Abstract
In industrialized countries, overweight and obesity account for approximately 13.8% and 24.9% of the kidney disease observed in men and women, respectively. Moreover, obesity-associated glomerulopathy is now considered as “an emerging epidemic.” Kidney function can be negatively impacted by obesity through several mechanisms, either direct or indirect. While it is well established that obesity represents the leading risk factor for type 2 diabetes and hypertension, awareness that obesity is associated with direct kidney damage independently of hypertension and diabetes is still not widespread. In this paper we will discuss the emerging role of adipose tissue, particularly in the visceral depot, in obesity-induced chronic kidney damage.
Keywords: hyperfiltration, kidney disease, obesity
Introduction
Obesity-related glomerulopathy is a slow and progressive impairment of renal function, usually occurring over a period of months or years (1). The role of obesity as a relevant risk factor for kidney disease has been increasingly recognized, and obesity-associated glomerulopathy is now considered as “an emerging epidemic” (2). In a recent meta-analysis, Wang et al. reported that, in industrialized countries, overweight and obesity account for approximately 13.8% and 24.9% of kidney disease observed in men and women, respectively, with an even higher estimate in the United States (24.2% and 33.9%, respectively) (3).
Kidney function can be negatively impacted by obesity through several mechanisms, either direct or indirect, the latter resulting from obesity-associated complications (hypertension, diabetes mellitus, hyperlipidemia). Several lines of evidence have demonstrated a link between obesity and endothelial dysfunction, a condition associated with unopposed vasoconstriction (4–8) and a proatherosclerotic phenotype (9), in which inflammatory mechanisms are known to play a central role (10). In addition, our group has shown the contribution of gut-derived hormones in the modulation of vascular function in obesity and metabolic syndrome (11–15).
Endothelial dysfunction is the likely link whereby obesity may directly promote kidney damage through a mechanism of hyperfiltration. The “theory of hyperfiltration” (16,17) is based on the observation that an impaired function and the structural adaptations of the kidney can be a consequence of a reduced number of performing nephrons. It must be pointed out that this theory may not apply to all clinical situations, as a significant number of patients showing severe reduction of renal mass do not develop kidney disease. A number of mechanisms have been postulated to account for the link between obesity and kidney disease where the adipocytokines, hormone-like peptides with multiple biological actions, appear to play a critical role.
The hyperfiltration theory
Brenner et al. first showed that unilateral nephrectomy can lead to proteinuria, glomerulosclerosis in the contralateral kidney, and progressive renal failure in rats (16,17). The theory of hyperfiltration is based on the hypothesis that a decline in the number of healthy nephrons leads to functional and structural adaptations, which eventually cause further nephron loss and impairment of residual kidney function in a self-perpetuating cycle. Following renal ablation, residual glomeruli undergo hemodynamic adaptations, including vasodilation of afferent glomerular arterioles, vasoconstriction of efferent glomerular arterioles, and increased filtration fraction and glomerular transcapillary hydraulic pressure. The compensatory glomerular hyperfiltration, which contributes to preserve glomerular filtration rate (GFR), is accompanied by glomerular volume expansion and an increase in the matrix components as well as the number of endothelial and mesangial cells. However, adult podocytes are unable to proliferate, leaving gaps in the glomerular basement membrane (GBM) and predisposing to the development of proteinuria (18). However, it must be pointed out that a significant number of patients (>80%) with bilateral renal carcinomas who underwent renal parenchyma reduction did not show these features during the follow-up (19). The same discrepancy has also been described in renal agenesis, where a substantial percentage of cases show regular renal function without proteinuria.
Role of leptin
Besides its classical function as an energy depot, adipose tissue controls several pathophysiological processes through the production of several mediators including adipocytokines, clotting factors, and complement products (19). Among adipokines, leptin, a class 1 cytokine, appears to play a broad spectrum of actions in different organ settings, including the kidneys. Most biological effects of leptin are mediated by Ob–Rb receptors. In the brain, these receptors are highly expressed in the hypothalamus, where they regulate the balance between appetite and caloric intake by activating the expression of proopiomelanocortin (POMC). In turn, POMC leads to the production of α-melanocyte-stimulating hormone (MSH), which binds to melanocortin receptor 3 and melanocortin receptor 4 (MC3/MC4-R) thereby reducing appetite and increasing energy expenditure (20–22).
In lean individuals, fasting decreases leptin concentration whereas hyperalimentation increases its levels. In contrast, in obese patients this physiological mechanism is impaired and even in the presence of high leptin levels there is blunted decline of appetite or increase in energy consumption (23). This observation suggests a resistance to leptin at the level of the hypothalamus and of metabolic organs. The organ-selectivity of leptin resistance can be possibly explained by potential defects in the leptin transfer at the level of the blood-brain barrier (24) and by a possible reduced activation of the signaling transduction pathways involved. Interestingly, this resistance appears to be organ-specific and may not involve the autonomic nervous system (ANS) and the kidneys. Thus, the increased risk of developing an obesity-associated glomerulosclerosis has been related to increased serum leptin concentration. Of note, leptin can also increase the production of the extracellular matrix (ECM) and participate in the pathogenesis of diabetic nephropathy, consistent with the observed association between higher urine leptin levels and increased albuminuria in Pima Indians (25).
Leptin and albuminuria
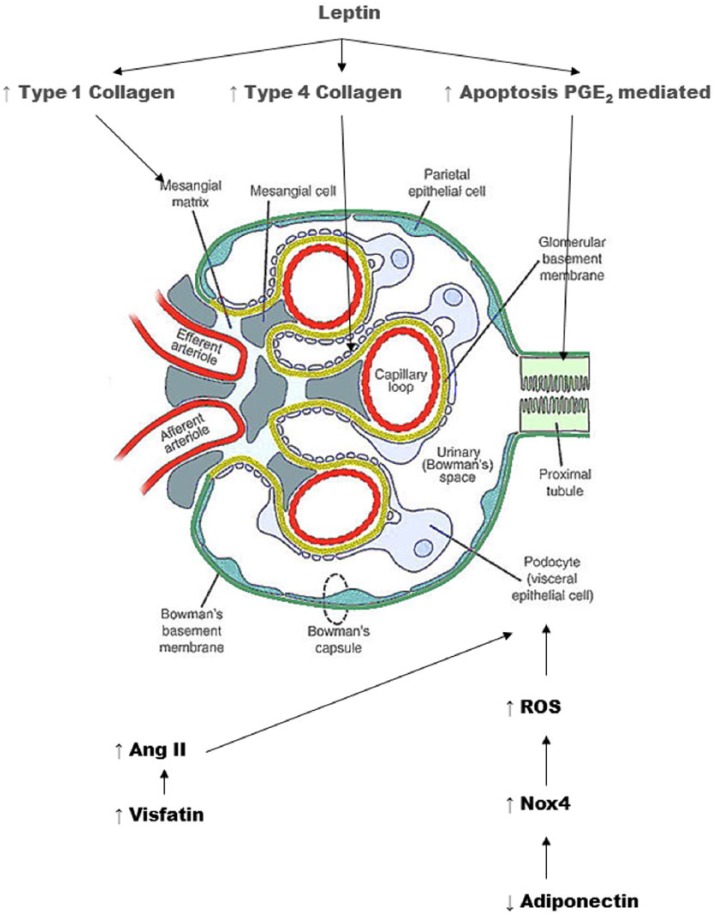
Kidney cells mainly express the shorter isoform of Ob–Ra receptor, although the Ob–Rb isoform is also present to a lesser extent (26). Short-term exposure to leptin is able to induce the synthesis of transforming growth factor-α1 (TGF-α1), leading to the proliferation of renal glomerular endothelial cells (27). Moreover, long-term infusion with the cytokine has been shown to upregulate glomerular expression of type I and type IV collagen in mesangial and glomerular endothelial cells, with ECM deposition, glomerulosclerosis, and a significant increase in urinary protein excretion independent of blood pressure increase (28). Interestingly, in a diet-induced obese mouse model, leptin-induced switch of mesangial cell to a fibrotic phenotype is mediated by thrombospondin 1 (TSP1) (29). TSP1 is expressed by mesangial, endothelial, and tubular cells as well as podocytes in the kidney, where it plays a critical role in the development of the diabetic kidney disease through the induction of the pro-fibrogenic transforming growth factor-β (TGF- β) (30,31). The critical role of TSP1 in the switch to a fibrotic cell phenotype has been demonstrated for the leptin-mediated induction of TGF-β1, fibronectin, and collagen type IV in TSP1-wild-type mesangial cells, while this stimulatory effect of leptin has not been described in TSP1-deficient mesangial cells (29).
Leptin and renal tubular damage
A critical feature of chronic kidney disease is the involvement of the interstitial tubule resulting in either cellular damage mediated by the protein reabsorption or direct damage of the tubular epithelium.
Long-term leptin treatment induces apoptosis in rat renal tubular cells (NRK-52E) in vitro (32) through an increase in the expression of cyclooxygenase 2 (COX-2)-mediated prostaglandin E2 (PGE2). It must be pointed out that while prolonged treatment with leptin has been associated with apoptosis of tubular cells, a short exposure to the hormone has been described as protective to gentamicin-induced apoptosis, suggesting a dichotomous scenario dependent on the time-length of cell exposure to leptin (33). In particular, in opossum renal tubular cells, 2-h exposure to leptin is able to reduce protein synthesis rate (34), likely through m-TOR (the mammalian target of rapamycin)-dependent mechanisms. However, this effect has been observed only in the presence of low glucose levels, suggesting that other mechanisms may be involved in the presence of hyperglycemia.
Leptin and transactivation of ErbB receptor family
An emerging mechanism linking hyperleptinemia and kidney damage involves the transactivation of the ErbB receptor family. These receptors are part of the tyrosine kinase subclass I (RTK) superfamily, which includes epidermal growth factor (EGF) receptors (EGFR) HER1 (ErbB1), HER2/neu (ErbB2), HER3 (ErbB3), and HER4 (ErbB4) (35).
The binding of specific ligands to ErbB receptors induces conformational changes and the creation of different receptor homo- or heterodimers, which in turn activate selective signaling pathways. Several ErbB receptors ligands have been identified including EGF, TGF-α, and the heparin-binding EGF-like growth factor (HB-EGF). Inactive transmembrane ErbB precursor ligands require the proteolytic cleavage of their ectodomain in order to be activated and released as mature soluble ligands. Although ADAM metalloprotease family members are the primarily responsible for cleavage (36), ErbB ligand cleavage can also be performed by several matrix metalloproteinases (37). G-protein coupled receptor (GPCR)-binding proteins such as angiotensin II (Ang II), which binds to the angiotensin type 1 receptor (AT1), can contribute to the ADAM-dependent EGFR ligand shedding. In particular, the ADAM-mediated activation of EGFR, induced by GPCR, has been defined as transactivation (38).
Depending on the tissue, different ADAMs may be involved in EGF receptor transactivation. In the kidney, ADAM17 has been implicated in the transactivation mediated by Ang II (39). The binding of different Erb-B ligands and the combination of Erb-B homo- and heterodimer receptors can activate distinct downstream pathways, including the mitogen activated protein kinase (MAPK) and the phosphatidylinosithol-3kinase (PI3K) signaling pathways (35), which are able to control cellular behavior by transducing cell signals to the nucleus, then influencing the activity of transcription factors. Based on these assumptions, Beltowski et al. have demonstrated the ability of high levels of leptin to transactivate the EGF receptor and activate the receptor Erb-B2 (40). Increased PI3K activity has been observed in the aortic media of adult male Wistar rats exposed to increasing doses of leptin for 10 days, which was markedly attenuated by the EGFR inhibitor AG1478 and by the ErbB2 receptor inhibitor AG825. These observations demonstrated that both EGFR and ErbB2 contribute to the activation of PI3K signaling pathway by leptin.
Leptin has also been shown to increase tyrosine phosphorylation of ErbB2, which is abolished either by AG1478 or AG825.
The ability of hyperleptinemia to transactivate ErbB receptors could be considered as an additional mechanism of fibrogenesis induction. Indeed, although constitutive EGFR expression has been observed in glomeruli, tubules, and interstitium of most normal human kidneys, EGFR increase has been detected in several forms of glomerulonephritis and in allograft nephropathy (41–43).
In particular, EGFR expression has been described in glomerular fibrotic lesions, while tubular EGFR levels have been found to correlate with the extent of interstitial fibrosis. HB-EGF is absent in normal kidneys while it is strongly expressed in the glomerular mesangium of patients with glomerulonephritis. Also, mesangial HB-EGF correlates with proliferation, and in a model of cultured mesangial cells recombinant HB-EGF is able to induce proliferation and synthesis of type I and III collagen (44). Ang II-induced EGFR transactivation has been shown to activate renal TGF-β signaling pathway, which demonstrates a link between two main effectors of fibrosis (45). Further investigation is needed to explore this potential pathophysiological role of hyperleptinemia.
Role of adiponectin
Adipocytokine abnormalities in obesity extend beyond leptin the role and include a reduced expression of adiponectin, a 30 kDa protein mainly secreted by adipocytes. In humans, plasma adiponectin is expressed as multimeric forms, from trimers to high molecular weight oligomers containing 12- to 18-mers at concentrations in the range of 5–30 μg/mL (46). Interestingly, a recombinant fragment of adiponectin containing the C-terminal globular head domain is able to interact with cellular adiponectin receptors, mimicking several activities of the full-length, oligomeric form of the protein. Both full-length forms of adiponectin (gAd and fAd, respectively) bind to AdipoR1 and AdipoR2 receptors, activating the 5′-AMP-activated protein kinase (AMPK) signaling and, possibly other intracellular pathways (47,48). AdipoR1 is widely expressed by different types of tissues, being particularly abundant in the liver and skeletal muscle (49). AdipoR2 expression is usually considered to be more restricted mainly to liver and muscle. Receptor subtypes are expressed in podocytes (50). The ligand-binding sites are slightly different for adipoR1 and R2: adipoR1 has higher affinity for globular adiponectin, while adipoR2 has intermediate affinity for both globular and full-length adiponectin (51).
Hypoadiponectinemia and microalbuminuria
A correlation between hypoadiponectinemia and microalbuminuria has been described in subjects with high blood pressure as well as in Japanese and African-American obese patients (52,53).
Adiponectin deficiency is associated to microalbuminuria and alteration of podocyte pediculi expression in the presence of a normal endothelium in knockout mice (54) and treatment with the protein restores normal albuminuria and regular podocyte foot processes.
In the absence of adiponectin, mice podocytes display foot fusion processes and appear to be reduced in the number. Podocyte damage secondary to the absence of adiponectin has been possibly related to the reduced activity of AMPK (see above). This observation has proved a role for podocytes in the initial development of albuminuria, which in contrast seems to be independent of the tubular uptake of albumin. The binding of adiponectin to the AdipoR1 receptor expressed by podocytes and other renal cell types leads to a reduction of the Nox4-isoform of NAPDH oxidase through the AMPK pathway. Thus low levels of adiponectin can lead to unbalanced Nox activity in the kidney, potentially causing an overall increase in reactive oxygen species (ROS) and vascular injury.
In particular, it has been shown that high levels of adiponectin can contrast the generation of ROS induced by high glucose levels in human glomerular mesangial cells, partly through the stimulation of the AMPK signaling pathway (55). A marked renal damage progression has been described in patients with hypoadiponectinemy by Kacso et al. (56), in a study performed on 86 type 2 diabetic patients, with a GFR >30 mL/min. A possible protective role of adiponectin on podocytes has been proposed by Rutkowski et al. (57). These authors have created a strain of guinea pigs characterized by the potential expression of the mutant protein FKBP, which, if present in a dimeric form, is able to induce caspase 8-mediated podocyte apoptosis. Dimerization of FKBP is induced by the administration of the specific agent AP20187. The induction of caspase 8-mediated apoptosis is described by the acronym ATTAC (apoptosis through targeted activation of caspase 8): apoptosis appears to be dependent on the dose of the dimerising agent and is self-limited in time. The POD-ATTAC strain is characterized by inducible podocyte reduction, whose extent depends on the dose of the dimerising agent FKBP. This model can achieve various degrees of histological impairment, from minimal change detectable only by electron microscopy to damage mimicking focal segmental glomerulosclerosis (FSGS) with consequent changes in GFR and albuminuria. Using low or intermediate doses of FKBP it is then possible to induce reversible podocyte damage, with the potential for structural and functional recovery.
The role of adiponectin in podocyte loss and renal function has been investigated by crossing adiponectin knock out and adiponectin-overexpressing adipo Tg mice (58) with the POD-ATTAC (POD) mice, with the generation of adiponectin KO POD-ATTAC (POD-KO) and adipo Tg POD-ATTAC (POD-Tg) mice. POD-Tg POD-KO mice exhibited a rapid loss of podocytes after treatment with FKBP; however, the lack of adiponectin did not increase the extent of podocyte ablation as compared with POD mice exposed to the same treatment. POD-KO podocytes showed lower recovery capacity as compared with POD mice, with glomeruli appearing from normal to FSGS. While adiponectin overexpression has been shown to be highly cytoprotective in ATTAC mouse models, 15 PODTg mice showed podocyte impairment at 7 days. However, a clear recovery has been observed from day 28 to day 60 in the glomerular area of adiponectin-overexpressing POD POD-Tg mice, along with decreased albumin excretion.
Adiponectin and renal tubular cells
Adiponectin appears to exert protective effects not only on podocytes, but also on tubular cells. The presence of adiponectin receptors on human renal tubular cells has been demonstrated by Fang et al. (59), starting from the assumption that renin angiotensin system (RAS) activation and increased Ang II could contribute to the development and progression of the kidney disease and that one of the mechanisms responsible would be an increase in oxidative stress due to the activation of Ang II-mediated NADPH oxidase (60). Adiponectin has then been shown to mitigate the effect of Ang II on NADPH oxidase through a mechanism Adipo R1-mediated. The role of Ang II-induced activation of NF-κB, a key mediator of the cellular inflammatory response, has been demonstrated in renal tubular cells by gene expression reporter systems, along with the production of fibronectin, an extracellular matrix protein which accumulates in kidney injury. Both Ang II-mediated NF-κB activation and fibronectin production have been shown to be attenuated by adiponectin.
Role of alpha-2-Heremans Schmid glycoprotein (fetuin-A)
Alpha-2-Heremans Schmid glycoprotein (AHSG), also known as fetuin-A, is a 64-kDa circulating glycoprotein initially isolated from fetal bovine serum. Human AHSG, a member of the cystatin superfamily of cysteine protease inhibitors (61,62), is synthesized by hepatocytes and present in all extracellular fluids (63). AHSG induces insulin resistance in the skeletal muscle and the liver by inhibiting insulin receptor tyrosine kinase and downregulating insulin signal transduction (64,65). Lack of fetuin-A in null mice has been associated with increased skeletal muscle glycogen content and resistance to weight gain in the presence of a high-fat diet (66,67). High levels of fetuin-A have been associated with obesity and insulin resistance in humans with chronic kidney disease (CKD) (68). Fetuin-A suppresses adiponectin mRNA in cultured human adipocytes and reduces serum adiponectin levels in wild-type mice (69). This effect appears to be specific to adiponectin, as fetuin-A treatment does not affect mRNA levels of leptin and resistin. Collectively, these observations demonstrate a role for fetuin- A in the inhibition of adiponectin production by the adipose tissue.
Role of visfatin
Visfatin was originally identified as PBEF (pre-B-cell colony-enhancing factor) and has been associated to a variety of inflammatory states (70–72). Visfatin induces the expression of the inflammatory mediators TNF-α, IL-1β, and IL-6 (73), of profibrotic molecules such as TGF-β1 and type I collagen, and of the antifibrinolytic plasminogen activator inhibitor-1 (74). Visfatin is secreted by the adipocytes of visceral fat in the presence of hyperglycemia and exerts several insulin-like actions (75). Interestingly, visfatin increases mRNA levels of rennin, angiotensinogen (AGT), angiotensin-converting enzyme (ACE), AT1, and AT2 in rat mesangial cells in a dose-dependent manner. These observations suggest that the negative effects of visfatin on the kidney are possibly mediated by Ang II through the involvement of AT1.
Conclusions
As stated by The World Health Organization in 1997, “…obesity’s impact is so diverse and extreme that it should now be regarded as one of the greatest neglected public health problems of our time with an impact on health which may well prove to be as great as that of smoking”. There is a growing understanding of the role of adipose tissue, particularly in the visceral depot, in obesity-induced chronic kidney damage. An abnormal regulation of adipocytokines appears to mediate at least in part this detrimental effect of obesity on renal function. On the one side, increased leptin levels have been linked to both glomerular damage (by inducing glomerulosclerosis and increasing production of collagen by podocytes and mesangial cells) and tubulointerstitial injury (by promoting apoptosis of tubular cells). On the other hand, reduced level of adiponectin, which exerts protective effects on podocites through anti-apoptotic and antifibrotic mechanisms, has been associated with podocyte damage mediated by ROS. While it is well established that obesity represents the leading risk factor for hypertension, metabolic syndrome, and type 2 diabetes mellitus, the awareness that obesity is associated with direct kidney damage independently of hypertension and diabetes is still not widespread. Further studies are necessary to improve our knowledge of the mechanisms linking obesity-related adipose tissue dysfunction and renal damage, to identify potential targets for intervention in order to prevent kidney injury and decrease the cardiovascular risk associated with chronic kidney disease.
Figure 1.
The Hyperfiltration Model.
Figure 2.
Kidney Damage Mechanisms.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by a grant from the Fondazione Roma to Manfredi Tesauro
References
- 1. National Kidney Foundation (2002) K/DOQI clinical practice guideline for chronic kidney disease: Evaluation, classification and stratification. American Journal of Kidney Disease 39(2 Suppl 1): S1–266. [PubMed] [Google Scholar]
- 2. Kambham N, Markowitz GS, Valeri AM, et al. (2001) Obesity-related glomerulopathy: An emerging epidemic. Kidney International 59: 1498–1509. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Chen X, Song Y, et al. (2008) Association between obesity and kidney disease: A systematic review and meta analysis. Kidney International 73: 19–33. [DOI] [PubMed] [Google Scholar]
- 4. Campia U, Tesauro M, Di Daniele N, et al. (2014) The vascular endothelin system in obesity and type 2 diabetes: Pathophysiology and therapeutic implications. Life Sciences 118: 149–155. [DOI] [PubMed] [Google Scholar]
- 5. Campia U, Tesauro M, Cardillo C. (2012) Human obesity and endothelium-dependent responsiveness. British Journal of Pharmacology 165: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tesauro M, Cardillo C. (2011) Obesity, blood vessels and metabolic syndrome. Acta Physiologica (Oxford, England) 203: 279–286. [DOI] [PubMed] [Google Scholar]
- 7. Schinzari F, Tesauro M, Rovella V, et al. (2013) Leptin stimulates both endothelin-1 and nitric oxide activity in lean subjects but not in patients with obesity-related metabolic syndrome. Journal of Clinical Endocrinology and Metabolism 98: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 8. Schinzari F, Tesauro M, Rovella V, et al. (2010) Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. American Journal of Physiology. Endocrinology and Metabolism 299: E947–952. [DOI] [PubMed] [Google Scholar]
- 9. Tesauro M, Canale MP, Rodia G, et al. (2011) Metabolic syndrome, chronic kidney, and cardiovascular diseases: Role of adipokines. Cardiology Research and Practice 2011: 653182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iantorno M, Campia U, Di Daniele N, et al. (2014) Obesity, inflammation and endothelial dysfunction. Journal of Biological Regulators and Homeostatic Agents 28: 169–176. [PubMed] [Google Scholar]
- 11. Iantorno M, Campia U, Di Daniele N, et al. (2014) Gut hormones and endothelial dysfunction in patients with obesity and diabetes. International Journal of Immunopathology and Pharmacology 27: 433–436. [DOI] [PubMed] [Google Scholar]
- 12. Tesauro M, Schinzari F, Caramanti M, et al. (2010) Cardiovascular and metabolic effects of ghrelin. Current Diabetes Reviews 6: 228–235. [DOI] [PubMed] [Google Scholar]
- 13. Tesauro M, Schinzari F, Rovella V, et al. (2009) Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension 54: 995–1000. [DOI] [PubMed] [Google Scholar]
- 14. Tesauro M, Schinzari F, Iantorno M, et al. (2005) Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 112: 2986–2992. [DOI] [PubMed] [Google Scholar]
- 15. Tesauro M, Schinzari F, Adamo A, et al. (2013) Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care 36: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brenner BM. (1983) Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney International 23: 647–655. [DOI] [PubMed] [Google Scholar]
- 17. Deen WM, Maddox DA, Robertson CR, et al. (1974) Dynamics of glomerular ultrafiltration in the rat. VII: Response to reduced renal mass. American Journal of Physiology 227: 556–562. [DOI] [PubMed] [Google Scholar]
- 18. Chen HM, Liu ZH, Zeng CH, et al. (2006) Podocyte lesions in patients with obesity-related glomerulopathy. American Journal of Kidney Disease 48: 772–779. [DOI] [PubMed] [Google Scholar]
- 19. González E, Gutiérrez E, Morales E, et al. (2005) Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney International 68: 263–270. [DOI] [PubMed] [Google Scholar]
- 20. Castro MG, Morrison E. (1997) Post-translational processing of pro-opiomelanocortin in the pituitary and in the brain. Critical Reviews in Neurobiology 11: 35–57. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz MW, Woods SC, Porte D, Jr, et al. (2000) Central nervous system control of food intake. Nature 404: 661–671. [DOI] [PubMed] [Google Scholar]
- 22. Spiegelman BM, Flier JS. (2001) Obesity and the regulation of energy balance. Cell 104: 531–543. [DOI] [PubMed] [Google Scholar]
- 23. Montani JP, Antic V, Yang Z, et al. (2002) Pathways from obesity to hypertension: From the perspective of a vicious triangle. International Journal of Obesity 26: S28–S38. [DOI] [PubMed] [Google Scholar]
- 24. Morris DL, Rui L. (2009) Recent advances in understanding leptin signaling and leptin resistance. American Journal of Physiology 297: E1247–E1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson C, Nelson R, Nicolson M, et al. (1998) Plasma leptin concentrations: No difference between diabetic pima indians with and without nephropathy. Diabetologia 41: 861–862. [DOI] [PubMed] [Google Scholar]
- 26. Serradeil-Le Gal C, Raufaste D, Brossard G, et al. (1997) Characterization and localization of leptin receptors in the rat kidney. FEBS Letters 404: 185–191. [DOI] [PubMed] [Google Scholar]
- 27. Wolf G, Hamann A, Han DC, et al. (1999) Leptin stimulates proliferation and TGF-β expression in renal glomerular endothelial cells: Potential role in glomerulosclerosis. Kidney International 56: 860–872. [DOI] [PubMed] [Google Scholar]
- 28. Ballermann BJ. (1999) A role for leptin in glomerulosclerosis? Kidney International 56: 1154–1155. [DOI] [PubMed] [Google Scholar]
- 29. Cui W, Maimaitiyiming H, Qi X, et al. (2013) Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fatdiet-induced obesity. American Journal of Physiology. Renal Physiology 305: F871–F880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang S, Shiva S, Poczatek MH, et al. (2002) Nitric oxide and cGMP-dependent protein kinase regulation of glucosemediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. Journal of Biological Chemistry 277: 9880–9888. [DOI] [PubMed] [Google Scholar]
- 31. Wang S, Skorczewski J, Feng X, et al. (2004) Glucose upregulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. Journal of Biological Chemistry 279: 34311–34322. [DOI] [PubMed] [Google Scholar]
- 32. Hsu YH, Cheng CY, Chen YC, et al. (2012) Long-term leptin treatment exerts a pro-apoptotic effect on renal tubular cells via prostaglandin E2 augmentation. European Journal of Pharmacology 689: 65–71. [DOI] [PubMed] [Google Scholar]
- 33. Chen YC, Chen CH, Hsu YH, et al. (2011) Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. European Journal of Pharmacology 658: 213–218. [DOI] [PubMed] [Google Scholar]
- 34. Briffa JF, Grinfeld E, McAinch AJ, et al. (2014) Short term exposure to elevated levels of leptin reduces proximal tubule cell metabolic activity. Molecular and Cellular Endocrinology 382: 38–45. [DOI] [PubMed] [Google Scholar]
- 35. Holbro T, Hynes NE. (2004) ErbB receptors: Directing key signaling networks throughout life. Annual Review of Pharmacology and Toxicology 44: 195–217. [DOI] [PubMed] [Google Scholar]
- 36. Shah BH, Catt KJ. (2006) TACE-dependent EGF receptor activation in angiotensin-II-induced kidney disease. Trends in Pharmacological Sciences 27: 235–237. [DOI] [PubMed] [Google Scholar]
- 37. Roelle S, Grosse R, Aigner A, et al. (2003) Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. Journal of Biological Chemistry 278: 47307–47318. [DOI] [PubMed] [Google Scholar]
- 38. Ohtsu H, Dempsey PJ, Eguchi S. (2006) ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. American Journal of Physiology. Cell Physiology 291: C1–10. [DOI] [PubMed] [Google Scholar]
- 39. Lautrette A, Li S, Alili R, et al. (2005) Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nature Medicine 11: 867–874. [DOI] [PubMed] [Google Scholar]
- 40. Bełtowski J, Wójcicka G, Widomska S. (2011) Leptin stimulates phosphoinositide 3-kinase in the vascular wall: Role of ErbB receptors. Adipobiology 3: 53–59. [Google Scholar]
- 41. Yoshioka K, Takemura T, Murakami K, et al. (1990) Identification and localization of epidermal growth factor and its receptor in the human glomerulus. Laboratory Investigation 63: 189–196. [PubMed] [Google Scholar]
- 42. Nakopoulou L, Stefanaki K, Boletis J, et al. (1994) Immunohistochemical study of epidermal growth factor receptor (EGFR) in various types of renal injury. Nephrology Dialysis Transplantation 9: 764–769. [PubMed] [Google Scholar]
- 43. Sis B, Sarioglu S, Celik A, et al. (2004) Epidermal growth factor receptor expression in human renal allograft biopsies: An immunohistochemical study. Transplant Immunology 13: 229–232. [DOI] [PubMed] [Google Scholar]
- 44. Takemura T, Murata Y, Hino S, et al. (1999) Heparin-binding EGF-like growth factor is expressed by mesangial cells and is involved in mesangial proliferation in glomerulonephritis. Journal of Pathology 189: 431–438. [DOI] [PubMed] [Google Scholar]
- 45. Chen J, Chen JK, Neilson EG, et al. (2006) Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. Journal of the American Society of Nephrology 17: 1615–1623. [DOI] [PubMed] [Google Scholar]
- 46. Scherer PE. (2006) Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 55: 1537–1545. [DOI] [PubMed] [Google Scholar]
- 47. Yamauchi T, Nio Y, Maki T, et al. (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature Medicine 13: 332. [DOI] [PubMed] [Google Scholar]
- 48. Ouedraogo R, Wu X, Xu SQ, et al. (2006) Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: Evidence for involvement of a cAMP signaling pathway. Diabetes 55: 1840–1846. [DOI] [PubMed] [Google Scholar]
- 49. Kadowaki T, Yamauchi T. (2005) Adiponectin and adiponectin receptors. Endocrine Reviews 26: 439–451. [DOI] [PubMed] [Google Scholar]
- 50. Sharma K. (2009) The link between obesity and albuminuria: Adiponectin and podocyte dysfunction. Kidney International 76: 145–148. [DOI] [PubMed] [Google Scholar]
- 51. Buechler C, Wanninger J, Neumeier M. (2010) Adiponectin receptor binding proteins–recent advances in elucidating adiponectin signaling pathways. FEBS Letters 584: 4280–4286. [DOI] [PubMed] [Google Scholar]
- 52. Tsioufis C, Dimitriadis K, Chatzis D, et al. (2005) Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. American Journal of Cardiology 96: 946–951. [DOI] [PubMed] [Google Scholar]
- 53. Yano Y, Hoshide S, Ishikawa J, et al. (2007) Differential impacts of adiponectin on low-grade albuminuria between obese and non obese persons without diabetes. Journal of Clinical Hypertension 9: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma K, Ramachandrarao S, Qiu G, et al. (2008) Adiponectin regulates albuminuria and podocyte function in mice. Journal of Clinical Investigation 118: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuan F, Li YN, Liu YH, et al. (2012) Adiponectin inhibits the generation of reactive oxygen species induced by glucose and promotes endothelial NO synthase formation in human mesangial cells. Molecular Medicine Reports 6: 449–453. [DOI] [PubMed] [Google Scholar]
- 56. Kacso IM, Bondor CI, Kacso G. (2012) Plasma adiponectin is related to the progression of kidney disease in type 2 diabetes patients. Scandinavian Journal of Clinical and Laboratory Investigation 72: 333–339. [DOI] [PubMed] [Google Scholar]
- 57. Rutkowski JM, Wang ZV, Park AS, et al. (2013) Adiponectin promotes functional recovery after podocyte ablation. Journal of the American Society of Nephrology 24: 268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Combs TP, Pajvani UB, Berg AH, et al. (2004) A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145: 367–383. [DOI] [PubMed] [Google Scholar]
- 59. Fang F, Liu GC, Kim C, et al. (2013) Adiponectin attenuates angiotensin II-induced oxidative stress in renal tubular cells through AMPK and cAMP-Epac signal transduction pathways. American Journal of Physiology. Renal Physiology 304: F1366–F1374. [DOI] [PubMed] [Google Scholar]
- 60. Onozato ML, Tojo A, Goto A, et al. (2002) Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEi and ARB. Kidney International 61: 186–194. [DOI] [PubMed] [Google Scholar]
- 61. Pedersen KO. (1944) Fetuin, a new globulin isolated from serum. Nature 154: 575. [Google Scholar]
- 62. Dziegielewska KM, Brown WM, Casey SJ, et al. (1990) The complete cDNA and amino acid sequence of bovine fetuin. Its homology with alpha 2HS glycoprotein and relation to other members of the cystatin superfamily. Journal of Biological Chemistry 265: 4354–4357. [PubMed] [Google Scholar]
- 63. Haglund AC, Ek B, Ek P. (2001) Phosphorylation of human plasma alpha2-Heremans-Schmid glycoprotein (human fetuin) in vivo. Biochemical Journal 357: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rauth G, Pöschke O, Fink E, et al. (1992) The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. European Journal of Biochemistry 204: 523–529. [DOI] [PubMed] [Google Scholar]
- 65. Srinivas PR, Wagner AS, Reddy LV, et al. (1993) Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Molecular Endocrinology 7: 1445– 1455. [DOI] [PubMed] [Google Scholar]
- 66. Rauth G, Pöschke O, Fink E, et al. (2006) Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochemical and Biophysical Research Community 350: 437–443. [DOI] [PubMed] [Google Scholar]
- 67. Mathews ST, Singh GP, Ranalletta M, et al. (2002) Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 51: 2450–2458. [DOI] [PubMed] [Google Scholar]
- 68. Axelsson J, Wang X, Ketteler M, et al. (2008) Is fetuin-A/alpha2-Heremans-Schmid glycoprotein associated with the metabolic syndrome in patients with chronic kidney disease? American Journal of Nephrology 28: 669–676. [DOI] [PubMed] [Google Scholar]
- 69. Hennige AM, Staiger H, Wicke C, et al. (2008) Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE 3: e1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen MP, Chung FM, Chang DM, et al. (2006) Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism 91: 295–299. [DOI] [PubMed] [Google Scholar]
- 71. Brentano F, Schorr O, Ospelt C, et al. (2007) Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis and Rheumatism 56: 2829–2839. [DOI] [PubMed] [Google Scholar]
- 72. Jia SH, Li Y, Parodo J, et al. (2004) Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis inexperimental inflammation and clinical sepsis. Journal of Clinical Investigation 113: 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moschen AR, Kaser A, Enrich B, et al. (2007) Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. Journal of Immunology 178: 1748–1758. [DOI] [PubMed] [Google Scholar]
- 74. Song HK, Lee MH, Kim BK, et al. (2007) Visfatin: A new player in mesangial cell physiology and diabetic nephropathy. American Journal of Physiology. Renal Physiology 295: 1485–1494. [DOI] [PubMed] [Google Scholar]
- 75. Fukuhara A, Matsuda M, Nishizawa M, et al. (2005) Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 307: 426–430. [DOI] [PubMed] [Google Scholar]