclerosis, we examined the percentage of vessels showing hyaline change or wall thickening. Wall thickening was evaluated as the ratio of luminal diameter to outer diameter and defined as a ratio less than 0.5.11 A pathologist without prior knowledge of any information concerning each patient performed these morphological evaluations.
Statistical analysis
All the results were expressed as mean and standard deviation (SD). Commercially software (SPSS version 22.0) was used for statistical analysis. The coefficient of skewness and the coefficient of kurtosis were used to evaluate normal distribution of data.
Multiple regression analysis was applied for the estimation of relationship of PI, RI, and S/D ratio with clinical features (e.g. age, SCr, CKD-EPI). Pearson product–moment correlation coefficient (r) or, as appropriate, Spearman’s rank correlation coefficient (r) was used for bivariate analysis. Group comparisons were made by Student’s unpaired two-tailed t-test or, as appropriate, by non-parametric Kruskal–Wallis test. P values <0.05 were considered significant.
Results
Clinical and histopathologic characteristics of the 100 patients are listed in Table 1. Patients included 39 men and 61 women (age, 52 ± 16 years). Primary glomerulonephritis diagnosed were 49%, secondary glomerulonephritis were 39%. Among secondary glomerulonephritis, the most common diagnosed were lupus nephritis (78%), dysgammaglobulinemia glomerulonephritis (20%), and metabolic glomerulonephritis (7.7%). Low-grade arteriolosclerosis was shown in 18% of patients, mild-grade arteriolosclerosis in 11%, high-grade arteriolosclerosis in 20%, while 51% of patients did not show arteriolosclerosis.
Table 1.
Clinical, ultrasonographic, and histopathologic characteristics of 100 patients.
| Clinical parameters | |
|---|---|
| Men:Women | 39:61 |
| Age (years) | 52 ± 16 |
| Proteinuria (g/24 h) | 3.4 ± 3.1 |
| Urea (mg/dL) | 66 ± 66 |
| sCr (mg/dL) | 2.1 ± 2.18 |
| eGFR (mL/min 1.73m2) | 58.66 ± 36.35 |
| Hypertension | 55 |
| Diabetes mellitus | 9 |
| Ultrasonographic parameters | |
| RI | 0.67 ± 0.1 |
| PI | 1.34 ± 0.41 |
| S/D | 3.33 ± 1.09 |
| Histopathologic parameters | |
| Primary glomerulonephritis (%) | 49 |
| Membranous nephropathy | 32.7 |
| Focal segmental glomerulosclerosis | 26.5 |
| Mesangial proliferative glomerulonephritis | 16.3 |
| IgA nephropathy | 10.2 |
| Membranoproliferative glomerulonephritis | 10.2 |
| Minimal change disease | 4.1 |
| Secondary glomerulonephritis (%) | 39 |
| Lupus nephritis | 78 |
| Dysgammaglobulinemia glomerulonephritis | 20 |
| Metabolic glomerulonephritis | 2 |
| Tubular interstitial nephritis (%) | 5 |
| Vascular nephropathies | 4 |
| Others | 3 |
| Glomerular sclerosis (%) | 77 |
| Crescents (%) | 29 |
| Arteriolosclerosis (%) | 49 |
| Mild (%) | 18 |
| Moderate (%) | 11 |
| Severe (%) | 20 |
The most predominant diagnosis was membranous glomerulonephritis (32.7%), followed by focal segmental glomerulosclerosis (26.5%), mesangial proliferative glomerulonephritis (16.3%), IgA nephropathy (10.2%), membranoproliferative glomerulonephritis (10.2%), and minimal change disease (4.1%).
Fifty-five patients had hypertension and nine diabetes. Medium value of sCr was 2.1 mg/dl, urea 65.9 mg/dl, eGFR with CKD-EPI was 58.7 ± 36.3 mg/dl, and 24 h proteinuria was 3.4 ± 3.1.
Age showed a positive correlation with PI (P <0.0001, r = 0.38), RI (P <0.01, r = 0.27) and S/D (P <0.0001, r = 0.46). We did not observe correlations between Doppler indices of renal parenchymal resistance and clinical variables (Table 2).
Table 2.
Result of regression analysis of Doppler indices of renal parenchymal resistance with clinical variables.
| Independent variable | Dependent variable | Beta coefficient | t | Sign |
|---|---|---|---|---|
| RI | Age | 0.621 | 2.689 | 0.010 |
| sCr | 0.171 | 1.204 | 0.23 | |
| CKD-EPI | −0.119 | −0.786 | 0.43 | |
| PI | Age | 0.738 | 4.270 | <0.0001 |
| sCr | 0.072 | 0.524 | 0.60 | |
| CKD-EPI | −0.237 | −1.614 | 0.11 | |
| S/D | Age | 0.405 | 3.934 | <0.0001 |
| sCr | 0.230 | 1.715 | 0.90 | |
| CKD-EPI | −0.13 | −0.093 | 0.90 |
Fifty-five patients had hypertension and 45 were normotensive. The mean value of RI not showed significant differences (P >0.05) in patients with hypertension (0.68 ± 0.12) and in patients without hypertension (0.66 ± 0.07).
A positive correlation exists between sCr and percentage of glomerular sclerosis (P <0.01, r = 0.26), conversely a negative correlation exists between CKD-EPI and percentage of glomerular sclerosis (P <0.0001, r = −0.35). The percentage of glomerular sclerosis correlate positively with PI (P <0.05, r = 0.21) and RI (P <0.05, r = 0.20). Any correlation was observed with S/D.
A negative correlation exists between percentage of crescents and CKD-EPI (P <0.05, r = −0.22). The percentage of crescents correlates positively with PI (P <0.05, r = 0.21) and RI (P <0.05, r = 0.20). Any correlation exists between percentage of crescents and S/D.
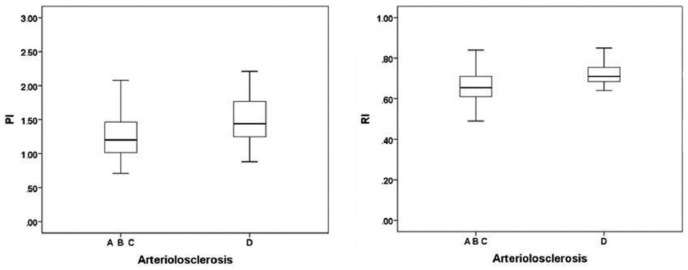
Classifying arteriolosclerosis according to a severity scale, PI (P <0.05) and RI (P <0.01) were significantly different in four groups of arteriolosclerosis (group A, absence of arteriolosclerosis; group B, mild arteriolosclerosis; group C, moderate arteriolosclerosis; group D, severe arteriolosclerosis) (Table 3).
Table 3.
Arteriolosclerosis and Doppler parameters of 100 patients.
| Arteriosclerosis |
PI
|
RI
|
||||
|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | |
| Group A | 1.20 | 0.71 | 2.49 | 0.65 | 0.49 | 0.84 |
| Group B | 1.25 | 0.92 | 2.70 | 0.68 | 0.04 | 0.87 |
| Group C | 1.29 | 0.81 | 1.71 | 0.68 | 0.57 | 0.78 |
| Group D | 1.44 | 0.88 | 2.21 | 0.71 | 0.58 | 0.85 |
S/D did not show any significant difference in the four groups. In the post hoc analysis, the median values of PI and RI were significantly higher in group D than in the others (Figure 1). Any difference between PI, RI, and groups A, B, and C was found.
Figure 1.
Correlations between arteriolosclerosis and PI/RI.
When we grouped patients in glomerular diseases (n = 88) and non-glomerular diseases (n = 9), the ROC curves (AUC% and 95% CI) demonstrated a poor accuracy of RI 41.1 (range, 25.5–56.7; P = 0.31).
Discussion
In our study glomerular sclerosis and crescents showed a positive correlation with sCr and a negative correlation with CKD-EPI. The Doppler indices (PI and RI) of renal parenchymal resistance increased with progression of glomerular sclerosis and in presence of crescents. Also, vascular damage (arteriolosclerosis) influences renal parenchymal resistance (PI and RI). In the literature, several studies have shown that renal ultrasound parameters such as RI and PI can be used as markers of the progression of renal damage, taking account of extrinsic factors that can influence these parameters.12 Also, an increased RI has been reported to correlate with glomerulosclerosis, tubulointerstitial damage, and vascular lesions.13 However, the results are not consistent, renal histology has been investigated in small populations14 and the majority of the studies have concerned transplanted kidneys.15 Our study was performed on a larger sample of patients than previous studies and only on native kidneys. Elevated RI and PI show greater severity of histopathological features with signs of chronicity and vascular involvement.
Hypertension and age are two other well-known parameters that influence renal resistances. In our study, age represents an independent factor of increased RI; conversely, hypertension showed no influence on renal resistances. Ikee et al. demonstrated that increased RI may be attributed to vascular lesions, rather than systemic hypertension.14 Anyway the limitation of this study is related to the use in some patients of ACE inhibitors (ACE-Is) or Angiotensin II receptor blockers (ARBs). In fact, the vasodilatation secondary to ACE-Is/ARBs may influence RDU parameters.
RI has been found to be associated with renal parenchymal abnormalities. Platt et al. first demonstrated in 41 patients with renal disease the relationship between an increased RI and parenchymal and vascular abnormalities assessed by renal biopsies.16 Renal vasculitides and tubular-interstitial nephropathies are more frequently identified by conventional ultrasound than glomerular nephropathies, since glomerular component accounts only for 8% of the renal parenchyma, whereas the highest percentage is occupied by vascular and tubulo-interstitial components.17
Since Doppler indices of renal parenchymal resistance increase with the progression of vascular (arteriosclerosis) and parenchymal (glomerular sclerosis and crescents) renal damage assessed by renal biopsy, we can assume that the Doppler indices can be used in association with biopsy and glomerular function (sCr and CKD-EPI) for the evaluation of renal damage in patients with glomerulonephritis.
Larger prospective studies are needed to demonstrate the role of Doppler indices of renal parenchymal resistance in glomerulonephritis assessment.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Cianci R, Martina P, Cianci M, et al. (2010) Ischemic nephropathy: Proteinuria and renal resistance index could suggest if revascularization is recommended. Renal Failure 32(10): 1167–1171. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Covarrubias L, Martinez A, Morales-Buenrostro LE, et al. (2010) Parameters of Doppler ultrasound at five days posttransplantation as predictors of histology and renal function at one year. Transplantation Proceedings 42(1): 262–265. [DOI] [PubMed] [Google Scholar]
- 3. Parolini C, Noce A, Staffolani E, et al. (2009) Renal resistive index and long-term outcome in chronic nephropathies. Radiology 252(3): 888–896. [DOI] [PubMed] [Google Scholar]
- 4. Conti F, Ceccarelli F, Gigante A, et al. (2014) Ultrasonographic evaluation of renal resistive index in patients with lupus nephritis: correlation with histologic findings. Ultrasound in Medicine & Biology 40(11): 2573–2580. [DOI] [PubMed] [Google Scholar]
- 5. Gigante A, Rosato E, Liberatori M, et al. (2014) Autonomic dysfunction in patients with systemic sclerosis: Correlation with intrarenal arterial stiffness. International Journal of Cardiology 177(2): 578–580. [DOI] [PubMed] [Google Scholar]
- 6. Rosato E, Barbano B, Gigante A, et al. (2014) Intrarenal arterial stiffness may predict the occurrence of new digital ulcers in systemic sclerosis. Arthritis Care & Research 66(9): 1380–1385. [DOI] [PubMed] [Google Scholar]
- 7. Rosato E, Gigante A, Barbano B, et al. (2013) Doppler indices of intrarenal arterial stiffness are useful in monitoring scleroderma renal crisis. Scandinavian Journal of Rheumatology 42(1): 80–81. [DOI] [PubMed] [Google Scholar]
- 8. Pontremoli R, Viazzi F, Martinoli C, et al. (1999) Increased renal resistive index in patients with essential hypertension: A marker of target organ damage. Nephrology, Dialysis, Transplantation 14: 360–365. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, et al. (2009) CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sis B, Mengel M, Haas M, et al. (2010) Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. American Journal of Transplantation 10: 464–471. [DOI] [PubMed] [Google Scholar]
- 11. Katafuchi R, Kiyoshi Y, Oh Y, et al. (1998) Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clinical Nephrology 49: 1–8. [PubMed] [Google Scholar]
- 12. Prabahar MR, Udayakumar R, Rose J, et al. (2008) Prediction of tubulo-interstitial injury by Doppler ultrasound in glomerular diseases: Value of resistive and atrophic indices. Journal of the Associated Physicians of India 56: 21–26. [PubMed] [Google Scholar]
- 13. Sugiura T, Nakamori A, Wada A, et al. (2004) Evaluation of tubulointerstitial injury by Doppler ultrasonography in glomerular diseases. Clinical Nephrology 61: 119–126. [DOI] [PubMed] [Google Scholar]
- 14. Ikee R, Kobayashi S, Hemmi N, et al. (2005) Correlation between the resistive index by Doppler ultrasound and kidney function and histology. American Journal of Kidney Disease 46(4): 603–609. [DOI] [PubMed] [Google Scholar]
- 15. Naesens M, Heylen L, Lerut E, et al. (2013) Intrarenal resistive index after renal transplantation. New England Journal of Medicine 369(19): 1797–1806. [DOI] [PubMed] [Google Scholar]
- 16. Platt JF, Ellis JH, Rubin JM, et al. (1990) Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: Correlation of resistive index with biopsy findings. American Journal of Roentgenology 154(6): 1223–1227. [DOI] [PubMed] [Google Scholar]
- 17. Quaia E, Bertolotto M. (2002) Renal parenchymal diseases: Is characterization feasible with ultrasound? European Radiology 12: 2006–2020. [DOI] [PubMed] [Google Scholar]