Abstract
The goals of this study were to determine if the biomarkers of head injury, NSE and S100B, increased in serum following an American football game. Serum creatine kinase (CK) and cortisol levels were also measured to determine muscle damage and stress caused by the football game. NSE, S100B, CK, and cortisol were measured in the serum of 17 football players before and after a collegiate junior varsity football game. No head injuries were reported by the players, athletic training staff, or coaches yet both NSE (Pre-game: 7.0 μg•L–1 ± 2.2 versus Post-game: 13.1 μg•L–1 ± 7.0, P <0.001) and S100B (Pre-game: 0.013 μg•L–1 ± 0.012 versus Post-game: 0.069 μg•L–1 ± 0.036, P <0.001) increased significantly. Neither CK (Pre-game: 90.5 U•L–1 ± 41.9 versus Post-game: 120.2 U•L–1 ± 62.7, P = 0.116) nor cortisol (Pre-game: 369.2 nmoles•L-1 ± 159.8 versus Post-game: 353.0 nmoles•L–1 ± 170.5, P = 0.349) increased significantly following the football game. There was little correlation found between S100B and body mass (R2 = 0.029) or CK (R2 = 0.352) levels. Although serum NSE and S100B increase as a result of playing in an American football game, the values are similar to or lower than levels found following competition in other contact and non-contact sports. Furthermore, the lack of correlation between S100B and body mass or CK indicates that S100B increases independent of body mass or muscle injury.
Keywords: cortisol, creatine kinase, neuronal specific enolase, protein S100B, subconcussion
Introduction
Concussion injury is defined as a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces,1 and is the most common head injury experienced by American football players.2 However, head impacts commonly occur during American football games in which visible signs or symptoms of neurological dysfunction may not develop,3–6 despite those impacts having the potential for neurological injury.7,8 Studies have shown that even a minor head injury with no concussive symptoms (subconcussion) can lead to abnormal results in neuropsychological tests, abnormal functional magnetic resonance imaging (fMRI), and chronic traumatic encephalopathy (CTE).7–11
This raises the question of whether or not ordinary American football play, without incidence of concussion, may cause immediate brain injury. Therefore, the purpose of the current study was to determine if serum concentrations of biochemical markers of brain injury are elevated following an American football game in athletes who sustained subconcussive head impacts not significant enough to cause clinical symptoms of brain injury. Numerous biomarkers of head injury have been discovered and their efficacy of detecting and predicting severity of traumatic brain injury have been tested in the clinical setting.12–14 However, few of these discovered biomarkers have been analyzed in their ability to detect sports-related head injury.15 Since S100B and NSE have been monitored quite frequently and appear to correlate well with number of head impacts, they were chosen as the biomarkers for this study.16–19 Creatine kinase (CK) and cortisol serum levels were also measured to estimate muscle damage and stress, respectively. It was hypothesized that NSE, S100B, and CK would increase significantly as a result of the football game, while cortisol levels would not increase significantly.
Materials and methods
Participants
Seventeen (four offensive linemen, three defensive linemen, three running backs, three linebackers, two quarterbacks, one defensive back, and one punter) male Division III collegiate American football players who were members of the junior varsity team participated in this study. Players had a mean age of 19.5 years (SD = 0.94), height of 1.83 m (SD = 0.07), and lean mass of 70.6 kg (SD = 10.0). Informed written consent was obtained from all participants and the University of Wisconsin-Platteville Institutional Review Board for the protection of human participants (Platteville, WI, USA) approved the study (IRB Protocol #2015-16-15).
Study design
Prior to the first and only junior varsity football game of the season (8 weeks following the start of the varsity season), anthropometric data were collected from all participants. Body weight and height were measured using a physician scale and height rod (DETECTO, Webb City, MO, USA), while dual-energy X-ray absorptiometry (DXA) whole-body scans were performed on all participants using a Hologic Horizon Wi bone densitometer (Hologic, Inc., Marlborough, MA, USA) to obtain lean mass measures.
Two days prior and approximately 1 h following the junior varsity football game, venous blood was collected from all participants. Pre-game blood was drawn and each participant acted as his own control. Blood was drawn from a prominent vein in the antecubital space using a 21-gauge butterfly needle with a 7-inch luer lock extension (Becton-Dickinson, Franklin Lakes, NJ, USA) connected to a vacutainer adapter (Becton-Dickinson, Franklin Lakes, NJ, USA). The blood was collected into a 4 mL serum separation vacutainer tube (Becton-Dickinson, Franklin Lakes, NJ, USA).
Biochemical analysis
Following blood collection all samples were allowed to incubate at room temperature for 1 h. Immediately after the 1 h incubation period, each sample was centrifuged at 3500 RPM for 10 min at 4°C using a Thermo IEC Centra CL3 centrifuge (Thermo Fisher Scientific, Inc., Walthum, MA, USA). Serum was then removed from each sample and placed in serum transfer tubes for subsequent analysis of NSE, S100B, and CK. Samples were then stored at 5°C for cortisol analysis, which occurred within 72 h of initial storage.
Neuronal specific enolase
NSE was measured using a commercially available enzyme immunometric assay (EIA) kit (CanAg NSE EIA 420-85, Fujirebio Diagnostics, Inc., Göteborg, Sweden). This is a solid phase, non-competitive immune assay utilizing two monoclonal antibodies (MAb) derived from mice. The monoclonal antibodies are specific for the γ-subunit of NSE and therefore detect both the γγ and αγ isoenzymes of NSE. The serum samples and calibrators were first added to streptavidin-coated microstrips and then were incubated with both a biotinylated anti-NSE MAb and an anti-NSE MAb labeled with horseradish peroxidase (HRP). Following this incubation period, a hydrogen peroxide (H2O2) and 3, 3’, 5, 5’ tetramethyl-benzidine (TMB) substrate solution was added. The HRP–H2O2 oxidizes the TMB forming a blue color in which the absorbance of the color at 620 nm is directly proportional to the concentration of NSE. All absorbance measurements were made using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
The known standards were then used to develop a calibration curve which was used to determine the NSE concentration in each serum sample. The sensitivity of the NSE assay was in the range of 1–150 μg·L−1. Analysis of standards and samples were all performed in duplicate. The NSE assay performed before the football game gave a calibration curve with an R2 = 0.9977 and the intra-assay coefficient of variation (CV) was 4.3%. After the football game, the NSE assay calibration curve was generated with an R2 = 0.9996 and the intra-assay CV was 9.4%. Inter-assay CV between the pre-game and post-game NSE assays was 6.3%.
Protein S100B
Protein S100B was measured using a commercially available EIA kit (CanAg S100 EIA 708-85, Fujirebio Diagnostics, Inc., Göteborg, Sweden). This EIA is a solid-phase, two-step, non-competitive immunoassay utilizing two MAb from mice that are specific to the B monomer of protein S100 such that the assay identifies both protein S100BB and protein S100A1B. The assay requires that the serum samples and calibrators are incubated in streptavidin-coated strips with a biotinylated Anti-S100B MAb. Following this incubation period, an Anti-S100B MAb conjugated with HRP is added to the strips and allowed to incubate before adding an H2O2 buffered TMB substrate. HRP–H2O2 then metabolizes the TMB forming a blue color at which the intensity is directly proportional to serum S100B concentration. The absorbance of this blue color was measured at 620 nm using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). The known standards were then used to develop a calibration curve which was used to determine the S100B concentration in each serum sample. The sensitivity of the S100B assay was in the range of 10–3500 ng·L−1. Analysis of standards and samples were all performed in duplicate. The S100B assay performed before the football game gave a calibration curve with an R2 = 0.9942 and the intra-assay CV was 3.6%. After the football game, the S100B assay calibration curve was generated with an R2 = 0.9936 and the intra-assay CV was 4.9 %. Inter-assay CV between the pre-game and post-game S100B assays was 5.2%.
Creatine kinase
CK was measured using a commercially available CK activity assay kit (Catalog No. MAK116; Sigma-Aldrich, St. Louis, MO, USA). CK is a dimeric protein made up of both muscle (M) and brain (B) subunits; however, this assay does not distinguish between types of CK. All serum samples and a calibrator were incubated with adenosine diphosphate (ADP), phosphocreatine (PC), hexokinase, glucose, glucose-6-phosphate dehydrogenase, and nicotinamide adenine dinucleotide phosphate (NADP). The CK present in each sample converts ADP and PC to ATP and creatine. The ATP is then used by hexokinase to phosphorylate glucose to glucose-6-phosphate. Glucose-6-phosphate is then oxidized by glucose-6-phosphate dehydrogenase forming NADPH and 6-phospho-D-gluconate. The production of NADPH was measured at 340 nm using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). The increase of absorbance at 340 nm is directly proportional to the activity of CK. One unit of CK is the amount of enzyme that will transfer 1 μmole of phosphate from PC to ADP per minute at a pH of 6.0. This assay was carried out over a period of 20 min where the absorbance was read immediately after plate preparation (initial) and then after a 20 min incubation period (final). The following formula was used to calculate CK activity in Units·L−1.
Where (A340)final = the final absorbance of NADPH at 340 nm, (A340)initial = the initial absorbance of NADPH at 340 nm, (A340)calibrator = the absorbance of the calibrator at 340 nm, (A340)blank = the absorbance of the blank (deionized water) at 340 nm, and 150 = the equivalent activity (Units·L−1) of the calibrator when the assay is read at 20 min and 40 min. The sensitivity of the CK assay was in the range of 30–1800 U·L−1. The intra-assay CV was 3.8% before the football game. After the football game the intra-assay CV was 1.6%. Inter-assay CV between the pre-game and post-game S100B assays was 3.6%.
Cortisol
Cortisol was measured using a commercially available enzyme-linked immunsorbent (ELISA) assay kit (Catalog No. SE120037; Sigma-Aldrich, St. Louis, MO, USA). This ELISA is a solid-phase, competitive immunoassay utilizing two anti-cortisol MAb. Serum samples and standards were incubated in anti-cortisol MAb coated wells with anti-cortisol MAb enzyme conjugate. TMB substrate was then added to the wells and the intensity of color is inversely proportional to cortisol concentration. The intensity of color was measured at an absorbance of 450 nm using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). Analysis of standards and samples were all performed in duplicate. The sensitivity of the cortisol assay was in the range of 0–800 ng·mL−1. The intra-assay CV was 4.7%. Since the cortisol assay for both pre- and post-game samples were performed on the same plate at the same time, there was no inter-assay CV. The cortisol standard curve is not linear; therefore, neither an R2 value nor a predictive equation could be generated. Because of this, the cortisol concentrations were estimated visually based off the standard curve.
Statistical analysis
All statistical analyses were generated using SPSS version 22 (SPSS Inc., Chicago, IL USA). Means of NSE, S100B, CK, and cortisol serum concentration pre game were compared to post game using two-tailed paired-samples t-tests for parametric data. Normality was analyzed using a Shapiro–Wilk test with an alpha level of 0.05, while homogeneity of variance was analyzed using Levene’s test with an alpha level of 0.05. A Wilcoxon rank-sum test was used for data not meeting the assumption for normality and a Welch t-test was used for data not meeting the assumption for homogeneity of variance. A 95% confidence interval (CI) was used to assign bounds of expected discrepancy between the sample mean and the population mean. A Cohen’s d (d) test using the original standard deviations (as opposed to the pooled standard deviations)20 was performed to determine the effect size that the football game had on each variable. A two-tailed Pearson Correlation Coefficient was used to measure the relationship between S100B and body mass, along with S100B and CK. Data are reported as mean (SD) and alpha was set at 0.05 to determine statistical differences between means.
Results
Mean serum NSE concentration was significantly different between pre game and post game (P <0.001; d = 1.2; observed power = 0.915). Pre-game serum NSE concentration was an average of 7.0 μg·L−1 (SD = 2.2; 95% CI, 5.8–8.1 μg·L−1) while mean serum NSE post game was 13.1 μg·L−1 (SD = 7.0; 95% CI, 9.5–16.7 μg·L−1). Figure 1 shows mean ± SD NSE pre-game and post-game data. The average serum concentration of S100B was also significantly greater post game as compared to pre game (P <0.001; d = 2.1; observed power = 1.000). Pre-game serum S100B concentration was an average of 0.013 μg·L−1 (SD = 0.012; 95% CI, 0.007–0.019 μg·L−1) while mean serum S100B post-game concentration was 0.069 μg·L−1 (SD = 0.036; 95% CI, 0.050–0.087 μg·L−1). Figure 2 shows mean ± SD S100B pre-game and post-game data.
Figure 1.
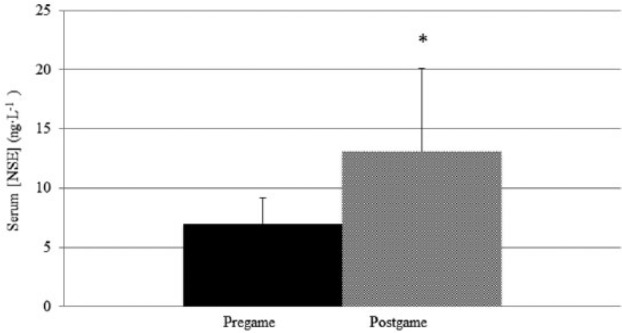
Mean ± SD serum neuronal specific enolase concentration pre game compared to post game. *Significant difference compared to pre game, P <0.05.
Figure 2.
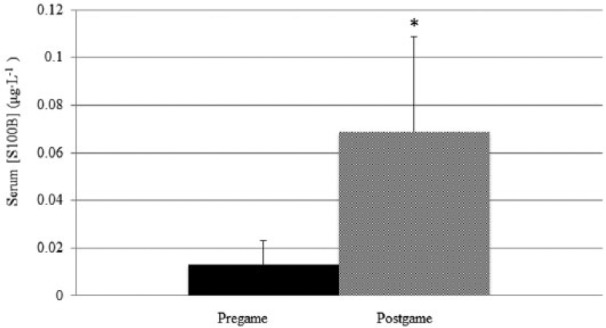
Mean ± SD serum protein S100B concentration pre game compared to post game. *Significant difference compared to pre game, P <0.05.
Neither serum CK (P = 0.116; d = 0.56; observed power = 0.349) nor serum cortisol concentration (P = 0.876; d = 0.12; observed power = 0.059) changed significantly from pre game to post game. Pre-game serum CK concentration was an average of 90.5 U·mL−1 (SD = 41.9; 95% C, 70.0–112.1 U·mL−1) while mean serum CK post-game concentration was 120.2 U·mL−1 (SD = 62.7; 95% CI, 87.9–152.4 U·mL−1). Figure 3 shows mean ± SD CK pre game and post game. Pre-game serum cortisol concentration was an average of 369.2 nmoles·L−1 (nM) (SD = 159.8; 95% CI, 287.0–451.4 nM) while mean serum cortisol post-game concentration was 353.0 nM (SD = 170.5; 95% CI, 265.3–440.6 nM). Figure 4 shows mean ± SD cortisol pre-game and post-game data. Figure 5 shows that little correlation was found between post-game S100B serum concentration and body mass (R2 = 0.029, P = 0.911) and low correlation was found between post-game S100B serum concentration and post-game CK serum concentration (R2 = 0.353, P = 0.165).
Figure 3.
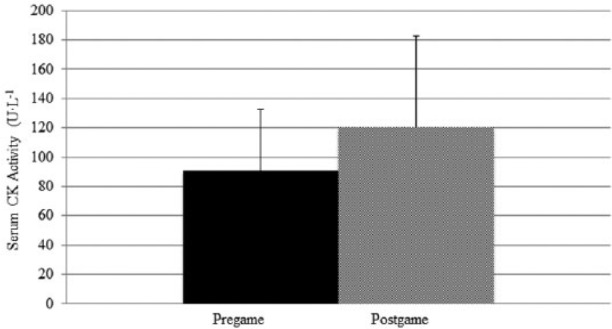
Mean ± SD serum CK concentration pre game compared to post game.
Figure 4.
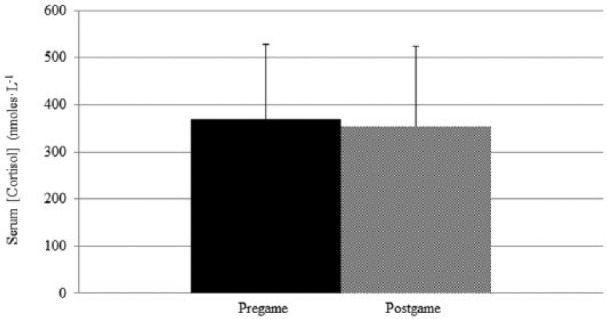
Mean ± SD serum cortisol concentration pre game compared to post game.
Figure 5.
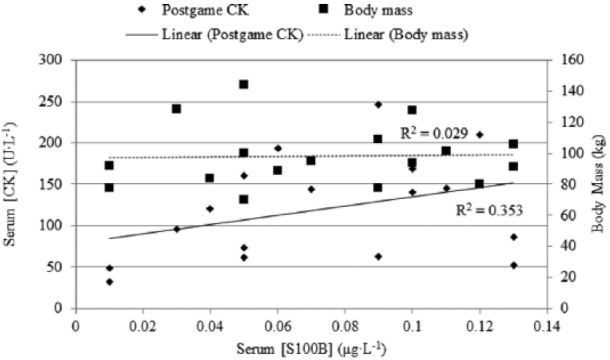
Correlation between post-game serum protein S-100B concentration and body mass, as well as correlation between post-game serum protein S-100B and post-game serum CK concentration.
Discussion
This research was conducted as a pilot study to determine if head injury occurs during one American football game due to subconcussive impacts without the occurrence of concussion or mild traumatic brain injury symptoms. During the football game, no concussions or traumatic head injuries were reported by the players or observed by the athletic training staff. Despite no clinical evidence of brain injury, both serum NSE and S100B increased significantly following the football game. The increase in serum levels of NSE and S100B following the football game coincide with previous studies analyzing these two biomarkers before and after athletic competition.16,17,21–26 Surprisingly, CK levels did not rise significantly following the football game as has been reported in past studies following athletic competition.16,17,26,27 Cortisol has been shown to increase following head injury,28 including boxing bouts with punches to the head,16 but has not been reported to increase significantly following an American football game27 when head injury is not present. The findings of cortisol not increasing following a sporting event in which no head injuries have occurred agrees with the results of our study.
The post-game serum concentration of 13.1 μg·L−1 for NSE is around the normal serum range of 12.5 μg·L−1 for healthy humans14 and similar to those levels found following a game or match in other contact sports.23–25 As expected, the concentration of 13.1 μg·L−1 following the football game was lower than that found in individuals following a boxing match (31.1 μg·L−1)16 or karate event (20.2 μg·L−1)17 in which punches and kicks to the head were prevalent. Interestingly, the median NSE concentration of 11.2 μg·L−1 found after the football game in our study was greater than the median concentration of 6.5 μg·L−1 found after concussion injury in ice hockey players.22 This low level of NSE concentration in the serum of concussed ice hockey players has brought the usefulness of NSE as a marker of sports-related head injury into question.22
The presence of NSE in thrombocytes and erythrocytes14 also brings the usefulness of measuring NSE to assess head injury into question. There is concern of erythrocyte lysis during competition29 and analysis30 which may cause a false interpretation of elevated NSE. Indeed, a limitation to our study is that we did not measure hemolysis of blood samples. However, due to immediate processing and analyzing of the serum samples, and the normal serum concentrations of NSE observed in the pre-game samples, it is unlikely that significant hemolysis due to our handling of the blood specimens occurred.30 In addition, Skogseid et al. have reported that serum NSE levels in patients with multiple extracerebral injury have still been shown to correlate only with brain injury,31 implying that the brain is the main source of NSE. Further research into the usefulness of NSE as a biomarker of head injury caused by sports is warranted.
Post game, the mean S100B serum concentration of 0.069 μg·L−1 was within the normal serum concentration of 0.2 μg·L−1 found in healthy humans.14 This level was also lower than the values found post competition in swimmers,32 runners,18,33 soccer players,24,25 ice hockey players,23 basketball players,23 boxers,16,18 karate practitioners,17 and concussed ice hockey players.22 However, the post-game serum concentration was greater than found in participants following cycling exercise.18 The change of serum S100B levels from pre to post game (0.056 μg·L−1) was similar to that found in other non-concussed football players.21,34 As with NSE, the usefulness of S100B as a biomarker for head injury in sports has been called into question22,32,33 due to extra cranial sources of S100B in adipocytes, melanocytes, chondrocytes, and skeletal muscle tissue.35
Support for the idea that S100B is a marker of muscle damage and not glial damage comes from studies that have found similar increases in both CK and S100B following running exercise26,33 along with increases in S100B after swimming.32 There is also evidence that participants with greater body mass have greater levels of serum S100B.36 However, other studies have reported no increases in serum S100B in the absence of impacts to the brain,18,26,37,38 and Pham et al. have reported that extracranial sources of S100B do not affect serum levels.39 Our results support the idea of serum S100B not being affected by extracranial sources as S100B was not correlated with CK or body mass.
In conclusion, the effect of the football game on levels of NSE (d = 1.2) and S100B (d = 2.1) were large. However, the low NSE and S100B levels in the serum of participants following this football game likely indicate two different possibilities: (1) that American football may be no more dangerous than other sports that cause axial vibrations or direct impacts to the head; or (2) NSE and S100B may not be the ideal biomarkers for assessing head injury.
Several limitations must be taken into consideration with this study. First of all, since this was a pilot study, there was a small number of participants and lack of a separate control group doing no exercise, a group performing non-contact exercise, and a group that did not receive any playing time during the game. Furthermore, these data were collected from one game within a cohort of athletes who have not played any other games during the season. This was a junior varsity game in which many substitutions occurred to give the majority of players a chance to compete and prove their talent to the coach. In contrast, starting offensive and defensive varsity players participate in approximately 13 games per year and typically remain in the game for the full duration of offensive or defensive play, which may result in greater NSE, S100B, and CK serum concentrations in participants following varsity games. Additional research must also be conducted in varsity athletes and National Football League (NFL) players to determine if the findings of this study are similar when elite athletes with a greater body mass play the game of American football for a longer time period.
There may also be biomarker differences by position which can be alluded to in our results but must be studied with a greater number of participants per position for statistical inference. The use of equipment to gain biomechanical data such as: number of head and body impacts, magnitude of impacts, and location of impacts would also be of valuable assessment in conjunction with biomarker data. Lastly, although the results of this study indicate that American football does not inherently cause immediate brain injury, there is likely occurrence of brain injury with reported concussions, which may be detrimental to participation. Although the data presented here are interesting and unique, future studies should consider the use of larger participant numbers and a control group.
Acknowledgments
We thank head football coach Michael Emendorfer and the football players who participated in this study for being open to the advancement of research in this area. We offer a special thanks to lab technician Andres Young for assisting in data collection. We also express gratitude to the undergraduate researchers: Abigail Fischer, Srimant Vaddadi, Sydney Keuler, Mackenzie Novak, Mariah Henderson, and Rachel Bruesewitz for all their help with data collection.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. McCrory P, Meeuwisse HM, Aubry M, et al. (2013) Consensus statement on concussion in sport: the 4th international conference on concussion in sport held in Zurich, November 2012. British Journal of Sports Medicine 47: 250–258. [DOI] [PubMed] [Google Scholar]
- 2. Boden BP, Tacchetti RL, Cantu RC, et al. (2007) Catastrophic head injuries in high school and college football players. The American Journal of Sports Medicine 35: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 3. Duma SM, Manoogian SJ, Bussone WR, et al. (2005) Analysis of real-time head accelerations in collegiate football players. Clinical Journal of Sports Medicine 15: 3–8. [DOI] [PubMed] [Google Scholar]
- 4. Guskiewicz KM, Mihalik JP, Shankar V, et al. (2007) Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery 61: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 5. Mihalik JP, Bell DR, Marshall SW, et al. (2007) Measurement of head impacts in collegiate football players: an investigation of positional and event-type differences. Neurosurgery 61: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 6. Pellman EJ, Viano DC, Tucker AM, et al. (2003) Concussion in professional football: Reconstruction of game impacts and injuries. Neurosurgery 53: 799–814. [DOI] [PubMed] [Google Scholar]
- 7. Bailes JE, Petraglia AL, Omalu BI, et al. (2013) Role of subconcussion in repetitive mild traumatic brain injury. Journal of Neurosurgery 119: 1235–1245. [DOI] [PubMed] [Google Scholar]
- 8. Omalu BI, DeKosky ST, Hamilton RL, et al. (2006) Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery 59: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 9. Dashnaw ML, Petraglia AL, Bailes JE. (2012) An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurgical Focus 33: 1–9. [DOI] [PubMed] [Google Scholar]
- 10. Jordan BD. (2013) The clinical spectrum of sport-related traumatic brain injury. Nature Reviews. Neurology 9: 222–230. [DOI] [PubMed] [Google Scholar]
- 11. Nagahiro S, Yoshifumi M. (2014) Current topics in sports-related head injuries: a review. Neurologia Medico-Chirgica 54: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cairelli MJ, Fiszman M, Zhang H, et al. (2015) Networks of neuroinjury semantic predications to identify biomarkers for mild traumatic brain injury. Journal of Biomedical Semantics 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carpenter KLH, Czosnyka M, Jalloh I, et al. (2015) Systemic, local, and imaging biomarkers of brain injury: more needed, and better use of those already established? Frontiers in Neurology 6: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hergenroeder GW, Redell JB, Moore AN, et al. (2008) Biomarkers in the clinical diagnosis and management of traumatic brain injury. Molecular Diagnosis and Therapy 12: 345–358. [DOI] [PubMed] [Google Scholar]
- 15. Papa L, Ramia MM, Edward D, et al. (2015) Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. Journal of Neurotrauma 32: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham MR, Myers T, Evans P, et al. (2011) Direct hits to the head during amateur boxing is associated with a rise in serum biomarkers for brain injury. International Journal of Immunopathology and Pharmacology 24: 119–125. [DOI] [PubMed] [Google Scholar]
- 17. Graham MR, Pates J, Davies B, et al. (2015) Should an increase in cerebral neurochemicals following head kicks in full contact karate influence return to play? International Journal of Immunopathology and Pharmacology 28: 539–546. [DOI] [PubMed] [Google Scholar]
- 18. Otto M, Holthusen S, Bahn E, et al. (2000) Boxing and running lead to a rise in serum levels of S-100B protein. International Journal of Sports Medicine 21: 551–555. [DOI] [PubMed] [Google Scholar]
- 19. Zetterberg H, Tanriverdi F, Unluhizarci K, et al. (2009) Sustained release of neuron-specific enolase to serum in amateur boxers. Brain Injury 23: 723–726. [DOI] [PubMed] [Google Scholar]
- 20. Dunlap WP, Cortina JM, Vaslow JB, et al. (1996) Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods 1: 170–177. [Google Scholar]
- 21. Marchi N, Bazarian JJ, Puvenna V, et al. (2013) Consequences of repeated blood-brain barrier disruption in football players. PLoS One 8: e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahim P, Tegner Y, Wilson DH, et al. (2014) Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurology 71: 684–692. [DOI] [PubMed] [Google Scholar]
- 23. Stålnacke BM, Tegner Y, Sojka P. (2003) Playing ice hockey and basketball increases serum levels of S-100B in elite players: a pilot study. Clinical Journal of Sports Medicine 13: 292–302. [DOI] [PubMed] [Google Scholar]
- 24. Stålnacke BM, Tegner Y, Sojka P. (2004) Playing soccer increases serum concentrations of the biochemical markers of brain damage S-100B and neuron-specific enolase in elite players: a pilot study. Brain Injury 18: 899–909. [DOI] [PubMed] [Google Scholar]
- 25. Stålnacke BM, Ohlsson A, Tegner Y, et al. (2006) Serum concentrations of two biochemical markers of brain tissue damage S-100B and neurone specific enolase are increased in elite female soccer players after a competitive game. British Journal of Sports Medicine 40: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stocchero CM, Oses JP, Cunha GS, et al. (2014) Serum S100B level increases after running but not cycling exercise. Applied Physiology, Nutrition, and Metabolism 39: 340–344. [DOI] [PubMed] [Google Scholar]
- 27. Sterczala AJ, Flanagan SD, Looney DP, et al. (2014) Similar hormonal stress and tissue damage in response to National Collegiate Athletic Association Division I football games played in two consecutive seasons. Journal of Strength and Conditioning Research 28: 3234–3238. [DOI] [PubMed] [Google Scholar]
- 28. King LR, McLaurin RL, Lewis HP, et al. (1970) Plasma cortisol levels after head injury. Annals of Surgery 172: 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sikora J, Orlov SN, Furuya K, et al. (2014) Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 124: 2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramont L, Thoannes H, Volondat A, et al. (2005) Effects of hemolysis and storage condition on neuronspecific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clinical Chemistry and Laboratory Medicine 43: 1215–1217. [DOI] [PubMed] [Google Scholar]
- 31. Skogseid IM, Nordby HK, Urdal P, et al. (1992) Increased serum creatine kinase BB and neuron specific enolase following head injury indicates brain damage. Acta Neurochirurgica 115: 106–111. [DOI] [PubMed] [Google Scholar]
- 32. Dietrich MO, Tort AB, Schaf DV, et al. (2003) Increase in serum S100B protein level after a swimming race. Canadian Journal of Applied Physiology 28: 710–719. [DOI] [PubMed] [Google Scholar]
- 33. Hasselblatt M, Mooren FC, von Ahsen N, et al. (2004) Serum S100beta increases in marathon runner reflect extracranial release rather than glial damage. Neurology 62: 1634–1636. [DOI] [PubMed] [Google Scholar]
- 34. Puvenna V, Brennan C, Shaw G, et al. (2014) Significance of ubiquitin carboxy-terminal hydrolase l1 elevations in athletes after sub-concussive head hits. PLoS One 9: e96296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raabe A, Kopetsch O, Woszczyk A, et al. (2003) Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restorative Neurology and Neuroscience 21: 159–169. [PubMed] [Google Scholar]
- 36. Steiner J, Schiltz K, Walter M, et al. (2010) S100B serum levels are closely correlated with body mass index: an important caveat in neuopsychiatric research. Psychoneuroendocrinology 35: 321–324. [DOI] [PubMed] [Google Scholar]
- 37. Kiechle K, Bazarian JJ, Merchant-Borna K, et al. (2014) Subject-specific increases in serum s-100b distinguish sports-related concussion from sports-related exertion. PLoS One 9: e84977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulte S, Schiffer T, Sperlich B, et al. (2011) Serum concentrations of S100B are not affected by cycling to exhaustion with or without vibration. Journal of Human Kinetics 30: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pham N, Fazio V, Cucullo L, et al. (2010) Extracranial sources of S100B do not affect serum levels. PLoS One 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


