Abstract
Diethylnitrosamine (DEN)-induced liver cancer normally develops in stages that progress from cirrhosis and carcinoma. Increased oxidative stress is suggested to play a role in DEN-induced carcinogenicity. Blueberries (BB) contain high antioxidant capacity. We investigated the effect of BB supplementation on development of DEN-induced cirrhosis and neoplastic lesions in the liver. Rats were injected with DEN (200 mg/kg; i.p.) three times with an interval of 15 days at 4, 6, and 8 weeks and sacrificed 8 weeks after the last DEN injection. They were also fed on 8% BB (w/w) containing chow for 16 weeks. Hepatic damage markers in serum were determined together with hepatic histopathological examinations. Hydroxyproline (HYP), malondialdehyde (MDA), diene conjugate (DC), protein carbonyl (PC), and glutathione (GSH) levels, and CuZn-superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activities, and their mRNA expressions were measured. Protein and mRNA expressions of glutathione transferase-pi (GST-pi) were evaluated as a marker of preneoplastic lesions. BB supplementation decreased hepatic damage markers in serum and hepatic MDA, DC, and PC levels, but SOD, CAT, and GSH-Px activities and their mRNA expressions remained unchanged in DEN-treated rats. BB attenuated cirrhotic changes and decreased hepatic HYP levels and GST-pi expressions. Our results indicate that BB is effective in decreasing development of DEN-induced hepatic cirrhosis and preneoplastic lesions by acting as an antioxidant (radical scavenger) itself without affecting activities and mRNA expressions of antioxidant enzymes.
Keywords: blueberry, cirrhosis, diethylnitrosamine, oxidative stress, preneoplastic lesions
Hepatocellular carcinoma (HCC) is the sixth most commonly encountered cancer in the world. Pre-existing fibrosis has been observed to present in most of the patients. Progression from fibrosis to cirrhosis and cancer was detected in almost 80% of them.1 The molecular mechanism underlying the generation of hepatocarcinogenesis in fibrotic/cirrhotic livers is not clearly understood.1,2
Among hepatocarcinogenic agents, diethylnitrosamine (DEN) is known to exert its carcinogenic activity powerfully. Alterations in DNA structure and increased oxidative stress play a role in DEN-induced carcinogenicity.1,2–6 Since hepatic tumor production by DEN is observed to proceed similar to human liver cancer, DEN-induced hepatocarcinogenesis happens to be an ideal model to investigate in rodents.2,7–9 Liver damage leading to fibrosis/cirrhosis and finally to HCC can be manipulated by DEN dosage and time of application. Since three stages as inflammation, cirrhosis, and HCC are present in the progression of the disease, cirrhosis without evidence of HCC, HCC without cirrhosis, or cirrhosis and HCC may be produced by DEN with different doses and models of application in rats.2,7–9
As it is known, there is no effective chemotherapy available for the treatment of cirrhosis and HCC. Therefore chemoprevention may be useful for the treatment of these diseases.10,11 Hytochemicals have received significant attention in this field. A large number of these agents have displayed chemo-preventive action in DEN-induced HCC.10–13
Blueberries (BB; Vacciniumcorymbosum L.) are among the fruits with high antioxidant power and contain anthocyanins, polyphenols, and flavonoids. The use of BB was suggested as useful in inflammation, cancer, diabetes mellitus, and hepatic, cardiovascular, and neuronal disorders. This state is attributable to powerful antioxidant actions of constituents in BB.14,15
In the current study, we wanted to investigate whether a preventive effect of BB treatment on DEN-induced oxidative stress, and cirrhotic and precancerous lesions in the liver. For this reason, hepatic damage markers in serum were determined together with hepatic histopathological examinations. Hepatic hydroxyproline (HYP), malondialdehyde (MDA), diene conjugate (DC), protein carbonyl (PC), and glutathione (GSH) levels, CuZn-superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activities, and their mRNA expressions were measured. Protein and mRNA expressions of glutathione transferase-pi (GST-pi) were also evaluated as a marker of preneoplastic lesions.
Materials and methods
Chemicals, materials
DEN and other chemicals were supplied from Sigma-Aldrich (St Louis, MO, USA).
Animals
Male Wistar rats weighing 200–250 g were used in the study. They were obtained from the Experimental Medical Research Institute of Istanbul University. Rats were housed in a light- and temperature-controlled room on a 12/12-h light/dark cycle. The animals were allowed free access to food and water, and were kept in wire-bottomed stainless steel cages. The experimental procedure used in this study met the guidelines of the Animal Care and Use Committee of the Istanbul University.
Preparation of foods
Fresh Northern highbush “Patriot” BB (Vacciniumcorymbosum L.) were donated by Gedik Flora (Kartal-Istanbul) and they were stored at −35°C until used. Then, BB were homogenized for 5 min using a blender.
A part of BB homogenates was used for the determination of total phenolic and total flavonoid content. They were diluted by distilled water and total phenolic compounds were determined with the Folin-Ciocalteu reagent and expressed as mg gallic acid equivalent per 100 g BB.16 The total flavonoid levels were measured with aluminum chloride colorimetric method. The results are presented as mg quercetin equivalents per 100 g BB.17 Total soluble phenolic compounds and total flavonoid levels were detected as 260 mg gallic acid equivalents and 105 mg catechin equivalents per 100 g BB, respectively.
BB homogenates were mixed with powdered rat chow by using a mixer for 15 min. Then, this mixture was dried and prepared as a pellet chow containing 8% BB (w/w) by Barbaros Denizeri AŞ (Istanbul).
Experimental design
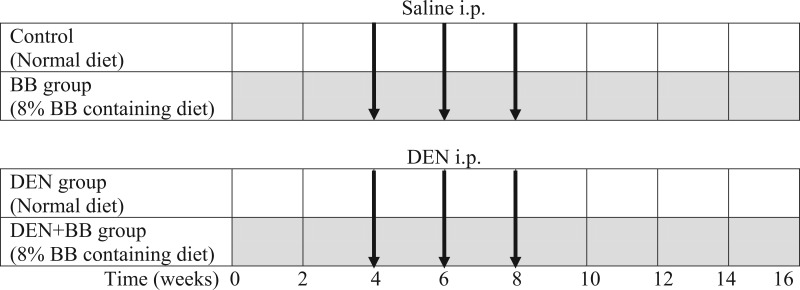
Rats were randomly divided into four groups as follows: (1) Control group (n = 6): Animals were fed with commercial rat chow; (2) DEN group (n = 8): Rats were injected with DEN (200 mg/kg; i.p.) three times with an interval of 15 days at 4, 6, and 8 weeks, and killed 8 weeks after the last DEN injection. They were fed with control diet during experimental period; (3) DEN + BB group (n = 8): Rats were injected with DEN (200 mg/kg; i.p.) three times at an interval of 15 days and they were fed with 8% BB containing diet for 16 weeks; (4) BB group (n = 6): Rats were fed with 8% BB containing rat chow. Experimental design is shown in Figure 1.
Figure 1.
Schematic representation of experimental procedure followed. Black arrows indicate injection moment; gray rows indicate blueberry (BB) treatment.
Blood and tissue samples
At the end of the treatment period, all rats were sacrificed by taking blood via cardiac puncture under sodium thiopental anesthesia (50 mg/kg, i.p.). Blood was collected in dry tubes. The livers were rapidly removed, washed in 0.9% NaCl and kept in ice. The materials were stored at −80°C until they were analyzed.
Determinations in serum
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) measurements were performed on Cobas Integra 800 autoanalyzer (Roche Diagnostics, Mannheim, Germany).
Determination of liver HYP levels
Liver HYP levels were measured by the method described by Bergman and Loxley.18 Briefly, 100 mg of liver tissue were hydrolyzed in 4 mL of 6 N HCl at 108°C for 16 h in a glass tube with teflon stopper. The hydolysate was filtered and 0.5 mL of hydrolysate was neutralized with approximately 0.4 mL of 6 N NaOH. Then, 0.5 mL of neutral or faintly acid solution was pipetted into clean tubes. Standards and samples were incubated with 1.0 mL oxidizing reagent containing chloramine T whereas, blank samples were incubated with same amount of oxidizing reagent without chloramine T for 5 min. One milliliter of Ehrlich’s reagent was added to all tubes, mixed and incubated at 60°C for 45 min. Absorbances were read at 570 nm with spectrophotometer. Results were calculated from standard curve generated from known quantities of HYP.
Determination of lipid peroxides and PC levels
Liver tissue was homogenized in ice-cold 0.15 M KCl (10%; w/v). Lipid peroxidation was assessed by two different methods in the tissue homogenates. First, the levels of MDA were measured the method of Ohkawa et al.19 The breakdown product of 1,1,3,3-tetraethoxypropane was used as a standard. Results were expressed as nmol/g tissue. Hepatic lipids were extracted with chloroform:methanol (2:1)20 and DC levels were determined in tissue lipid extracts at 233 nm spectrophotometrically and calculated using a molar extinction coefficient of 2.52 × 104 M−1cm−1. DC results were expressed as µmol/g tissue.21
The oxidative protein damage was measured by the quantification of carbonyl groups based on spectrophotometric detection of the reaction with 2,4-dinitrophenylhydrazine with PC to form protein hydrazones. Hepatic PC levels were calculated from the maximum absorbance (360 nm) using a molar extinction coefficient of 22,000 M−1cm−1. The results were expressed as nmol carbonyl per mg protein.22
Determination of GSH levels and antioxidant enzyme activities
GSH levels were measured in the homogenates with 5,5-dithiobis-(2-nitrobenzoate) at 412 nm according to the method of Beutler et al.23
Liver homogenates were centrifuged at 600 ×g for 10 min at 4°C to remove crude fractions and supernatants were used for the determination of CAT activity. To determine SOD, GSH-Px and GST activities, liver homogenates were also centrifuged at 10,000 ×g for 20 min and activities were determined in postmitochondrial fraction.
SOD activity was assayed by its ability to increase the effect of riboflavin-sensitized photo-oxidation of o-dianisidine.24 CAT activity was measured by using hydrogen peroxide as substrate.25 The disappearance of H2O2 was followed spectrophotometrically at 240 nm. One unit of CAT was considered the amount of enzyme needed to degrade 1 μmol H2O2 per min at 25°C. GSH-Px activity was measured using cumene hydroperoxide as substrate.26 GST activity was determined 1-chloro-2,4-dinitrobenzene as substrate.27 Protein levels were determined by using bicinchoninic acid.28
Determination of mRNA expressions of antioxidant enzymes
mRNA expressions of SOD, CAT, GSH-Px, and GST-Pi were determined using by real-time quantitative polymerase chain reaction. All reagents and equipment for mRNA/cDNA preparation were purchased from Roche Applied Science Diagnostics (Mannheim, Germany). Total RNA was extracted from the liver tissues using a commercially available RNA extraction kit (High Pure PCR Template Preparation Kit) according to manufacturer’s instructions. RNA concentration and purity were detected by using UV-Vis spectrophotometer (Shimadzu, MD, USA). cDNA was synthesized with a reverse transcription (RT) kit (Transcriptor High Fidelity cDNA synthesis kit) using 250 ng RNA in a total volume of 20 µL.
qPCR was performed by using the “TaqMan Master kit” in the LightCycler 2.0 system. Real-time ready-probes were used for SOD (CuZnSOD; #100023905), CAT (#100047547), GSH-Px (#100047787), and GST-Pi (#100047529). For the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), universal probe library (UPL)-probe was used (#05046220001). All PCR reactions were performed for 45 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 1 s. Each sample was quantified by measuring its fluorescence resonance energy with the LightCycler quantification software. GAPDH served as a control for RNA and relative quantification.
Histopathological studies
Liver tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) for histological examination. The Gomori Reticulin stain was also performed to show reticulin fibers of fibrotic areas. Fibrosis was classified by using H&E staining according to Ishak’s stage:29 0, no fibrosis; 1, fibrous expansion of some portal areas, with or without short fibrous septa; 2, fibrous expansion of most portal areas, with or without short fibrous septa; 3, fibrous expansion of most portal areas with occasional portal to portal (P-P) bridging; 4, fibrous expansion of portal areas with marked bridging (P-P as well as portal to central [P-C]) to portal [P-P] as well as portal to central [P-C]); 5, marked bridging (P-P and/or P-C) with occasional nodules (incomplete cirrhosis); and 6, cirrhosis, probable or definite.
Immunohistochemistry for GST-Pi
Hepatic GST-Pi expressions were examined by immunohistochemical procedure as a marker of preneoplastic lesions. Slides containing 5-mm slices were rehydrated through graded alcohol series and immersed in citrate buffer solution (Citrate Buffer, Thermo Scientific, Germany) and placed in a commercial microwave oven for antigen retrieval for 20 min. Endogen peroxidase activity was blocked by incubating with 3% hydrogen peroxide. To prevent non-specific staining, slides were treated with blockage solution for 15 min (Super Block, Scytek Laboratories, USA). Primary antibodies of GST-Pi (#311-H; Anti-GST-P rabbit polyclonal antibody, MBL, Nagoya, Japan) were applied to the specimens then incubated for 60 min. After this process, biotinylated secondary antibody (goat anti-rabbit IgG; Santa Cruz Biotechnology, Heidelberg, Germany), streptavidin peroxidase, and substrate-chromogen (AEC) solution were applied, respectively. Nuclear staining was performed with hematoxylin. Staining intensity was defined as a percentage and given a score in the range of 1–3: 5–30% (+), 30–60% (++), and >60% (+++).
Statistical analysis
The results were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post-hoc test was used for equal variances. Kruskal–Wallis test (post-hoc Mann–Whitney U-test) was performed for unequal variances. In all cases, a difference was considered significant when P <0.05.
Results
Body weight and liver weight
There were no significant differences in daily food and water consumption and final body weights between control and experimental groups. Liver weights (g) increased in DEN (9.07 ± 0.42) and DEN+BB (8.30 ± 0.38) groups in comparison with the control group (7.13 ± 0.44). However, there was no significant difference in liver weights of rats in DEN and DEN + BB groups.
Hepatic function tests in serum
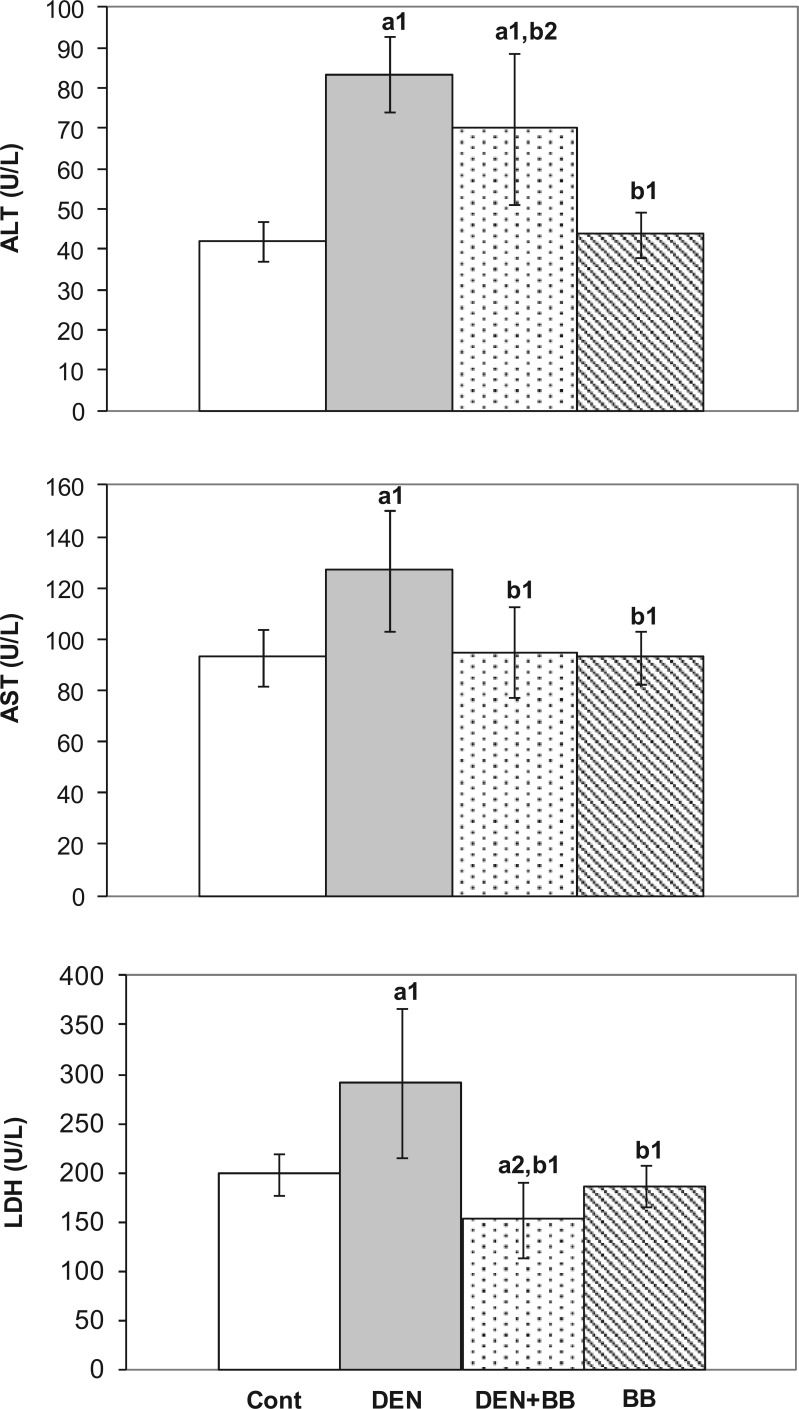
Hepatocellular damage is as evident by a significant elevation in serum activities of ALT, AST, and LDH activities which have been used as reliable markers of hepatotoxicity. DEN treatment indeed caused significant increases in serum ALT, AST, and LDH activities. These enzyme activities were observed to decrease in DEN-treated rats due to BB treatment (Figure 2).
Figure 2.
The effects of blueberry (BB) supplementation on serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) activities in diethylnitrosamine (DEN)-treated rats. Values are expressed as mean ± SD of duplicates (n = 6 for control and BB groups; n = 8 for DEN and DEN + BB groups). a1P <0.01, a2P <0.05 as compared to controls; b1P <0.01, b2P <0.05 as compared to DEN.
Hepatic HYP, MDA, DC, and PC levels
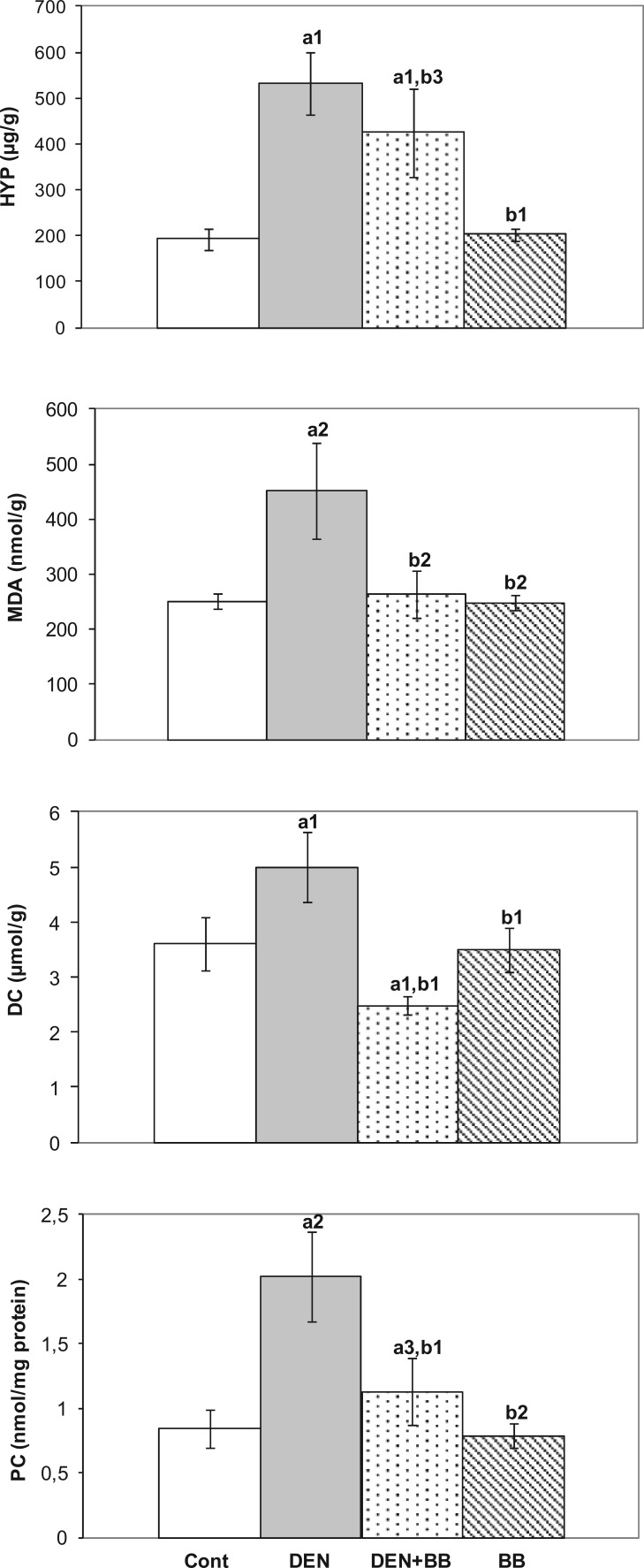
Hepatic HYP levels were analyzed to evaluate hepatic collagen production after DEN administration. Significant increases in hepatic HYP levels were found in DEN-treated rats as compared to controls. However, high levels of HYP levels decreased due to BB treatment in DEN-treated rats (Figure 3).
Figure 3.
The effects of blueberry (BB) supplementation on hepatic hepatic hydroxyproline (HYP) and malondialdehyde (MDA), diene conjugate, and protein carbonyl (PC) levels in diethylnitrosamine (DEN)-treated rats. Values are expressed as mean ± SD of duplicates (n = 6 for control and BB groups; n = 8 for DEN and DEN + BB groups). a1P <0.001, a2P <0.01, a3P <0.05 as compared to controls; b1P <0.001, b2P <0.01, b3P <0.05 as compared to DEN.
To examine the effects of DEN administration on lipid peroxidation and protein oxidation, MDA, DC, and PC levels were determined. Hepatic MDA, DC, and PC levels increased significantly due to DEN treatment. Significant decreases in these parameters were detected due to BB treatment as compared to DEN-treated rats (Figure 3).
Hepatic antioxidants and mRNA expressions
Although GSH levels and GST activities were observed to increase, but SOD, CAT, and GSH-Px activities decreased in the liver of DEN-treated rats. However, BB supplementation did not alter these parameters in the liver of DEN-treated rats (Table 1). mRNA expressions of hepatic SOD, CAT, and GSH-Px enzymes were found to decrease in DEN-treated rats. These expressions remained unchanged due to BB treatment. However, DEN treatment caused remarkable increases in mRNA expressions of GST-pi as compared to control group. High GST-pi mRNA expression in DEN group decreased after BB supplementation (Table 2).
Table 1.
The effects of blueberry (BB) supplementation on hepatic glutathione (GSH) levels and superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione transferase (GST) activities in diethylnitrosamine (DEN)-treated rats (mean ± SD).
| Control (n = 6) | DEN (n = 8) | DEN + BB (n = 8) | BB (n = 6) | |
|---|---|---|---|---|
| GSH (μmol/g) | 6.24 ± 0.35 | 9.54 ± 1.42a1 | 8.89 ± 0.80a1 | 6.36 ± 0.23b1 |
| SOD (U/mg protein) | 7.24 ± 1.13 | 5.26 ± 0.92a1 | 6.20 ± 0.88 | 7.17 ± 0.87b1 |
| CAT (μmol/min/mg protein) | 495.2 ± 70.4 | 347.9.0 ± 69.4a1 | 381.7 ± 55.1a2 | 486.2 ± 63.0b1 |
| GSH-Px (nmol/min/mg protein) | 825.5 ± 73.8 | 675.7 ± 60.6a2 | 617.1 ± 139.1a1 | 817.7 ± 73.4b2 |
| GST (nmol/min/mg protein) | 841.6 ± 65.7 | 1029.7 ± 81.0a1 | 952.0 ± 133.2 | 838.4 ± 66.6b1 |
P <0.01, a2P <0.05 as compared to control; b1P <0.01, b2P <0.05 as compared to DEN.
Table 2.
Relative mRNA expressions of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione transferase-pi (GST-Pi) in the liver of diethylnitrosamine (DEN)-treated rats.
| Control | DEN | DEN + BB | BB | |
|---|---|---|---|---|
| SOD | 0.89 ± 0.13 | 0.67 ± 0.05a2 | 0.65 ± 0.11a2 | 0.94 ± 0.16b2 |
| CAT | 1.05 ± 0.05 | 0.69 ± 0.22a1 | 0.63 ± 0.11a1 | 1.06 ± 0.07b1 |
| GSH-Px | 1.11 ± 0.09 | 0.82 ± 0.16a2 | 0.84 ± 0.22a2 | 1.05 ± 0.06b2 |
| GST-pi | 0.99 ± 0.06 | 23.7 ± 6.90a1 | 14.3 ± 5.41a1,b1 | 1.01 ± 0.09b2 |
The amounts of target genes mRNA were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and the results were expressed as fold change compared to that of normal rat livers which was assigned a value of 1. The data shown represent two independent experiments (mean ± SD; n = 6; each).
P <0.01, a2P <0.05 as compared to controls; b1P <0.01, b2P <0.05 as compared to DEN.
Histopathological observations
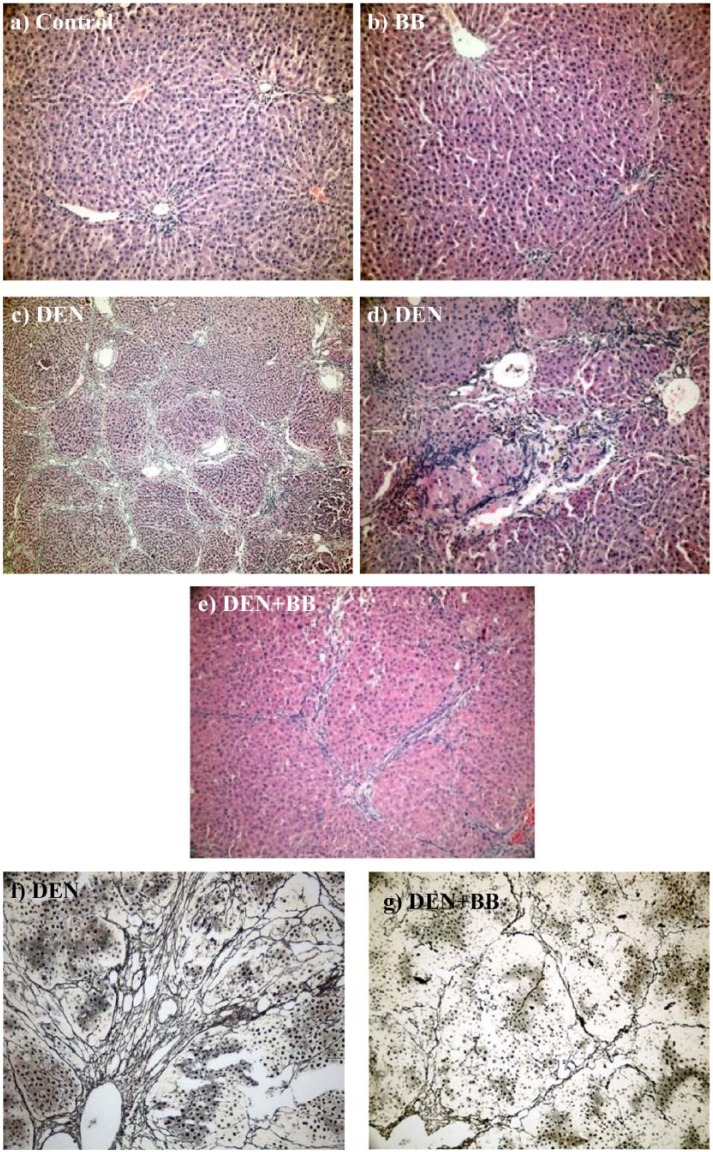
The Ishak stages for fibrosis and GSP-Pi expression scores for the DEN and DEN + BB groups were summarized in Table 3. In histopathologic examination, normal histologic appearance of liver was observed in both control and BB groups (Figure 4a, b). According to the Ishak stages, the fibrosis score in the DEN + BB group (2.66 ± 0.81) was significantly lower than the DEN-treated group (4.00 ± 1.09). Remarkable fibrosis P-P and P-C areas were determined in the DEN group. In three examples of the group the incomplete cirrhosis that circumscribed as a nodular formation of liver tissue was shown (Figure 4c, d). Inflammatory infiltration, parenchymal necrosis, and biliary stasis were also observed in these areas. In contrast to the DEN group, the DEN + BB group showed less liver cell damage. The sections displayed P-C fibrosis containing fibrous bands. Inflammation and parencyhmal cell necrosis were mildly determined (Figure 4e). The Gomori Reticulin stain exposed the reticulin fibers of fibrotic areas clearly in the DEN and DEN + BB groups (Figure 4f, g).
Table 3.
Ishak stages and GST-Pi scores in the DEN and DEN + BB groups.
| Ishak stage | GST-Pi | |
|---|---|---|
| DEN group | ||
| 1 | 3 | 3 |
| 2 | 5 | 3 |
| 3 | 5 | 3 |
| 4 | 5 | 3 |
| 5 | 3 | 3 |
| 6 | 3 | 3 |
| DEN + BB group | ||
| 1 | 2 | 2 |
| 2 | 3 | 2 |
| 3 | 4 | 2 |
| 4 | 2 | 1 |
| 5 | 3 | 2 |
| 6 | 2 | 2 |
Figure 4.
The representative hepatic histopathology: (a, b) Control and blueberry (BB) groups: Normal histologic appearance of liver (H&E ×200); (c, d) DEN group: Lobular architecture, parenchymal nodules, distinct fibrosis in portal-portal and portal-central areas, inflammatory cells, necrotic parenchymal cells, indicating incomplete cirrhosis (H&E ×100 and ×200; respectively); (e) DEN + BB group: Portal-central and portal-portal fibrosis as thin fibrous septa (H&E ×200). According to Ishak stages, fibrosis scores were significantly (P <0.05) decreased in the DEN + BB group (2.66 ± 0.81) as compared to the DEN group (4.00 ± 1.09). The Gomori Reticulin stain exposed the reticulin fibers of fibrotic areas clearly in DEN (f) and DEN + BB (g) groups (×200). However, DEN + BB group showed weaker reticulin frame work than DEN group.
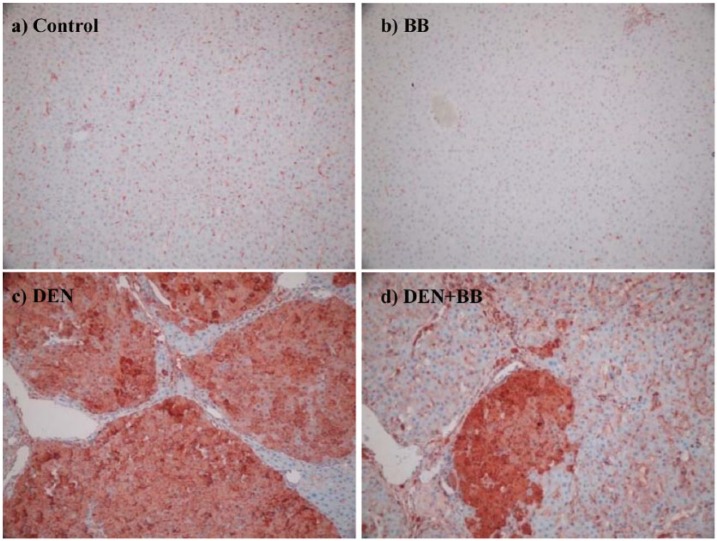
There was no GST-Pi expression in hepatocytes of BB and control groups (Figure 5a, b). DEN and DEN + BB groups showed positivity with GST-Pi antibody. Diffuse and strong GST-Pi positivity was observed at nodular structures in DEN group (Figure 5c). The DEN + BB group also displayed positivity with GST-Pi antibody, however the staining intensity and positive-nodular structure number was decreased in comparison to the DEN group (Figure 5d).
Figure 5.
Immunohistochemical detection of hepatic glutathione transferase-Pi (GST-Pi) expression in diethylnitrosamine (DEN) treatment with and without blueberry (BB) supplementation (×200). (a, b) No expression of GST-Pi in hepatocytes and non-specific staining in other cells at the control and BB groups, respectively; (c) Strong positive reactivity of GST-Pi in the liver tissue among fibrous septa in DEN-treated rats; (d) GST-Pi positive cells as small groups of hepatocytes among thin fibrous septa and nonspecific-stained cells in periphery in the DEN + BB group. GST-Pi expressions were significantly decreased (P <0.001) in the DEN + BB group (1.83 ± 0.40) when compared to the DEN group (3.00 ± 0.00).
Discussion
DEN is a carcinogenic agent known to produce hepatic tumors similar to human HCC. In most models, initiation and promotion steps are important in producing HCC. Initiation by DEN application is usually promoted by phenobarbital, carbon tetrachloride (CCl4), and partial hepatectomy.2,7,30 These models require a long induction period in the range of 12–18 months and produce only HCC without liver cirrhosis.2,7–9 However, in experimental cirrhotic models produced by CCl4, thioacetamide, common bile duct ligation, and alcohol, spontaneous HCC generation during the induction of liver cirrhosis is not possible.7 However, some investigators have introduced a modified DEN treatment protocol that can generate HCC and liver cirrhosis simultaneously.7,8,31,32 Repeated injections of DEN were found to cause significant fibrosis in 8–10 weeks and the existence of both cirrhosis and preneoplastic lesions in 12–16 weeks and HCC in 20 weeks.2,8,9,32,33 Compared to the other models, reproduction of cirrhosis and HCC sequentially as similar to human liver disease becomes possible with this protocol and this may enable the usage of some agents to prevent the progression of cirrhosis to advanced stages.2,7,34
In the current study, rats were injected with DEN (200 mg/kg; i.p.) three times with an interval of 15 days at 4, 6, and 8 weeks, as previously reported,33,35 and sacrificed 8 weeks after the last DEN injection. This treatment caused increased serum ALT, AST, and LDH activities together with cirrhotic changes and increased HYP levels in the liver. However, no HCC nodule was detected either at macroscopic examination or at histological analyses. GST-pi is clearly known to be a strong marker enzyme for rat preneoplastic lesions.33,35–37 In our study, protein and mRNA expressions of hepatic GST-pi were observed to increase in DEN-treated rats. Accordingly, our findings indicate that DEN treatment resulted in precancerous liver cirrhosis in rats.
Oxidative stress was reported to play an important role in development and progression of liver cirrhosis.38 Several researchers have reported increased oxidative stress parameters such as MDA, DC, PC, and DNA damage together with decreased non-enzymatic and enzymatic antioxidants in the liver due to DEN treatment.3–6,34,39,40 In the current study, increases in hepatic MDA, DC, and PC levels and decreases in SOD, CAT, and GSH-Px activities were due to DEN treatment. However, GSH levels and GST activities were observed as increased due to DEN treatment. On the other hand, in the current study, mRNA expressions of SOD, CAT, and GSH-Px were also decreased in the liver of DEN-treated rats. These findings agree with the results of previous studies.7,33–35
BB contains polyphenols, flavonoids, and other active components and its powerful antioxidant actions may be due to free radical scavenger properties.14,15 BB consumption was reported to be useful in oxidative stress-related conditions.14,15 It has been reported that BB exerts protective effects on endotoxin plus D-galactosamine,41 acetaminophen,42 and cadmium43 -induced liver injury. BB supplementation reduced oxidative stress and liver injury in hypercholesterolemic guinea pigs44 and D-galactose-treated rats45 by acting as an antioxidant. Wang et al.46,47 have reported that BB also showed an antifibrotic effect in CCl4-induced hepatic fibrosis. These authors have observed that BB treatment was associated with elevated hepatic expression of metallothionein, increased SOD activity, reduced inflammation, oxidative stress, and α-smooth muscle actin and collagen III expressions in the liver.
There are two studies related to the effect of BB on DEN-induced acute and chronic hepatic injury. In our previous acute study, rats were fed with a diet containing 5% and 10% BB for 6 weeks and a single dose of DEN (200 mg/kg; i.p.) was applied 2 days before the end of this period.40 BB treatment was detected to have an inhibiting effect on acute liver injury by reducing apoptosis, necrosis, proliferation, oxidative, and nitrosative stress in the acute DEN-treated rats. Sadık et al.48 have investigated the effect of BB on chronic DEN-induced hepatocarcinogenesis. DEN (10 mg/kg; p.o., 5 times per week for 15 weeks) administration caused significant increases in alpha-fetoprotein (AFP), a tumor-associated fetal protein and histopathological changes indicating hepatocarcinogenesis. A diet containing 4% freeze-dried BB for 15 weeks improved the histopathological changes and decreased serum AFP levels. According to this, authors have proposed that BB may be useful in the prevention of DEN-induced hepatocarcinogenesis.
In the current study, we wanted to investigate whether BB treatment had a preventive effect on the development of DEN-induced oxidative stress, cirrhotic, and precancerous lesions in the liver. The individual phenolic compounds in BB have strong antioxidant activities, but the antioxidant activities of the combination of phenolics may be better than the individual phenolics. Therefore, in the current study, whole fresh BB supplemented diets were used and their concentrations were chosen according to previous studies.40,41,44,45 According to our results, BB treatment decreased high activities of serum ALT, AST, and LDH activities and hepatic MDA, DC, and PC levels, but GSH levels together with SOD, CAT, and GSH-Px activities and their mRNA expressions did not change. The incomplete cirrhosis which was seen in the DEN group was not observed in the DEN + BB group. In this group, the fibrosis containing thin bands was limited to P-C areas. Indeed, hepatic HYP levels were also found to decrease due to BB treatment. On the other hand, BB treatment decreased high levels of protein and mRNA expressions of GST-pi in DEN-treated rats. According to this, BB treatment reduces the formation of cirrhosis, especially the onset of preneoplastic lesions in rats with cirrhosis. These effects of BB may be attributed to free radical scavenger properties of polyphenols and flavonoids and other active components.
In conclusion, our results indicate that BB have a preventive effect against the formation of DEN-induced hepatic cirrhosis and preneoplastic lesions by acting as an antioxidant (radical scavenger) itself without affecting activities and mRNA expressions of antioxidant enzymes.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The present work was supported by Research Fund of Istanbul University (Project no. 16705).
References
- 1. De Minicis S, Kisseleva T, Francis H, et al. (2013) Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Digestive and Liver Disease 45: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borbath I, Stärkel P. (2011) Chemoprevention of hepatocellular carcinoma. Proof of concept in animal models. Acta Gastro-enterologica Belgica 74: 34–44. [PubMed] [Google Scholar]
- 3. Arul D, Subramanian P. (2013) Inhibitory effect of naringenin (citrus flavonone) on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biochemical and Biophysical Research Communications 434: 203–209. [DOI] [PubMed] [Google Scholar]
- 4. MadanKumar P, NaveenKumar P, Manikandan S, et al. (2014) Morin ameliorates chemically induced liver fibrosis in vivo and inhibits stellate cell proliferationin vitro by suppressing Wnt/β-catenin signaling. Toxicology and Applied Pharmacology 277: 210–220. [DOI] [PubMed] [Google Scholar]
- 5. Paula Santos N, Colaço A, Gil da Costa RM, et al. (2014) N-diethylnitrosamine mouse hepatotoxicity: Time-related effects on histology and oxidative stress. Experimental and Toxicologic Pathology 66: 429–436. [DOI] [PubMed] [Google Scholar]
- 6. Kumar AK, KV (2015) Protective effect of Punicagra-natum peel and Vitisvinifera seeds on DEN-induced oxidative stress and hepatocellular damage in rats. Applied Biochemistry and Biotechnology 175: 410–420. [DOI] [PubMed] [Google Scholar]
- 7. Huang KW, Huang YC, Tai KF, et al. (2008) Dual therapeutic effects of interferon-alpha gene therapy in a rat hepatocellular carcinoma model with liver cirrhosis. Molecular Therapy 16: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 8. Tan Y, Yin P, Tang L, et al. (2012) Metabolomics study of stepwise hepatocarcinogenesis from the model rats to patients: Potential biomarkers effective for small hepatocellular carcinoma diagnosis. Molecular & Cellular Proteomics 11: M111.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma G, Bai R, Jiang H, et al. (2013) Assessment of hemodynamics in a rat model of liver cirrhosis with precancerous lesions using multislice spiral CT perfusion imaging. BioMed Research International 2013: 813174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chatterjee R, Mitra A. (2015) An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. International Immunopharmacology 24: 335–345. [DOI] [PubMed] [Google Scholar]
- 11. Glauert HP, Calfee-Mason K, Stemm DN, et al. (2010) Dietary antioxidants in the prevention of hepatocarcinogenesis: A review. Molecular Nutrition & Food Research 54: 875–889. [DOI] [PubMed] [Google Scholar]
- 12. Miller PE, Snyder DC. (2012) Phytochemicals and cancer risk: A review of the epidemiological evidence. Nutrition in Clinical Practice 27: 599–612. [DOI] [PubMed] [Google Scholar]
- 13. Stagos D, Amoutzias GD, Matakos A, et al. (2012) Chemoprevention of liver cancer by plant polyphenols. Food and Chemical Toxicology 50: 2155–2170. [DOI] [PubMed] [Google Scholar]
- 14. Neto CC. (2007) Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Molecular Nutrition & Food Research 51:652–664. [DOI] [PubMed] [Google Scholar]
- 15. Zafra-Stone S, Yasmin T, Bagchi M, et al. (2007) Berry anthocyanins as novel antioxidants in human health and disease prevention. Molecular Nutrition & Food Research 51: 675–683. [DOI] [PubMed] [Google Scholar]
- 16. Liu LC, Wang CJ, Lee C, et al. (2010) Aqueous extract of Hibiscus sabdariffa L. Decelerates acetaminophen-induced acute liver damage by reducing cell death and oxidative stress in mouse experimental models. Journal of the Science of Food and Agriculture 90: 329–337. [DOI] [PubMed] [Google Scholar]
- 17. Yin J, Heo SI, Wang MH. (2008) Antioxidant and antidiabetic activities of extracts from Circiumjaponicum roots. Nutrition Research and Practice 2: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergman I, Loxley R. (1963) Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Analytical Chemistry 35: 1961–1965. [Google Scholar]
- 19. Ohkawa H, Ohishi N, Yagi K. (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95: 351–358. [DOI] [PubMed] [Google Scholar]
- 20. Folch J, Lees M, Sloane Stanley GH. (1956) A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226: 497–509. [PubMed] [Google Scholar]
- 21. Buege JA, Aust JD. (1956) Microsomal lipid peroxidation. Methods in Enzymology 52: 302–310 [DOI] [PubMed] [Google Scholar]
- 22. Reznick AZ, Packer L. (1994) Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods in Enzymology 233: 357–363. [DOI] [PubMed] [Google Scholar]
- 23. Beutler E, Duron O, Kelly BM. (1979) Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine 61: 882–888. [PubMed] [Google Scholar]
- 24. Mylorie AA, Collins H, Umbles C, et al. (1986) Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicology and Applied Pharmacology 82: 512–520. [DOI] [PubMed] [Google Scholar]
- 25. Worthington V. (1993) Catalase. In: Worthington Enzyme Manual: enzymes and related biochemicals. Lakewood, NJ: Worthington Biochemical Corporation, p.77–80. [Google Scholar]
- 26. Lawrence RA, Burk RF. (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochemical and Biophysical Research Communications 71:952–958. [DOI] [PubMed] [Google Scholar]
- 27. Habig WH, Jacoby WB. (1981) Assays for differentiation of glutathione S-transferases. Methods in Enzymology 77: 398–405. [DOI] [PubMed] [Google Scholar]
- 28. Smith PK, Krohn RI, Hermanson GT, et al. (1985) Measurement of protein using bicinchoninic acid. Analytical Biochemistry 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 29. Goodman ZD. (2007) Grading and staging systems for inflammation and fibrosis in chronic liver diseases. Journal of Hepatology 47: 598–607. [DOI] [PubMed] [Google Scholar]
- 30. Shen T, Khor SC, Zhou F, et al. (2014) Chemoprevention by lipid-soluble tea polyphenols in diethylnitrosamine/phenobarbital-induced hepatic pre-cancerous lesions. Anticancer Research 34: 683–693. [PubMed] [Google Scholar]
- 31. Liu YF, Zha BS, Zhang HL, et al. (2009) Characteristic gene expression profiles in the progression from liver cirrhosis to carcinoma induced by diethylnitrosamine in a rat model. Journal of Experimental & Clinical Cancer Research 28:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schiffer E, Housset C, Cacheux W, et al. (2005) Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 41: 307–314. [DOI] [PubMed] [Google Scholar]
- 33. Khan MS, Devaraj H, Devaraj N. (2011) Chrysin abrogates early hepatocarcinogenesis and induces apoptosis in N-nitrosodiethylamine-induced preneoplastic nodules in rats. Toxicology and Applied Pharmacology 251: 85–94. [DOI] [PubMed] [Google Scholar]
- 34. Ghosh D, Choudhury ST, Ghosh S, et al. (2012) Nanocapsulated curcumin: Oral chemo preventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chemico-biological Interactions 195: 206–214. [DOI] [PubMed] [Google Scholar]
- 35. Khan MS, Halagowder D, Devaraj SN. (2011) Methylated chrysin induces co-ordinated attenuation of the canonical Wnt and NF-kB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chemico-biological Interactions 193:12–21. [DOI] [PubMed] [Google Scholar]
- 36. Furuta K, Sato S, Miyake T, et al. (2008) Anti-tumor effects of cimetidine on hepatocellular carcinomas in diethylnitrosamine-treated rats. Oncology Reports 19: 361–368. [PubMed] [Google Scholar]
- 37. Zhang CL, Zeng T, Zhao XL, et al. (2013) Garlic oil attenuated nitrosodiethylamine-induced hepatocarcinogenesis by modulating the metabolic activation and detoxification enzymes. International Journal of Biological Sciences 9: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tell G, Vascotto C, Tiribelli C. (2013) Alterations in the redox state and liver damage: Hints from the EASL Basic School of Hepatology. Journal of Hepatology 58: 365–374. [DOI] [PubMed] [Google Scholar]
- 39. Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, et al. (2010) Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxidative Medicine and Cellular Longevity 3: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bingül İ, Başaran-Küçükgergin C, Tekkeşin MS, et al. (2013) Effect of blueberry pretreatment on diethylnitrosamine-induced oxidative stress and liver injury in rats. Environmental Toxicology and Pharmacology 36: 529–538. [DOI] [PubMed] [Google Scholar]
- 41. Osman N, Adawi D, Ahrne S, et al. (2007) Endotoxin and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Digestive and Liver Disease 39: 849–856. [DOI] [PubMed] [Google Scholar]
- 42. Ozcelik E, Uslu S, Burukoglu D, et al. (2014) Chitosan and blueberry treatment induces arginase activity and inhibits nitric oxide production during acetaminophen-induced hepatotoxicity. Pharmacognosy Magazine 10: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gong P, Chen FX, Wang L, et al. (2014) Protective effects of blueberries (Vacciniumcorymbosum L.) extract against cadmium-induced hepatotoxicity in mice. Environmental Toxicology and Pharmacology 37: 1015–1027. [DOI] [PubMed] [Google Scholar]
- 44. Çoban J, Evran B, Özkan F, et al. (2013) Effect of blueberry feeding on lipids and oxidative stress in the serum, liver and aorta of guinea pigs fed on a high-cholesterol diet. Bioscience Biotechnology Biochemistry 77: 389–391. [DOI] [PubMed] [Google Scholar]
- 45. Çoban J, Betül-Kalaz E, Küçükgergin C, et al. (2014) Blueberry treatment attenuates D-galactose-induced oxidative stress and tissue damage in rat liver. Geriatrics & Gerontology International 14: 490–497. [DOI] [PubMed] [Google Scholar]
- 46. Wang YP, Cheng ML, Zhang BF, et al. (2010) Effects of blueberry on hepatic fibrosis and transcription factor Nrf2 in rats. World Journal of Gastroenterology 7: 2657–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Y, Cheng M, Zhang B, et al. (2013) Dietary supplementation of blueberry juice enhances hepatic expression of metallothionein and attenuates liver fibrosis in rats. PLoS One 8: e58659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sadik NAH, El-Maraghy SA, Ismail MF. (2008) Diethylnitrosamine-induced hepatocarcinogenesis in rats: Possible chemoprevention by blueberries. African Journal of Biochemical Research 2: 81–87. [Google Scholar]