Abstract
Nanomaterials present in cosmetics and food additives are used for industrial applications. However, their safety profile is unclear. Amorphous silica nanoparticles (nSPs) are a widely used nanomaterial and have been shown to induce inflammatory cytokines following intratracheal administration in mice. The current study investigated the adjuvant effect of nSP30 (nSP with a diameter of 33 nm) on T helper (Th)1, Th2, and Th17 immune responses as well as immunoglobulin (Ig) levels in mice. BALB/c mice were intraperitoneally administered ovalbumin (OVA) with or without varying doses and varying sizes of nSPs. The adjuvant effect of nSPs was investigated by measuring OVA-specific IgG antibodies in sera, OVA-specific proliferative responses of splenocytes, and the production of Th1, Th2, and Th17 cytokines. Aluminum hydroxide was used as a positive adjuvant control. Anti-OVA IgG production, splenocyte proliferative responses, and secretion of IFN-γ, IL-2, IL-4, IL-5, and IL-17 were increased significantly in mice receiving a combined injection of nSP30 (30 or 300 µg) with OVA compared with OVA alone or a combined injection with nSP30 (3 µg). The responses were nSP30 dose-dependent. When different sized nSPs were used (with 30, 100, and 1000 nm diameters), the responses to OVA were enhanced and were size-dependent. The smaller sized nSP particles had a greater adjuvant effect. nSPs appear to exert a size-dependent adjuvant effect for Th1, Th2, and Th17 immune responses. Understanding the mechanisms of nSP adjuvanticity might lead to the development of novel vaccine adjuvants and therapies for allergic diseases caused by environmental factors.
Keywords: adjuvant, amorphous silica, antibody, cytokine, nanomaterial
Introduction
Recent progress in nanotechnology has allowed the development of nanomaterials (NMs) with a diameter ⩽100 nm. The tissue permeability and electrical properties of NMs are different from those of traditional submicron scale materials with a diameter ⩾100 nm.1 Therefore, NMs have gained increasing attention as next-generation materials because of their innovative functions. NMs are already in practical use in cosmetic materials, food additives, and industrial applications. Thus, NMs are essential for our modern lifestyle, but the safety of NMs has not been fully determined. Indeed, the innovative functions of NMs might induce unexpected toxicity.2 Concerns regarding the unexpected biological effects of NMs have increased worldwide, as recent studies have described carcinogenicity and inflammatory diseases caused by carbon nanotubes and titanium dioxide nanoparticles (nTiO2).3,4 Notably, immune cells are likely to recognize NMs as foreign agents, indicating that NMs might have unknown effects on the immune system.4
Amorphous silica nanoparticles (nSPs) are one of the most widely used NMs and are commonly incorporated into cosmetic materials, including sunscreens, foundation, diaphoretics, body powders, tooth dental powder, and filling material, as well as food additives, including fluidizers and anticoagulants.5 The optimization of nSP particle size and dispersibility has progressed rapidly because of their improved availability and hygroscopicity owing to a decrease in particle size. Therefore, increased numbers of products containing nSPs will lead to a more frequent exposure to nSPs via various routes. A recent study demonstrated that nSPs applied to the skin penetrated the skin barrier, entered keratinocytes and Langerhans cells, migrated into the blood stream, and entered regional immune organs including the lymph nodes and spleen, although traditional submicron scale SPs were not absorbed transdermally.6 nSPs were also suggested to have a strong proinflammatory potential because the intraperitoneal administration of nSPs induced a significant increase in numbers of intraperitoneal cells and the production of inflammatory cytokines and chemokines.6 Therefore, the influence of nSPs on the immune system is of concern worldwide.
The immune system responds to exogenous antigens via both innate and adaptive immunity. T lymphocytes (T cells) and B lymphocytes (B cells) express cell surface antigen-specific receptors that have pivotal roles in adaptive immunity. CD4+ T helper lymphocytes (Th cells) are classified into three subsets, Th1, Th2, and Th17 cells based on their distinct cytokine profiles.7 Th cells contribute to immunoglobulin (Ig) class switching, antibody responses and cell-mediated responses, followed by innate immunity. Th1 cells predominately secrete proinflammatory cytokines such as interleukin (IL)-2 and interferon (IFN)-γ, and are involved in the activation of cytotoxic T cells and the promotion of delayed hypersensitivity reactions. Th2 cells produce cytokines including IL-4, IL-5, and IL-13, which regulate the production of IgG1 and IgE. Notably, IL-4 induces IgG1 and IgE class switching. IL-5 regulates the proliferation of eosinophils and IgE antibodies cause anaphylaxis. Thus, Th2 cytokines are considered mediators of allergic diseases. Th17 cells, which secrete IL-17, were identified as a new lineage of effector Th cells in 2000. Th17 cells play an important role in immune responses in infectious and autoimmune diseases including encephalomyelitis by producing various inflammatory mediators.7 For instance, high levels of IL-17 were observed in inflamed areas in rheumatoid arthritis and asthma.8 Furthermore, Th cell subsets suppress each other by the action of cytokines.7 For instance, IFN-γ inhibits the proliferation of Th2 cells and IL-4 suppresses Th1 development and secretion of IFN-γ. Both IFN-γ and IL-4 inhibit Th17 differentiation and the production of IL-17 by Th17 cells and IL-17 suppresses Th1 differentiation. Furthermore, the balance of Th1, Th2, and Th17 immune responses depends on the type of foreign pathogen present.
Representative immunotoxicity caused by substances in the environment includes the increased prevalence of allergic diseases caused by air pollutants. For example, the combined injection of diesel exhaust particles with an antigen enhances immune responses against the antigen.9 Diesel exhaust particles are not antigens but have an adjuvant effect that sensitizes the immune system to augment antibody production and lymphocyte proliferation against antigens.9 Exposure to antigens with adjuvants might excessively enhance the immune system and cause allergic diseases. Therefore, the assessment of environmental substance adjuvanticity is important. Environmental substances including diesel exhaust particles and bisphenol-A, an environmental hormone affect the immune system, especially the Th1/Th2 balance.9 Therefore, it is important to evaluate the effects of NMs on immune responses.
In the present study, SPs were intraperitoneally (i.p.) administered to BALB/c mice with ovalbumin (OVA) antigen, and the production of OVA-specific antibodies and proliferative responses of splenocytes to OVA were measured to evaluate the effect of SP dose and particle size on the immune system. In addition, the effect of SPs on Th1, Th2, and Th17 immune responses in mice was investigated by measurement of OVA-specific IgG subclasses and cytokines after nSP and OVA exposure. The BALB/c mouse model was chosen because this model is commonly used to evaluate immune responses to foreign substances.9
Material and methods
Animals
BALB/c mice (male, 8 weeks old) were purchased from Japan SLC, Inc. (Haruno Breeding branch, Shizuoka, Japan) and acclimated for 1 week before use. Body weight was in the range of 18.9–21.7 g at arrival. During the acclimation period, the animals were observed once daily for clinical signs and body weight was measured. There were no abnormalities in clinical signs or body weight in any animals (data not shown). Based on the data obtained during the acclimation period, all animals were judged suitable for the study. Consequently, the mice were assigned to the study based on a body weight-stratified sequenced randomization on Day −1. Each treatment group consisted of five mice. Throughout the study, the mice were maintained in polycarbonate cages in a pathogen-free environment maintained at 20–26°C with 30–70% relative humidity and a 12-h light-dark cycle. All mice had ad libitum access to standard rodent chow (CRF-1; Oriental Yeast Co., Ltd, Tokyo, Japan) and filtered UV-irradiated water. All experiments were performed according to the Ethical Guidelines established by Shionogi & Co., Ltd and were approved by the Institutional Animal Care and Use Committee of Shionogi & Co., Ltd. The mice were weighed using an electronic balance (UX2200H, Shimadzu Corporation, Kyoto, Japan) on Days −3, 0, 1, 3, 7, 14, and 21 of the study period.
Administration of antigen and SPs
OVA (Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved in phosphate-buffered saline (PBS). nSPs with a diameter of 30 nm (nSP30) or 100 nm (nSP100) and microsilica particles with a diameter of 1000 nm (mSP1000) were purchased from Micromod Partikeltechnologie GmbH (Rostock, Germany). Each SP suspension was stored at room temperature, sonicated (400 W) for 5 min at 25°C, and then vortexed for 1 min immediately before use. SP suspensions and the antigen solution were combined in equal volumes using a vortex mixer for several seconds. Mice were then immediately immunized by intraperitoneal injection of 500 µL of PBS containing SPs and/or OVA (100 µg) on Day 0. Imject aluminum hydroxide (Alum, Thermo Fisher Scientific Inc., Rockland, IL, USA), a commonly used adjuvant, was used as a positive control. The antigen solution was added to Alum dropwise with constant mixing at a 1:1 volume ratio of Alum to antigen. After continuous mixing for 30 min, 500 µL of Alum/antigen solution was administered i.p. All experiments were repeated three times and 195 mice in total were used.
Physicochemical examinations of SPs
Silica particles were diluted with PBS to 0.25 mg/mL (nSP30) or 0.5 mg/mL (nSP100 and mSP1000). The mean size and size distribution of SPs were measured using a dynamic light scattering method as described previously.10
Measurement of OVA-specific antibodies
The assay to measure OVA-specific antibodies was performed as described previously.9 IgG, IgG1, IgG2a, and IgE antibodies specific for OVA were measured by ELISA. To measure the levels of IgG, IgG1, and IgG2a antibodies, 100 µL/well of OVA (100 µg/mL) was incubated overnight at 4°C in 96-well flat-bottomed microtiter plates, and the wells were then washed three times with PBS. The wells were blocked with 200 µL PBS containing 1% (w/v) casein (Sigma) by incubating at 37°C for 1 h. After the wells were washed, each received either 100 µL of a 1:10,000 (for IgG or IgG1 measurements) or 1:50 (for IgG2a) dilution of each serum sample and the plates were incubated at 37°C for 1 h. Thereafter, the wells were gently washed and 100 µL/well of solution containing alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (1:1000 dilution; Sigma) or AP-conjugated rat anti-mouse IgG1 (or IgG2a) (1:1000 dilution; BD Pharmingen, San Diego, CA, USA) was added, and the plates were incubated at 37°C for 1 h. The wells were then gently washed and 100 µL of a 3 mM p-nitrophenyl phosphate solution (BioRad, Hercules, CA, USA) was added to each well and the plates were incubated in the dark (at room temperature) until a bright yellow reaction product was observed. The absorbance in each well was measured at 405 nm with a VersamMax microplate reader (MDS Inc., Sunnyvale, CA, USA). All results were expressed as the mean absorbance at OD405 (A405) ± SEM.
To measure IgE, 100 µL of 10 µg/mL rat anti-mouse IgE antibody (Monosan, Uden, the Netherlands) was loaded into each well of a 96-well flat-bottomed microtiter plate and incubated at 37°C for 1 h. After the wells were washed three times with SuperBlock Blocking Buffer (Pierce Biotechnology, Inc., Rockford, IL, USA), 100 µL of each serum sample (diluted 1:5) was added to the wells, and the plate was incubated for 1 h at 37°C. The wells were then washed with PBS containing 0.05% (v/v) Tween-20 before each received 100 µL of a biotinylated-OVA (1 µg/mL) solution. After incubation at 37°C for 1 h, the wells were washed and 100 µL of a 1 µg/mL solution of streptavidin conjugated with horseradish peroxidase (HRP; Vector Laboratories, Inc., Burlingame, CA, USA) was added to each well. The plate was incubated at 37°C for 1 h, the wells were washed, 100 µL of TMB (3,3′,5,5′-tetramethylbenzidine) peroxide substrate solution (Pierce) was added to each well, and the plate was incubated for 30 min at room temperature. The reaction in each well was stopped by the addition of 100 µL of 2 M sulfuric acid and then absorbance values were measured at 450 nm in a microplate reader. All results were expressed as the mean absorbance at OD450 (A450) ± SEM.
Proliferation assay
Spleens removed on Day 21 were processed to yield cell suspensions as previously described.11 Erythrocytes in the suspensions were lysed with a Tris-NH4Cl solution (0.75% [w/v] NH4Cl and 0.2% Tris [pH 7.2]; room temperature, 5 min) and then all non-erythrocytes were collected by centrifugation and resuspension in complete medium, (RPMI 1640 containing 1 mM l-glutamine, 40 µM 2-mercaptoethanol (all Sigma), 100 U penicillin/mL, 100 µg streptomycin/ml (both Life Technologies Co., Carlsbad, CA, USA), and 10% [v/v] heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc., Waltham, MA, USA)). After these cells were counted and viability assessed (using trypan blue) from this suspension, 5 × 105 cells (in 0.1 mL complete medium were placed into each well of a 96-well plate. The cells were then untreated or treated with a bolus of OVA (20, 100, or 500 µg OVA/mL final concentration in each well); unstimulated cells received medium alone in place of the OVA bolus. After 48 h in culture at 37°C (in 5% CO2 atmosphere), each well was pulsed with 0.5 µCi of [3H]-thymidine (GE Health-care UK Ltd., Buckinghamshire, UK) and the cells were cultured for a further 6 h. The cells in each well were then harvested onto fiberglass filters with a multi-harvester (Perkin Elmer, Waltham, MA, USA) and the total [3H]-radioactivity was determined by liquid scintillation counting. All results were expressed as the mean total counts per minute (cpm) of triplicate cultures of cells pooled from five mice.
Measurement of cytokines
Samples of RPMI 1640 (1 mL) containing 5 × 106 splenocytes from OVA-tolerized mice treated with or without nSP30 were placed in 24-well tissue culture plates and then received medium alone or medium containing 20, 100, and 500 µg OVA/mL. After 4 days of incubation at 37°C, the supernatant in each well was harvested and stored at −20°C until assay. Formation/secretion of IFN-γ, IL-2, IL-4, IL-5, and IL-17 by the cells was quantified using commercial ELISA kits (Endogen, Inc., Woburn, MA, USA for IFN-γ, IL-4, and IL-5; R&D Systems Inc., Minneapolis, MN, USA for IL-2 and IL-17), according to the manufacturers’ protocols. The level of sensitivity of the kits was 10 pg IFN-γ/mL, 3 pg IL-2/mL, 5 pg IL-4/mL, 5 pg IL-5/mL, and 5 pg IL-17/mL.
Statistical analysis
All data are reported as means ± SEM. A one-way analysis of variance (ANOVA) followed by a Dunnett’s parametric multiple t-test was used to compare the data obtained from the samples in each group with those from the PBS/PBS or OVA/PBS control groups. All analyses were performed using Prism Software v.6.03 for Windows (GraphPad, La Jolla, CA, USA). A P value <0.05 was accepted as significant.
Results
Material characteristics
Prior to undertaking this study, we first analyzed the physicochemical properties of SPs. Based on a dynamic laser scattering analysis, the mean particle diameters of these silica particles were determined (Table 1). SPs remained stable, dispersed well, and did not aggregate in phosphate-buffered saline (PBS). Therefore, they were considered ideally suited to evaluate the biological effects of nSP in this study.
Table 1.
Summary of the physicochemical properties of amorphous SPs.
| Primary particle size (nm)a | Diameter in PBS (nm) | |
|---|---|---|
| mSP1000 | 1000 | 1074 ± 29.8 |
| nSP100 | 100 | 105 ± 3.1 |
| nSP30 | 30 | 33 ± 2.6 |
Particle sizes in PBS solution are expressed as mean ± standard deviation (n = 3).
Information from technical datasheet of products.
PBS, phosphate-buffered saline; SP, silica particle.
Effects of nSP30 on body weight
To investigate the effects of nSP30 on body weight, mice were weighed on Days −3, 0, 1, 3, 7, 14, and 21 of the study period. The changes in body weight of mice administered nSP30 and/or OVA are shown in Figure 1. No significant differences in body weight were observed in any group, although the administration of nSP30 (30 and 300 µg) and Alum with OVA led to a transient and mild weight loss after its administration. However, this was not considered to have affected the study results because: (1) no significant differences in body weight were observed in any group; (2) weight loss was transient and very mild; and (3) no abnormal clinical signs were observed in the animals during the study.
Figure 1.
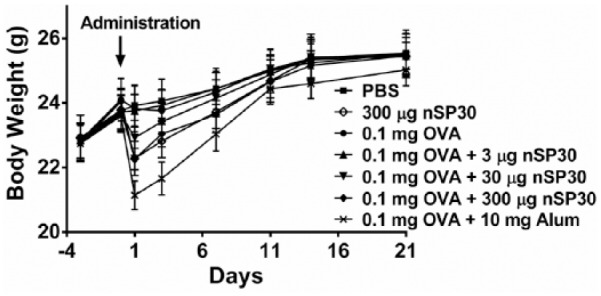
Effect of nSP30 administration on body weight. Mice were weighed on Days −3, 0, 1, 3, 7, 14, and 21 of the study period. Values are expressed as the mean (n = 5).
Effect of nSP30 on the production of anti-OVA IgG antibodies and proliferative responses of splenocytes to OVA
The effect of nSP30 on the production of OVA-specific antibodies was determined using seven experimental groups and anti-OVA IgG antibodies were measured by ELISA in sera collected on Day 21. As shown in Figure 2a, anti-OVA IgG production in the combined injection groups of nSP30 (30 and 300 µg) with OVA was significantly and dose-dependently enhanced compared with that in the OVA-alone group, whereas antibody production in the combined injection group of nSP30 (3 µg) with OVA was very low. The serum levels of anti-OVA IgG in mice administered OVA with 30 and 300 µg of nSP30 were 2.0- and 4.1-fold higher, respectively, than that in animals administered OVA alone. The combined injection of the positive control (Alum) with OVA induced marked anti-OVA IgG production, whereas antibody production was low in mice treated with OVA alone. In addition, splenocyte proliferative responses to OVA in the nSP30 (30 and 300 µg) and OVA groups were significantly and dose-dependently increased compared with that in the OVA-alone group, whereas the splenocyte proliferative response to OVA in the combined injection group of nSP30 (3 µg) with OVA was similar to that in the OVA-alone group (Figure 2b). The rates of cell proliferation to OVA (500 µg/mL) in mice treated with 30 and 300 µg nSP30 plus OVA were 920 and 1670%, respectively.
Figure 2.
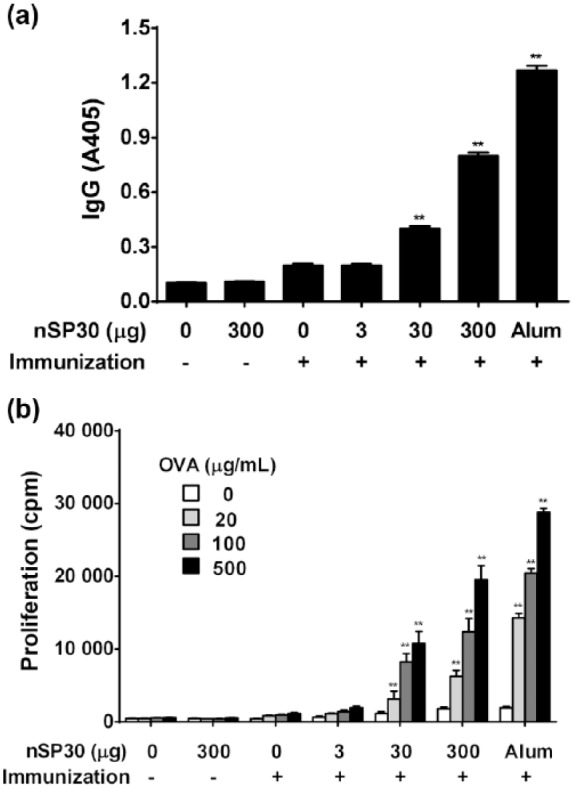
Effect of nSP30 on anti-OVA IgG antibody production and proliferation of splenocytes to OVA. Mice were i.p. injected with 100 µg of OVA with or without 3, 30, or 300 µg of nSP30 or aluminum hydroxide (Day 0). PBS or 300 µg of nSP30 alone was used as a control. (a) Effect of nSP30 on anti-OVA IgG antibody production. On Day 21, anti-OVA IgG antibodies in sera were determined by ELISA. Values are expressed as the mean ± SEM of five mice. (b) Effect of nSP30 on proliferation of splenocytes to OVA. On Day 21, spleens were removed, cells isolated, pooled and then incubated with 20, 100, or 500 µg OVA/mL to measure the proliferative capacity (as outlined in the Methods). Values shown are the mean ± SEM of triplicate cultures/cohort. cpm, counts per minute; PBS, phosphate-buffered saline; OVA, ovalbumin; Alum, aluminum hydroxide. **P <0.01 vs. OVA/PBS (Dunnett’s test).
Effect of nSP30 on the production of anti-OVA IgG1, IgE, and IgG2a antibodies
To assess the effect of nSP30 on Th1 and Th2 immune responses, serum anti-OVA IgG subclasses and IgE were measured by ELISA. The presence of IgG subclasses and IgE indicate Th1 and Th2 immune responses because IFN-γ secreted by Th1 cells promotes IgG2a production by B cells, whereas IL-4 secreted by Th2 cells promotes IgG1 and IgE production. Anti-OVA IgG1, IgE, and IgG2a production in the combined injection groups of nSP30 (30 and 300 µg) with OVA was significantly and dose-dependently enhanced compared with that in the OVA-alone group, whereas values in the combined injection group of nSP30 (3 µg) with OVA were similar to that in the OVA-alone group (Figure 3). The combined injection of Alum with OVA was reported to induce strong anti-OVA IgG1 and IgE production compared with anti-OVA IgG2a production12,13 in accordance with the results in the current study. These findings suggest that nSP30 exerts a stronger adjuvant effect for Th1 than Alum, because the IgG2a/IgG1 ratios in the nSP30 (300 µg) and Alum group were 1.79 and 0.57, respectively, compared with the OVA-alone group.
Figure 3.
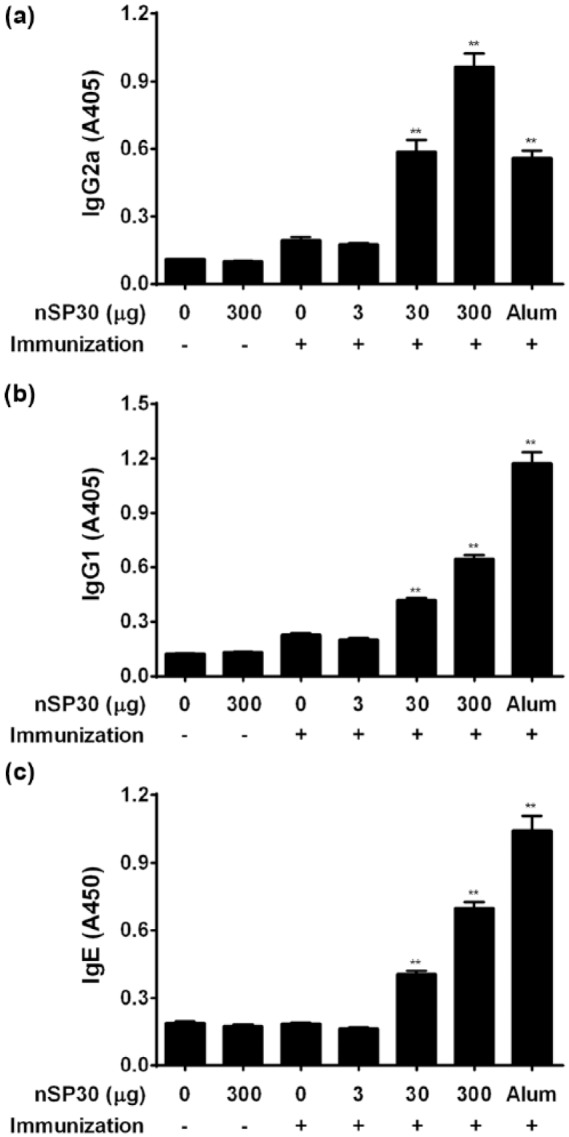
Effect of nSP30 on anti-OVA IgG2a, IgG1, and IgE antibody production. Mice were i.p. injected with 100 µg of OVA with or without 3, 30, or 300 µg of nSP30 or aluminum hydroxide (Day 0). PBS or 300 µg of nSP30 alone was used as a control. On Day 21, anti-OVA IgG2a (a), IgG1 (b), and IgE (c) antibodies in sera were determined by ELISA. Values are expressed as the mean ± SEM of five mice. **P <0.01 vs. OVA/PBS (Dunnett’s test).
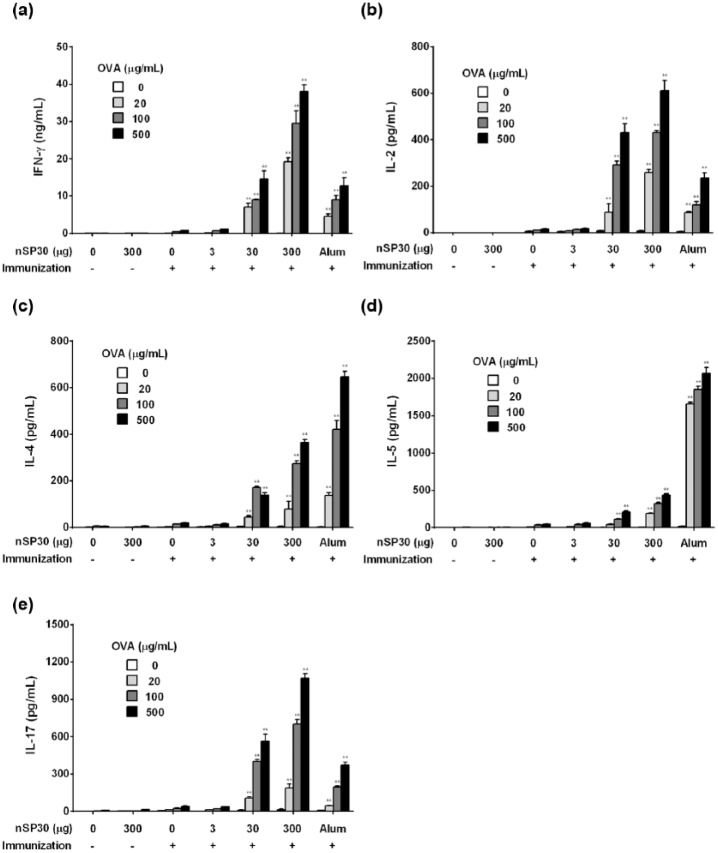
Effect of nSP30 on cytokine secretion
To assess the type of immune response observed in terms of cytokine levels, IFN-γ and IL-2 (Th1 cytokines), IL-4 and IL-5 (Th2 cytokines), and IL-17 (Th17 cytokine) were measured from immunized splenocytes stimulated with OVA in vitro. The secretion of IFN-γ, IL-2, IL-4, IL-5, and IL-17 was enhanced in mice that received the nSP30 (30 and 300 µg) plus OVA treatments compared with other groups (Figure 4). These results were consistent with Ig levels. Higher levels of IL-4 and IL-5 secretion and lower levels of IFN-γ, IL-2, and IL-17 were observed when mice were administered a combination of OVA and Alum compared with the nSP30 (300 µg) plus OVA group.
Figure 4.
Effect of nSP30 on IFN-γ, IL-2, IL-4, IL-5, and IL-17 production. Mice were i.p. injected with 100 µg of OVA with or without 3, 30, or 300 µg of nSP30 or aluminum hydroxide (Day 0). PBS or 300 µg of nSP30 alone was used as a control. On Day 21, spleens were removed and splenocytes were incubated with 20, 100, or 500 µg/mL of OVA followed by measurement of IFN-γ (a), IL-2 (b), IL-4 (c), IL-5 (d), and IL-17 (e) in the culture supernatant. Values are shown as the mean ± SEM of triplicate samples from culture supernatants of cell pooled from five mice. *P <0.05, **P <0.01 vs. OVA/PBS (Dunnett’s test).
Effect of variously sized SPs on the production of anti-OVA IgG antibodies and proliferative responses of splenocytes to OVA
To evaluate the effects of SP particle size on the adjuvant effect, five experimental groups were formed and serum anti-OVA IgG antibodies and proliferative responses of splenocytes to OVA in the SP (diameter of 100 or 1000 nm) treatment groups in addition to nSP30 were measured. The same assay protocol and experimental conditions were used as for the nSP30 assessment. Anti-OVA IgG production in the combined injection groups of SPs (30, 100, and 1000 nm) with OVA was enhanced in a size-dependent manner (Figure 5a). In addition, splenocyte proliferative responses to OVA in SPs (30, 100, and 1000 nm) with OVA groups were enhanced in a size-dependent manner (Figure 5b). The rates of cell proliferation to OVA (500 µg/mL) in mice treated with 30, 100, and 1000 nm nSP30 plus OVA were 1473, 1077, and 409%, respectively. These findings suggest that the smaller the particle size the greater the adjuvant effect.
Figure 5.
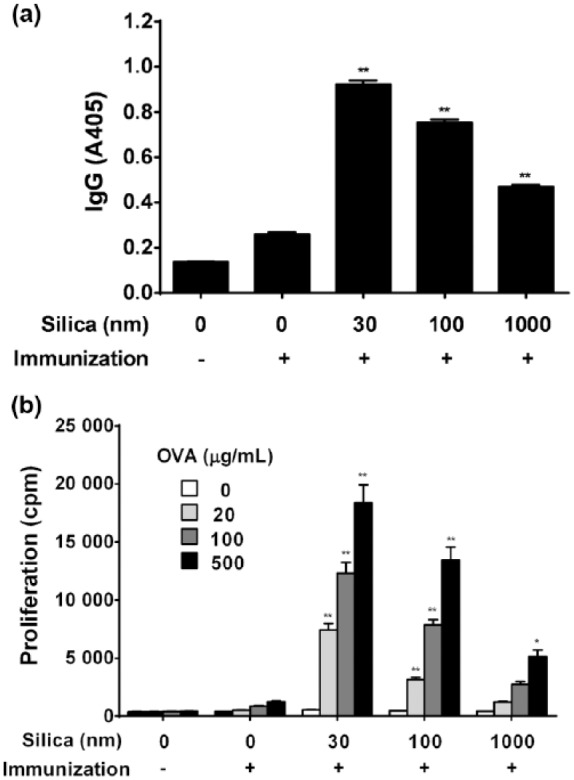
Effect of variously sized SPs on anti-OVA IgG antibody production and proliferation of splenocytes to OVA. Mice were i.p. injected with 100 µg of OVA with or without 30, 100, or 1000 nm of SPs (Day 0). PBS alone was used as a control. (a) On Day 21, anti-OVA IgG antibodies in sera were determined by ELISA. Values are expressed as the mean ± SEM of five mice. (b) Effect of variously sized SPs on proliferation of splenocytes to OVA. On Day 21, spleens were removed, cells isolated, pooled, and then incubated with 20, 100, or 500 µg OVA/mL to measure the proliferative capacity (as outlined in the Methods). Values are the mean ± SEM of triplicate cultures/cohort. cpm, counts per minute; PBS, phosphate-buffered saline; OVA, ovalbumin. *P <0.05, **P <0.01 vs. OVA/PBS (Dunnett’s test).
Effect of variously sized SPs on Th1 and Th2 immune response profiles
To assess the effect of variously sized SPs on Th1 and Th2 immune responses, serum anti-OVA IgG subclasses and IgE were measured by ELISA. Anti-OVA IgG1, IgE, and IgG2a production in the combined injection groups of SPs with OVA were enhanced in a size-dependent manner (Figure 6). These results suggest that SPs exert an adjuvant effect for both Th1 and Th2 immune responses regardless of particle size.
Figure 6.
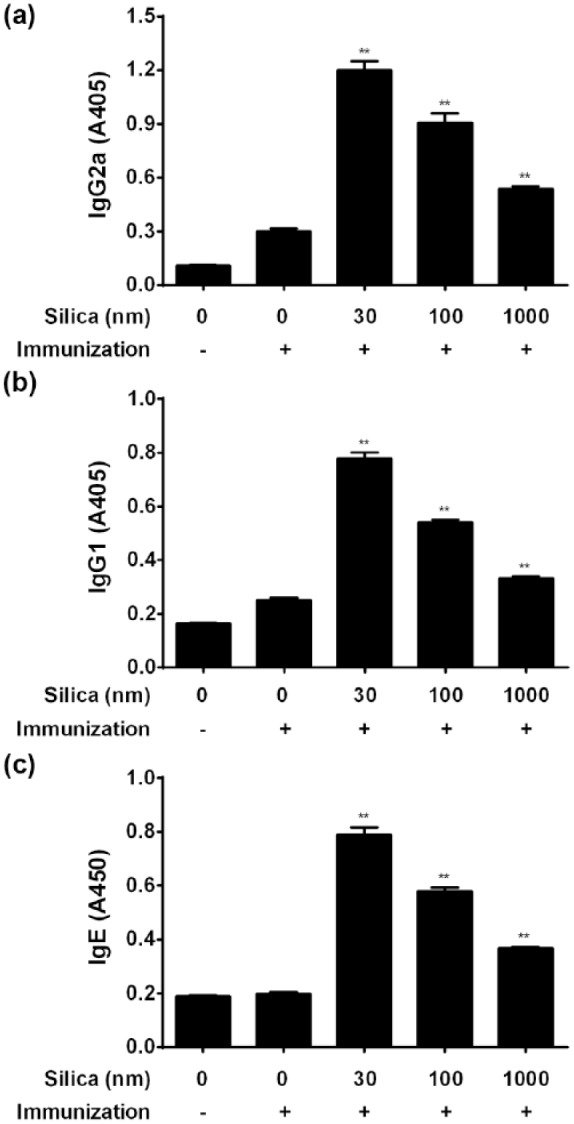
Effect of variously sized SPs on anti-OVA IgG2a, IgG1, and IgE antibody production. Mice were i.p. injected with 100 µg of OVA with or without 30, 100, or 1000 nm of SPs (Day 0). PBS alone was used as a control. On Day 21, anti-OVA IgG2a (a), IgG1 (b), and IgE (c) antibodies in sera were determined by ELISA. Values are expressed as the mean ± SEM of five mice. **P <0.01 vs. OVA/PBS (Dunnett’s test).
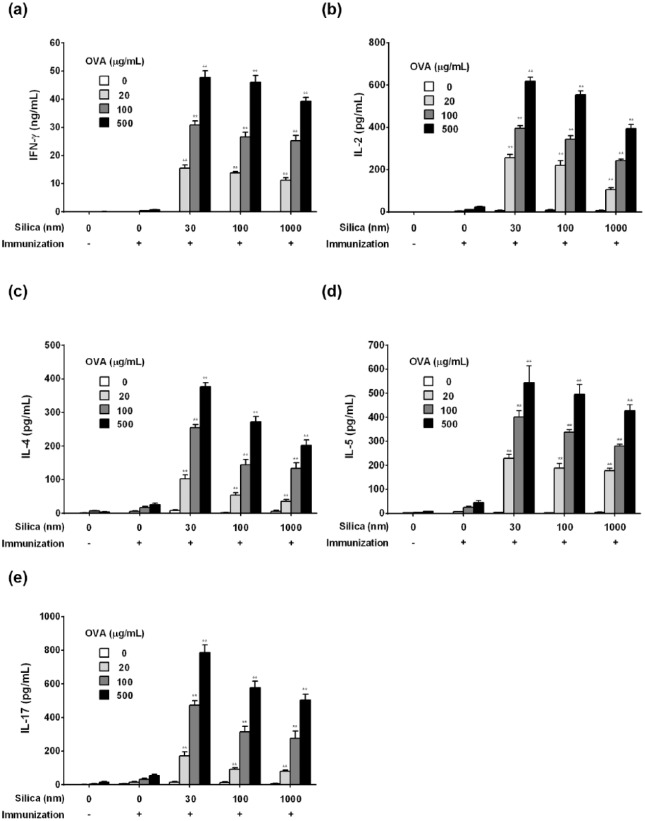
Effect of variously sized SPs on cytokine secretion
To assess the type of immune response in terms of cytokine levels, IFN-γ, IL-2, IL-4, IL-5, and IL-17 secreted from splenocytes were measured. The secretion of IFN-γ, IL-2, IL-4, IL-5, and IL-17 tended to be enhanced based on particle size (Figure 7). These results suggest that SPs exert adjuvant effects for Th1, Th2, and Th17 immune responses, regardless of particle size.
Figure 7.
Effect of variously sized SPs on IFN-γ, IL-2, IL-4, IL-5, and IL-17 production. Mice were i.p. injected with 100 µg of OVA with or without 30, 100, or 1000 nm of SPs (Day 0). PBS alone was used as a control. On Day 21, spleens were removed and splenocytes were incubated with 20, 100, or 500 µg/mL of OVA followed by measurement of IFN-γ (a), IL-2 (b), IL-4 (c), IL-5 (d), and IL-17 (e) in the culture supernatant. Values are shown as the mean ± SEM of triplicate samples from culture supernatants of cell pooled from five mice. **P <0.01 vs. OVA/PBS (Dunnett’s test).
Discussion
The present study showed that the production of anti-OVA IgG antibodies and proliferative responses of splenocytes to OVA were enhanced by the combined injection of nSP30 and OVA in a dose-dependent manner, whereas the injection of OVA or nSP30 alone failed to affect such immune responses. These findings suggest that nSP30 has an adjuvant effect and might play a role in the development of allergic diseases. Indeed, it was reported that a combined intradermal injection of nSP and antigen aggravated atopic dermatitis-like skin lesions mediated by IL-18 and thymic stromal lymphopoietin.10 In addition, many reports have already described that nSPs might affect the immune system. For instance, a murine macrophage cell line (RAW264.7) treated with nSP produced tumor necrosis factor (TNF)-α, suggesting nSPs might activate immune responses mediated by inflammatory cytokines.14 Furthermore, the intratracheal administration of nSP30 to mice caused lung inflammation mediated by inflammatory cytokines and neutrophil infiltration.14 These reports indicate that nSPs activate innate immunity in vitro and in vivo, and support our results that nSP30 has an adjuvant effect.
We also demonstrated that treatment with OVA plus nSP30 was associated with increased anti-OVA IgG1, IgG2a, and IgE antibody production. These increases in nSP30-treated mice suggested that i.p. injection of OVA and nSP30 induces both Th1 and Th2 immune responses, because IgG1 and IgE production is dependent on Th2 cells, whereas IgG2a production is dependent on Th1 cells. These findings are also supported by the finding that the secretion of Th1 cytokines (IFN-γ and IL-2) and Th2 cytokines (IL-4 and IL-5) was augmented by nSP30. These findings suggest that nSP30 has an adjuvant effect on Th1 and Th2 immune responses. Previous studies have already described that some fine particulate matters might have adjuvant effects. The most widespread fine particulate matter with adjuvant effects (particulate adjuvant) is Alum (the positive control used in the present study). Alum has been added to various vaccines as an adjuvant to induce humoral immunity, and was reported to induce dominant antigen-specific IgG1 and IgE production.15 In addition to Alum, monosodium urate crystals, a damage-associated molecular pattern molecule, are considered to be a causal factor of gout, and induce strong inflammatory responses and Th2-type responses.16 Furthermore, chemically synthesized particulate matters such as lactic acid, glycolic acid polymer, polystyrene particles, and carbon nanotubes induce antigen-specific IgG1 in mice.17 It is also clear that nickel oxide nanoparticles induce strong inflammatory responses and antigen-specific IgE.17 Most well-known particulate adjuvants induce Th2-type immune responses although it is unclear how particulate adjuvants activate immune systems. Therefore, particulate adjuvants are thought to have similar activation mechanisms. Nevertheless, nSP30 is different from other particle Th2 adjuvants including Alum, because it does not induce a specific Th subset, but rather, both Th1 and Th2 immune responses. The reason nSP30 induces both Th1 and Th2 immune responses might be because nSP induces both humoral immunity and cellular immunity by the induction of cross-presentation, which might explain the low specificity of nSPs for Th immune responses.18 The activation mechanisms of adjuvants have been categorized into two types: (1) carriers to antigen presenting cells including dendritic cells that incorporate antigens efficiently; and (2) immune modulators that stimulate various innate immune receptors expressed on cell membranes or endosomes including Toll-like receptors and RIG-I-like receptors to produce inflammatory cytokines and type I IFNs. Many well-known adjuvants are considered to use both mechanisms. Adjuvants are considered to stimulate individual different innate immune receptors to produce distinct cytokines leading to distinct Th responses. It remains unclear which innate immune receptors recognize SPs, although silica crystals induce immune responses mediated by the NALP3 inflammasome as well as Alum and asbestos.19 Therefore, further studies to determine the detailed mechanisms by which nSPs activate the immune system might explain the low specificity of nSP30 for inducing Th immune responses. Previous studies demonstrated that nSP induced the vacuolation of macrophages and caused cytotoxicity by i.p. administration in mice; therefore, the adjuvant effect of nSP30 is likely to induce danger signals via self-nucleic acid released from damaged cells as well as Alum.15
In the present study, i.p. injection of OVA and nSP30 induced not only Th1 and Th2 immune responses but also Th17 immune responses, because the secretion of the Th17 cytokine IL-17 was augmented by nSP30. Th17 cells are differentiated from naïve CD4+ T cells by IL-6 and transforming growth factor-β.7 Administration of nSP dose-dependently increased inflammatory cytokines including IL-6 and TNF-α.20 Therefore, the promotion of IL-6 production by nSPs might be involved in the differentiation of Th17 cells. Th17 cells are present exclusively in the small intestinal lamina propria mucosa and are induced by enteric bacteria such as segmented filamentous bacterium. Th17 cells secrete IL-17 and IL-22, which play an important role in the elimination of fungi and extracellular bacteria via neutrophil migration and release of antibacterial peptides from epithelial cells.21 Th17 cells have been recently recognized as an important component for the protective immunity produced by mucosal vaccination because Th17 cytokines have also been demonstrated to contribute to mucosal immunity.22 Thus, a Th17 adjuvant might be useful for the development of novel mucosal vaccines. Most vaccines are antibody-based vaccines (Th2-type response). Antibody-based vaccines usually have a limited protective spectrum because the responses are restricted to the vaccination strains. In contrast, T cell-based vaccines (Th1- and Th17-type responses) have the potential to provide serotype-independent protection by recognizing antigens conserved across species and thus have been recently investigated by many researchers. Indeed, in experimental models, vaccination induced significant Th17 responses in the lung yielding higher pathogen burden and mortality.22 Protection by immunization with whole-cell pertussis vaccine could also be mediated by enhancement of the Th17-dependent bactericidal activity of macrophages.23 A combination of Th1 and Th17 responses is necessary for protection against infection from pathogens such as Mycobacterium tuberculosis.24 Nanoemulsion adjuvant, a Th17 adjuvant, has safety concerns because elevated IL-17 and IL-22 production could be harmful and is well documented in autoimmune diseases.25 Although Th17 adjuvants have already prompted safety concerns, our enhanced understanding of the molecular mechanisms that control protective Th17 responses will greatly aid vaccine design. Elucidation of the mechanism of action of adjuvants might lead to the selection of more suitable adjuvants in terms of pathogenic microbes and disease, allowing for the appropriate direction of immune responses.
In the present study, the no-observed-adverse-effect level for the adjuvant after i.p. injection of OVA and nSP30 was suggested to be 3 µg because an nSP30 concentration of 3 µg or lower did not induce antigen-specific immune responses. This might not be of clinical significance because i.p. injection does not reflect the actual exposure route. However, continued monitoring of the safety of silica should be conducted because nSPs have rapidly become widespread in daily life, men and women of all ages (including pregnant women) cannot avoid exposure to them, and nSP accumulation in the body is of concern. Therefore, further investigations on the effect of nSP30 on the immune system using the correct exposure route and the relationship between the adjuvant effects and disposition, including the exposure level in blood and in various tissues and organs, might provide useful information in the future.
The adjuvant effect of nSP30 observed in the present study is likely to depend on particle size because many types of particulate matter with an external dimension of ⩽1 µm have adjuvant effects and enhance antigen-specific IgE antibody production, thus highlighting the relationship between particle size and adjuvant effects.26 Polymethylmethacrylate with a diameter ⩽1 µm affected the Th1/Th2 balance in mice, whereas polymethylmethacrylate with a diameter >1 µm had no effect on the immune system.26 In addition, particle size is involved in the activation of the innate immune system because it is likely the primary factor that regulates endocytosis of phagocytes, including macrophages.27 Therefore, this study investigated mice administered with OVA plus variously sized SPs (30, 100, and 1000 nm). The production of anti-OVA IgG antibodies and proliferative responses of splenocytes to OVA were enhanced in a size-dependent manner. However, the type of immune response was not dependent upon particle size because patterns of anti-OVA IgG subclasses and cytokines induced by SPs (100 and 1000 nm) were similar to those induced by nSP30. Thus, these findings suggest that a reduction in particle size of SPs enhanced adjuvant effects but did not affect the type of immune response. A report from an Organization of Economic Co-operation and Development workshop in 2009 described the characterization of NMs by mass and various dose metrics (e.g. particle surface area and particle number and concentration), which might be important because the surface area available for the reaction is increased with a reduction in particle size.28 A further understanding of the NM profile and improvements in risk estimation and safety assessment for NMs should be conducted based on weight, surface area, and particle number.
The current study showed that i.p. injections of SPs and OVA antigen enhanced the production of anti-OVA IgG antibodies and proliferative responses of splenocytes to OVA in a dose- and size-dependent manner (smaller particles had a greater adjuvant effect). Additionally, anti-OVA IgG1, IgG2a, and IgE production was enhanced in a dose- and size-dependent manner by the combined injection of SPs with OVA. Finally, there was also a dose and size-dependent enhancement in the production of IFN-γ, IL-2, IL-4, IL-5, and IL-17. Thus, SPs exerted an adjuvant effect for Th1, Th2, and Th17 immune responses and their effects correlated with particle size in terms of Ig and cytokine levels. However, further investigations on SP adjuvanticity are warranted because i.p. injection does not reflect the actual exposure route or various dose metrics (e.g. particle surface area and number concentration) that change with a reduction in particle size. Particulate adjuvants in our living environment have biological effects and might be precipitating factors in allergic diseases, despite their medical benefits as vaccine adjuvants. Elucidation of the mechanism of action of particulate adjuvants might lead to the development of a new generation of vaccine adjuvants as well as innovative therapies for allergic diseases caused by environmental factors. Further studies of the mechanisms by which nSPs activate the immune system are expected to provide more information about the design of safe and applicable forms of NMs.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Toda is an employee of Shionogi & Co., Ltd, Japan. Dr. Yoshino has no conflict of interest to declare. The authors alone are responsible for the content of this manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Jaganathan H, Godin B. (2012) Biocompatibility assessment of Si-based nano- and micro-particles. Advanced Drug Delivery News 64(15): 1800–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao Y, Xing G, Chai Z. (2008) Nanotoxicology: Are carbon nanotubes safe? Nature Nanotechnology 3(4): 191–192. [DOI] [PubMed] [Google Scholar]
- 3. Takagi A, Hirose A, Nishimura T, et al. (2008) Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. Journal of Toxicological Sciences 33(1): 105–116. [DOI] [PubMed] [Google Scholar]
- 4. Morishige T, Yoshioka Y, Tanabe A, et al. (2010) Titanium dioxide induces different levels of IL-1beta production dependent on its particle characteristics through caspase-1 activation mediated by reactive oxygen species and cathepsin B. Biochemical and Biophysical Research Communications 392(2): 160–165. [DOI] [PubMed] [Google Scholar]
- 5. Peters R, Kramer E, Oomen AG, et al. (2012) Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 6(3): 2441–2451. [DOI] [PubMed] [Google Scholar]
- 6. Nabeshi H, Yoshikawa T, Matsuyama K, et al. (2011) Systemic distribution, nuclear entry and cytotoxicity of amorphous nanosilica following topical application. Biomaterials 32(11): 2713–2724. [DOI] [PubMed] [Google Scholar]
- 7. Zhu J, Yamane H, Paul WE. (2010) Differentiation of effector CD4 T cell populations. Annual Review of Immunology 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin W, Dong C. (2013) IL-17 cytokines in immunity and inflammation. Emerging Microbes & Infections 2(9): e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshino S, Sagai M. (1999) Induction of systemic Th1 and Th2 immune responses by oral administration of soluble antigen and diesel exhaust particles. Cellular Immunology 192(1): 72–78. [DOI] [PubMed] [Google Scholar]
- 10. Hirai T, Yoshikawa T, Nabeshi H, et al. (2012) Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection. Particle and Fibre Toxicology 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamaki K, Uchida H, Harada Y, et al. (2003) Effect of the nonsteroidal anti-inflammatory drug indomethacin on Th1 and Th2 immune responses in mice. Journal of Pharmaceutical Sciences 92(8): 1723–1729. [DOI] [PubMed] [Google Scholar]
- 12. Brewer JM, Conacher M, Satoskar A, et al. (1996) In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. European Journal of Immunology 26(9): 2062–2066. [DOI] [PubMed] [Google Scholar]
- 13. Grun JL, Maurer PH. (1989) Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: The role of endogenous interleukin 1 in proliferative responses. Cellular Immunology 121(1): 134–145. [DOI] [PubMed] [Google Scholar]
- 14. Kusaka T, Nakayama M, Nakamura K, et al. (2014) Effect of silica particle size on macrophage inflammatory responses. PLoS One 9(3): e92634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marichal T, Ohata K, Bedoret D, et al. (2011) DNA released from dying host cells mediates aluminum adjuvant activity. Nature Medicine 17(8): 996–1002. [DOI] [PubMed] [Google Scholar]
- 16. Kool M, Willart MA, van Nimwegen M, et al. (2011) An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34(4): 527–540. [DOI] [PubMed] [Google Scholar]
- 17. Kuroda E, Coban C, Ishii KJ. (2013) Particulate adjuvant and innate immunity: Past achievements, present findings, and future prospects. International Reviews of Immunology 32(2): 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirai T, Yoshioka Y, Takahashi H, et al. (2012) Amorphous silica nanoparticles enhance cross-presentation in murine dendritic cells. Biochemical and Biophysical Research Communications 427(3): 553–556. [DOI] [PubMed] [Google Scholar]
- 19. Hornung V, Bauernfeind F, Halle A, et al. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunology 9(8): 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wottrich R, Diabaté S, Krug HF. (2004) Biological effects of ultrafine model particles in human macrophages and epithelial cells in mono- and co-culture. International Journal of Hygiene and Environmental Health 207(4): 353–361. [DOI] [PubMed] [Google Scholar]
- 21. Honda K, Littman DR. (2012) The microbiome in infectious disease and inflammation. Annual Review of Immunology 30: 759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar P, Chen K, Kolls JK. (2013) Th17 cell based vaccines in mucosal immunity. Current Opinion in Immunology 25(3): 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins SC, Jarnicki AG, Lavelle EC, et al. (2006) TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: Role of IL-17-producing T cells. Journal of Immunology 177(11): 7980–7989. [DOI] [PubMed] [Google Scholar]
- 24. Zenaro E, Donini M, Dusi S. (2009) Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, Mannose Receptor, and DC-SIGN. Journal of Leukocyte Biology 86(6): 1393–1401. [DOI] [PubMed] [Google Scholar]
- 25. Bielinska AU, Gerber M, Blanco LP, et al. (2010) Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Critical Reviews in Immunology 30(2): 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Granum B, Gaarder PI, Groeng E, et al. (2001) Fine particles of widely different composition have an adjuvant effect on the production of allergen-specific antibodies. Toxicology Letters 118(3): 171–181. [DOI] [PubMed] [Google Scholar]
- 27. Hirota K, Terada H. (2012) Endocytosis of particle formulations by macrophages and its application to clinical treatment. Molecular Regulation of Endocytosis. DOI: 10.5772/45820 Available at: http://cdn.intechopen.com/pdfs-wm/37733.pdf. [DOI]
- 28. The OECD Working Party on Chemicals, Pesticides and Biotechnology (2010) Report of the Workshop on Risk Assessment of Manufactured Nanomaterials in a Regulatory Context. Series on the Safety of Manufactured Nanomaterials No. 21. Paris: OECD. [Google Scholar]