Abstract
Xylaria nigripes (XN) is a medicinal fungus that was used traditionally as a diuretic, nerve tonic, and for treating insomnia and trauma. In this study, we elucidated possible mechanisms of neuroprotective effects of XN mycelia extracts. XN mycelia were produced by fermentation. Hot water extract and 70% ethanol extract of XN mycelia were evaluated on hydrogen peroxide (H2O2)-induced apoptosis in PC12, a rat pheochromocytoma cell line. Both XN extracts effectively protected PC12 cells against H2O2-induced cell damage by inhibiting release of lactate dehydrogenase, decreasing DNA damage, restoring mitochondrial membrane potential, and arresting abnormal apoptosis through upregulation of Bcl-2 and downregulation of Bax and caspase 3. Compared to water extract, ethanol extract showed not only greater neuroprotective effects but also a higher antioxidant activity by scavenging DPPH radicals, inhibiting lipid peroxidation, and reducing power. High phenolic content and antioxidant activity may provide the neuroprotective properties of XN ethanol extract.
Keywords: antioxidant, apoptosis, mitochondrial membrane potential, neuroprotection, PC12 cells, Xylaria nigripes
Introduction
Neurons in the central nervous system are the most fragile cells and are easily damaged.1 Oxidative stress may damage cells, induce untimely apoptosis of neurons, and eventually cause an onset of some neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases.2 In recent years, many researchers have demonstrated neuroprotective effects for different types of antioxidants.2
Xylaria nigripes (XN) is a traditional medicinal fungus belonging to the family Xylariaceae. Traditionally, XN is used to treat insomnia and trauma, and also as a nerve tonic.3 In vivo and in vitro studies have demonstrated the bioactivities of XN, including the following activities: antioxidant,4,5 immunomodulatory, anti-inflammatory,6 hepatoprotective,7 anti-tumor5 prevention of spatial memory impairment,8 anti-depressant activity in epileptic patients,9 enhancing insulin sensitivity,10 and neuroprotective activities.11 These bioactivities of XN are attributed to different chemical compounds reported in XN such as intracellular and extracellular polysaccharides, adenosine, total polyphenols, and triterpenoids in mycelia.4–6,12
Hydrogen peroxide (H2O2) can increase oxidative stress and induce apoptosis by initiating mitochondrial dysfunctions in PC12 cells.2 In the present study, PC12 cells treated with H2O2 were used as the cell model and XN mycelia extracts produced from mycelia by submerged fermentation were evaluated for their protective effects on neural cells affected by oxidative damage.
Materials and methods
Chemicals and reagents
All chemicals and solvents used were of analytical grade. Dulbecco’s Modified Eagle Medium (DMEM) was purchased from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Biological industries (Beit-Haemek, Israel). Soy lecithin was obtained from Wako Pure Chemical Industries (Osaka, Japan). Malt extract, Gallic acid, rutin hydrate, α-Diphenyl-β-picrylhydrazyl (DPPH), thiazolyl blue tetrazolium bromide (MTT), trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid), and low melting agarose were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). Lactate dehydrogenase (LDH) was from TaKaRa Bio Laboratories (Tokyo, Japan). Xylaria nigripes (Klotzsch) M.C. Cooke (BCRC No. 34219), and PC12 cell (rat adrenal pheochromocytoma cells) were purchased from the Bioresource Collection and Research Centre (BCRC; FIRDI, Hsinchu, Taiwan). FITC-Annexin V/propidium iodide (PI) apoptosis detection assay kit, primary antibodies including Bax, Bcl-2, caspase 3, and β-actin, HRP Goat Anti-Mouse Ig antibody and BD™ MitoScreen Flow Cytometry mitochondrial membrane potential detection kit were purchased from BD Bioscience (San Jose, CA, USA). RNase A was from Biokit (Miaoli, Taiwan). Immobilon™ western HRP substrate kit was purchased from Millipore (Billerica, MA, USA). Brown rice (Golden Rice Castle, Taitung, Taiwan) was obtained from a local shop in Taichung, Taiwan and ground into fine flour.
XN growth conditions, fermentation, and extraction
The XN culture provided by BCRC on malt extract agar (MEA, 20 g/L malt extract, 15 g/L Agar) plates was subcultured by cutting out 5 × 5 mm of the agar plate culture with a sterilized cutter and transferring the cut section to a fresh MEA plate. The plates were incubated at 25°C for 12–14 days. Stock cultures were prepared by submerging the subcultured XN (in an agar cube of 5 × 5 mm) in a tight-capped glass tube filled with sterilized Milli-Q water and stored at room temperature. The viability of the stock cultures was evaluated every month by culturing in a MEA plates.
The stock culture was activated twice in an MEA plate at 25°C for 12–14 days. To grow XN, the plate of MEA agar was cut into 5 × 5 mm and then inserted into a 250 mL flask containing 50 mL MEDB medium (2% malt extract, 1% glucose) and maintained at 25°C on a rotary shaker incubator (60 rpm) for seven days. For inoculum preparation, each 50 mL activated XN in MEDB was transferred into a 450 mL of seed medium (2% malt extract, 2% glucose) in a 1 L flask and incubated at 25°C with constant stirring 100 rpm for seven days. A total of 2 L inoculum was obtained by pooling all four flasks of seed cultures.
Fermentation was performed in an airlift fermenter (200 cm × –26.6 cm) with internal draft tube (120 cm × –17.8 cm) (Biotop Process and Equipment Inc., Taichung, Taiwan) containing 18 L of medium (2% brown rice flour, 0.5% malt extract in distilled water) with 2 L of inoculum, and the fermentation conditions were maintained under daylight under 25°C and 1 vvm airflow for ten days.
After fermentation, XN mycelia was harvested, washed several times with water to remove excess brown rice powder, and then dried using a vacuum freeze-dryer (Model FDU540, Eyela Co., Japan). The yield of dried XN mycelia was in the range of 4.25 ± 0.3 g/L. Dried XN mycelia was ground into fine powder and stored in airtight container until used for extraction.
XN mycelia powder was extracted with 90°C hot water or 70% ethanol under constant stirring for 1 h according to previous methods.4 After extraction, the samples were filtered through muslin cloth and centrifuged at 10,000 × g for 10 min. The supernatant was dried using a vacuum freeze-dryer, and the lyophilized samples stored at −20°C. For determination of bioactivities, XN extracts were dissolved in Milli-Q water/PBS and sonicated in an ultrasonic bath (100 W, 42 kHz, 3510R-MT, Branson Ultrasonic Corporation, CT, USA) for 10 min.
Determination of total polysaccharide, phenolics, and flavonoids
The content of total polysaccharide was determined spectrophotometrically by the phenol–sulfuric acid method. The total phenol content was estimated by the Folin–Ciocalteu reagent method and was expressed as mg gallic acid equivalents per gram of tested sample. The total flavonoid content was determined according to the method described by Stankovi13 and the flavonoid content was expressed as mg rutin per gram of tested sample.
DPPH scavenging activity
The free radical scavenging activity of XN mycelia extract on DPPH radicals was determined following the method. Briefly, an aliquot of the sample (0.1 mL, 0.39–12.5 mg/mL), 0.4 mL of 100 mM Tris-HCl buffer (pH 7.4), and 0.5 mL of DPPH solution (500 µM in ethanol) was mixed by shaking vigorously for 20 s. The mixture was kept in the dark at room temperature for 20 min and the absorbance determined at 517 nm using a spectrometer. Trolox (0.1 mL, 50–500 µM) was used as a positive control. The ability to scavenge DPPH radicals was calculated as follows: DPPH scavenging activity (%) = 1 – (absorbance of sample at 517 nm / absorbance of control at 517 nm) × 100.
Inhibition of lipid peroxidation
The inhibitory effect of XN mycelia extracts on lipid peroxidation was measured using a liposome model. Briefly, an aliquot of the XN mycelia extract (15 µL, 0.15–12.5 mg/mL) and 20 mM sodium phosphate buffer (165 µL, pH 7.2) were mixed with 300 µL of liposome prepared from soybean lecithin. After induction with FeCl3-ascorbate, liposome peroxidation was determined by measuring the formation of malondialdehyde-thiobarbituric acid (MDA-TBA) at 535 nm using a spectrometer. The inhibitory activity against liposome peroxidation was calculated as follows: Inhibition effect (%) = 1 – (absorbance of sample at 535 nm / absorbance of control at 535 nm) × 100.
Reducing power
Equal volumes (0.25 mL) of the XN mycelia extract (0.39–12.5 mg/mL), 0.2 M phosphate buffer (pH 6.6), and 1% potassium ferricyanide were mixed and incubated at 50°C for 20 min. After cooling, the mixture was added to 0.25 mL of 10% trichloroacetic acid and centrifuged at 4500 ×g for 10 min. The supernatant (0.5 mL) was mixed with 0.5 mL of distilled water and 0.1 mL of 0.1% ferric chloride solution. The reducing power of the tested samples was determined by measuring the absorbance at 700 nm. Ascorbic acid (0.005–0.100 mg/mL) was used a positive control.
Protection of PC12 cells against H2O2
PC12 cells were maintained in DMEM medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum, 100 units/mL of penicillin, and 100 µg/mL of streptomycin. Cell lines were incubated at 37°C in a humidified atmosphere with 5% CO2 and the medium was changed every other day. The PC12 cells were plated and grown for 24 h in cultured medium. Cells were pre-treated with XN mycelia extracts (10, 50, and 100 μg/mL) for 30 min prior to induction of stress by freshly prepared hydrogen peroxide (H2O2, final concentration 300 µM) and incubated for 24 h.
Protection effect on PC12 cells
PC12 cells (100 μL) were seeded in a Corning® CellBIND® Surface 96-well plate (Corning, NY, USA) at a density of 3 × 105 cells/mL and incubated at 37°C for 24 h in a CO2 incubator. The activity of the treated PC12 cells was determined using the MTT method.
Prevention of lactate dehydrogenase (LDH) release
The LDH assay was performed according to the manufacturer’s protocol. Supernatant (100 μL) of the cultured PC12 cells was mixed with 100 μL solution from the assay kit. The reaction mixture was incubated for 30 min at room temperature in the dark. Absorbance of the reaction mixture was measured at 490 nm with a microplate reader (Fluostar Optima, BMG Labtech, Germany). LDH leakage was expressed as the percentage (%) of the total LDH activity (LDH in the medium + LDH in the cell) as follows: % LDH released = (LDH activity in the medium / total LDH activity) × 100.
Prevention of apoptosis
Flow cytometric analysis was performed in order to detect cell death. Briefly, treated PC12 cells were harvested, washed twice with cold PBS, suspended in binding buffer, and incubated with 5 µL FITC-Annexin V and 5 µL propidium iodide (PI) for 15 min at room temperature in the dark. Then, 500 μL Annexin V binding buffer and 500 μL 2% formaldehyde were mixed and added to the reaction mixture. Samples were kept on ice for 10 min and 1 mL PBS was added to each tube. The mixture was centrifuged and the supernatant decanted. After treating with RNase (50 μg/mL) at 37°C for 15 min, the cells were centrifuged at 450 ×g for 5 min and examined using flow cytometry (FACScan, Becton Dickinson, USA) and analyzed by Cell Quest program (BD Biosciences). At least 10,000 cells were analyzed in cell sorting.
Protection of mitochondrial membrane
Mitochondrial membrane potential (MMP, ∆ψ) was detected using BD™ MitoScreen Flow Cytometry mitochondrial membrane potential detection kit as per manufacturer’s instructions. In brief, treated PC12 cells were centrifuged at 400 ×g for 5 min and the cell pellet was suspended in 0.5 mL of freshly prepared JC-1 Working Solution and kept at 37°C for 15 min in a CO2 incubator. Stained cells were washed twice with 1X assay buffer and analyzed by the flow cytometer (FACScan, Becton Dickinson, USA). In this assay, the ∆ψ of normal, healthy mitochondria is polarized and JC-1 is rapidly taken up by such mitochondria and measured in the Red (FL-2) channel. Green (FL-1) channel reflects the monomeric form of JC-1 which indicates depolarized ∆ψ (altered mitochondrial function which may be due to apoptosis).
DNA protection
To evaluate the integrity of DNA after cells were damaged by H2O2, alkaline comet assay (single cell gel electrophoresis assay) was performed according to a previous method. All slides were viewed using a fluorescence microscope and data were analyzed by OpenComet (an open-source software tool providing automated analysis of comet assay) using image J software.14 For each treatment, 20 cells were randomly selected and results were reported as tail length and % of Tail DNA.
Expression of Bcl-2, Bax, and caspase 3 in cytosolic fractions of PC12 cell
To analyze the regulators of apoptosis, western blot analysis was performed according to previous methods.2 Treated PC12 cells were mixed with 1 mL boiling lysis buffer (1% SDS, 1.0 mM sodium ortho-vanadate, 10 mM Tris pH 7.4) and an equal volume of 2X concentrated electrophoresis sample buffer (125 mM Tris pH 6.8, 4 % SDS, 10 % glycerol, 0.006 % bromophenol blue, 1.8 % ß-mercaptoethanol) was added. The samples were separated by 12.5% SDS–PAGE and transferred to a polyvinylidene difluoride membrane (Amersham Biosciences, USA). The membrane was incubated overnight with primary antibodies including Bax, Bcl-2, caspase 3, and β-actin (diluted 1:500) at 4°C, followed by HRP Goat Anti-Mouse Ig antibody. The bands were visualized using the Immobilon™ western HRP substrate kit. Chemiluminescence was detected using Fusion Fix imaging system (Vilber Lourmat) and analyzed with FusionCapt Advance software.
Statistical analysis
Fermentation and determinations were conducted in triplicate. Data are expressed as the mean ± standard deviation (n = 3). Values were evaluated by one-way ANOVA, followed by Duncan’s multiple range tests using the Statistical Analysis System (SAS institute, Cary, NC, USA). A significance level of 5% was adopted for all comparisons.
Results
Total polysaccharide, phenol, and flavonoid contents of XN mycelia extracts
The extraction yields of XN mycelia for hot water and 70% ethanol solution were 5.5 ± 0.1% and 3.4 ± 0.4%, respectively. The XN mycelia extracts contained high amount of total phenols (14.1 ± 0.81 mg gallic acid/g of hot water extract and 19.3 ± 0.62 mg gallic acid/g of 70% ethanol extract); flavonoids (4.64 ± 0.45 mg rutin/g of hot water extract and 1.97 ± 0.07 mg rutin/g of 70% ethanol extract) and total polysaccharides (448.0 ± 0.7 of hot water extract and 86.8 ± 1.4 mg/g of 70% ethanol extract). Hot water extract showed a significantly higher total polysaccharide and total flavonoid contents than the ethanol extract (P < 0.05). However, ethanol extract possessed significantly higher phenolic content than the hot water extract (P < 0.05).
Antioxidant activities of XN mycelia extracts
Table 1 shows antioxidant activities of XN mycelia extracts in the DPPH radical scavenging, lipid peroxidation inhibition, and reducing power assays. Compared to hot water extract, the ethanol extract showed significantly (P < 0.05) greater activities in the tested antioxidant-related assays.
Table 1.
Effect XN mycelia extracts on the DPPH radical scavenging activities, inhibition of lipid peroxidation, and reducing power activity.
| Antioxidant activities | Hot water extract | Ethanol extract | Positive control |
|---|---|---|---|
| DPPH radical scavenging IC50 (mg/mL) |
2.04 ± 0.06 | 1.13 ± 0.03* | Trolox 0.35 ± 0.01 |
| Lipid peroxidation inhibition IC50 (mg/mL) |
4.35 ± 0.09 | 1.08 ± 0.04* | ND |
| Reducing power (mg/mL, concentration needed to reach Absorbance700 = 0.5) | 10.45 ± 1.17 | 2.02 ± 0.10* | Ascorbic acid 0.02 ± 0.01 |
All data are the average of three independent experiments and each value represents the means ± SD.
Significant difference (P < 0.05) between hot water extract and ethanol extract sample as analyzed by Student’s paired t-test.
ND, not determined.
Protection effect of XN mycelia extracts on PC12 cells viability and oxidative damage
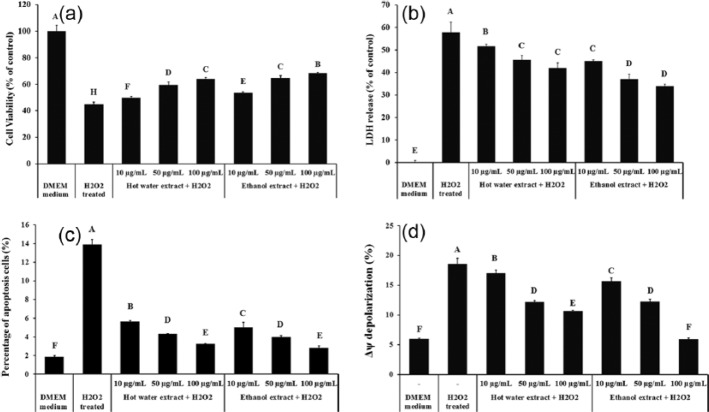
PC12 cells treated with H2O2 showed a 55.1% decrease in cell viability compared to control (Figure 1a). Both hot water and ethanol extracts significantly improved the cell viability (P < 0.05). A dose-dependent protection was observed and the cell viability was increased to 63.8% and 68.5% by 100 µg/mL of hot water extract and ethanol extract, respectively, indicating 18.9% and 23.6% recovery from H2O2-induced cell death.
Figure 1.
Protective effect of XN mycelia extracts on % PC 12 cells against H2O2 induced cell damage as analyzed by (a) MTT assay, (b) lactate dehydrogenase (LDH) release assay, flow cytometric assay for (c) the percentage of PC12 cells apoptosis, and (d) the mitochondrial membrane potential (MMP, Δψ) depolarization (%). Cell viability was determined by measuring MTT reduction and LDH release activity was measured using a colorimetric LDH assay kit. For apoptosis detection, cells were labelled with a combination of annexin V-FITC/PI and measured by flow cytometry, acquiring 10,000 gated events per fraction. For MMP (Δψ), cells were stained with JC-1, washed twice with 1× assay buffer and measured by flow cytometry, acquiring 20,000 gated events per fraction. Data are presented as the means ± SD (n = 3). A, B, and C indicate values with significant differences (P < 0.05) compared to controls, as analyzed by Duncan’s multiple range test.
When cells suffer serious damage, lactate dehydrogenase is rapidly released from cell cytoplasm into the cell culture supernatant. When treated with 300 µM H2O2, a total of 57.6% LDH was released to cell culture medium compared to untreated cells (Figure 1b). Both hot water and ethanol extracts significantly reduced the LDH leakage in a dose-dependent manner (P < 0.05). At the highest tested concentration (100 µg/mL), release of LDH were 42.1% and 33.9% in hot water extract and ethanol extract treated PC12 cells, respectively.
Effect of XN mycelia extracts on H2O2-induced apoptosis
Double staining with Annexin V/PI monitored with flow cytometric analysis was used to determine H2O2-induced apoptosis in PC12 cells (Figure 1c). Compared with the control group (DMEM medium), the percentage of apoptotic cells increased from 1.8 ± 0.14% to 13.9 ± 0.53% in H2O2-treated PC 12 cells. H2O2-induced apoptosis rates for PC 12 cells were 5.6 ± 0.10%, 4.3 ± 0.06% and 3.2 ± 0.04% when 10, 50, and 100 µg/mL of hot water extracts were added in the culture medium, respectively. Ethanol extract showed similarity effect on H2O2-induced apoptosis. The percentage of PC12 cells in apoptosis following treatment with 10, 50 and 100 µg/mL of ethanol extract were 5.1 ± 0.52%, 4.0 ± 0.11%, and 2.8 ± 0.22%, respectively. These results suggest that XN extracts inhibits H2O2-induced apoptosis in PC12 cells.
Effect of XN mycelia extracts on mitochondrial membrane potential (MMP)
Protective effect of XN mycelia extracts on the mitochondrial membrane potential (MMP) was monitored with flow cytometric analysis in H2O2-treated PC-12 cells. Cells were pre-treated with XN mycelia extracts (10, 50, and 100 μg/mL) for 30 min prior to induction of stress using freshly prepared H2O2 (300 μM). In the normal cells (without H2O2 treatment), a total of 6.0 ± 0.1% cells were under reduced MMP (Figure 1d). H2O2 treatment significantly increased the percentage of cells with reduced MMP (18.6 ± 0.9%). At the tested dose range, both hot water extract and ethanol extract protected mitochondrial membrane in a dose-dependent manner. When the PC12 cells were pre-treated with XN mycelia extracts prior to H2O2 addition, the percentage of cells with reduced MMP decreased to 10.7 ± 0.1% and 5.9 ± 0.3% at 100 μg/mL of hot water extract and 100 μg/mL of ethanol extract, respectively.
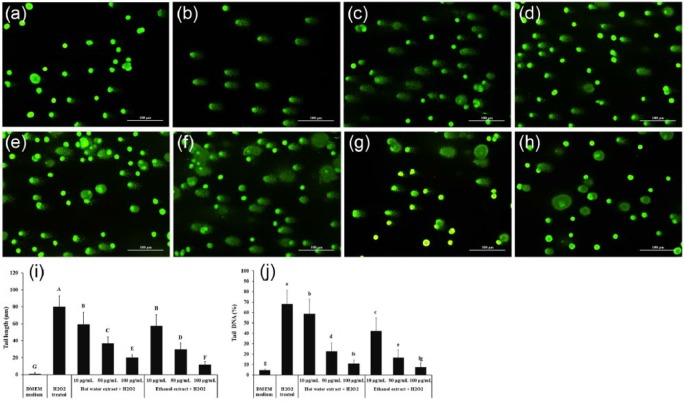
Protective effect of XN mycelia extracts on DNA damage
Compared with control (Figure 2a), H2O2 treatment induced DNA damage, as indicated by the presence of longer DNA tail length in PC12 cells (Figure 2b). A dose-dependent decrease in tail formation was observed following treatment with hot water extract (Figure 2c–e) and ethanol extract (Figure 2f–h). The tail length was increased from 1.1 ± 1.1 to 80.0 ± 13.0 (Figure 2i) and the percent of Tail DNA (Figure 2j) was increased from 4.4 ± 2.6% to 68.3 ± 10.1% after PC12 cells were treated with 300 µM H2O2. At the highest dose (100 µg/mL) of hot water extract and ethanol extract treatment, the tail lengths were 19.9 ± 3.5 and 11.6 ± 4.1, respectively (Figure 2i). The percentage of tail DNA was significantly decreased (P < 0.05) to 10.8 ± 3.4% and 7.6 ± 3.3% by 100 µg/mL of hot water extract and ethanol extract, respectively.
Figure 2.
Comet assay analysis of the prevention of H2O2-induced DNA damage in PC12 cells by XN mycelia extracts. (a) Untreated control; (b) H2O2 treated; (c) hot water extract (10 µg/mL) + H2O2; (d) hot water extract (50 µg/mL) + H2O2; (e) hot water extract (100 µg/mL) + H2O2; (f) ethanol extract (10 µg/mL) + H2O2; (g) ethanol extract (50 µg/mL) + H2O2; (h) ethanol extract (100 µg/mL) + H2O2; (i) tail length graph and (j) tail DNA percentage graph of Comet Assay. DNA damage was determined by comet assay and data analyzed using the OpenComet software tool in image J software. The data are presented as the mean ± SD (n = 3). A, B, and C represent tail length (µm) and a, b, c represent % tail DNA with significant differences (P < 0.05) compared to controls, as analyzed by Duncan’s multiple range test.
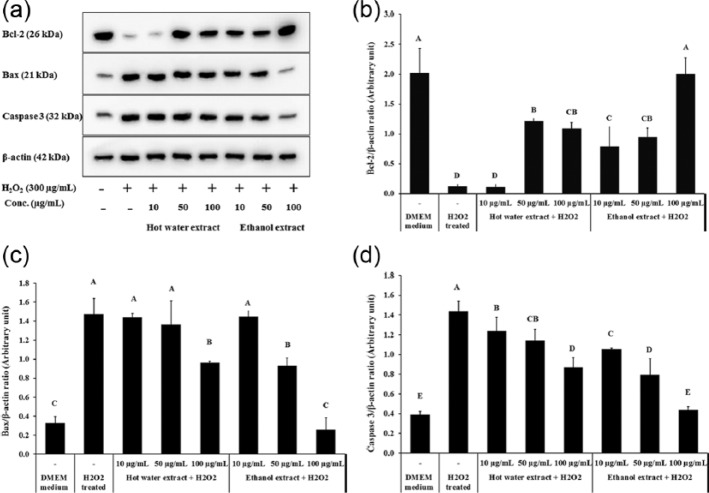
Effect of XN mycelia extracts on protein expression of Bcl-2, Bax, and caspase 3 in cytosolic fractions of PC12 cell
Western blotting was applied to determine the effects of XN mycelia extracts on expression of Bcl-2 family members in H2O2-treated PC12 cells. As shown in Figure 3, H2O2 treatment significantly decreased the expression level of Bcl-2, the pro-survival members, in PC12 cells (P < 0.05); while protein level of Bax and caspase 3, the pro-apoptotic members, were significantly increased by H2O2 treatment compared to untreated PC12 cells (Figure 3a) (P < 0.05). The effects of H2O2 treatment on Bcl-2 family members were weakened when XN mycelia extracts were applied simultaneously. The highest inhibition was observed in cells treated with at 100 µg/mL of the ethanol extract applied for 30 min prior to H2O2 delivery, causing the expression level of Bcl-2, Bax, and caspase 3 to completely recover to same level as the control group (Figure 3b–d).
Figure 3.
Effect of XN mycelia extracts on protein expression levels of Bcl-2, Bax, and caspase 3 in cytosolic fractions of PC12 cell. (a) The expression of Bcl-2, Bax, and caspase 3 in PC12 cells exposed to H2O2 and XN mycelia extracts, (b) quantitative analysis of Bcl-2 blots normalized to β-actin, (c) quantitative analysis of Bax blots normalized to β-actin, and (d) quantitative analysis of caspase 3 blots normalized to β-actin. Data are presented as the mean ± SD (n = 3). A, B, and C indicate values with significant differences (P < 0.05) compared to the control, as analyzed by Duncan’s multiple range test.
Discussion
In folk medicine, XN is used to treat central nervous system associated disorders, such as insomnia, anxiety, depression, and spatial memory impairment.3,4,8,10 Recently, bioactive compounds with neuroprotective, anti-neuroinflammatory, and cytotoxic properties were isolated from XN mycelia.11 However, no study has reported detailed mechanisms regarding the neuroprotection of XN mycelia.
This study demonstrates the neuroprotective effect of XN mycelia extracts on H2O2-induced cytotoxicity in PC12 cells through multiple mechanisms. Both XN hot water extract and 70% ethanol extract showed good antioxidant activities in the DPPH radical scavenging activity assay, inhibition of lipid peroxidation, and through reducing power. Compared to XN hot water extract, the XN ethanol extract exhibited a higher antioxidant activity which could be attributed to its higher phenolic content.4 Evidence that XN mycelia extracts confers neuroprotective activity in H2O2-treated PC12 cells includes the following observations: increased cell viability; arrest of abnormal apoptosis; restoration of MMPs; and decreased DNA damage. In PC12 cells treated with XN mycelia extracts and H2O2, abnormal apoptosis induced by H2O2 was reduced through upregulation of Bcl-2 and downregulation of Bax and caspase 3. Furthermore, the ethanol extract showed a better neuroprotective effect than the hot water extract. Our results provide some scientific support to the traditional applications of XN for treating central nervous system associated diseases. Our study also confirms reports by Martin et al.,15 who mentioned that many natural substances with antioxidant activities are capable of delaying reactive oxygen species (ROS)-induced apoptosis during neurodegenerative diseases.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the Ministry of Economic Affairs, Republic of China (Taiwan) (no. 101-EC-17-A-18-S1-227) and the Ministry of Science and Technology, Republic of China (Taiwan) (MOST 104-2320-B2-126-004-MY3).
References
- 1. Sastry PS, Rao KS. (2001) Apoptosis and the nervous system. Journal of Neurochemistry 74: 1–20. [DOI] [PubMed] [Google Scholar]
- 2. Huang CK, Lin Y, Su H, et al. (2014) Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 cell. Neurochemical Research 40: 27–35. [DOI] [PubMed] [Google Scholar]
- 3. Lin Y, Wang XY, Ye R, et al. (2013) Efficacy and safety of Wuling capsule, a single herbal formula, in Chinese subjects with insomnia: A multicenter, randomized, double-blind, placebo-controlled trial. Journal of Ethnopharmacology 145: 320–327. [DOI] [PubMed] [Google Scholar]
- 4. Ko H-J, Song A, Lai M-N, et al. (2009) Antioxidant and antiradical activities of Wu Ling Shen in a cell free system. American Journal of Chinese Medicine 37: 815–828. [DOI] [PubMed] [Google Scholar]
- 5. Ma YP, Mao DB, Geng LJ, et al. (2013) Production optimization, molecular characterization and biological activities of Exopolysaccharides from Xylaria nigripes. Chemical and Biochemical Engineering Quartery 27: 177–184. [Google Scholar]
- 6. Ko H-J, Song A, Lai M-N, et al. (2011) Immunomodulatory properties of Xylaria nigripes in peritoneal macrophage cells of Balb/c mice. Journal of Ethnopharmacology 138: 762–768. [DOI] [PubMed] [Google Scholar]
- 7. Song A, Ko H-J, Lai M-N, et al. (2011) Protective effects of Wu-Ling-Shen (Xylaria nigripes) on carbon tetrachloride-induced hepatotoxicity in mice. Immunopharmacology and Immunotoxicology 33: 454–60. [DOI] [PubMed] [Google Scholar]
- 8. Zhao Z, Li Y, Chen H, et al. (2014) Xylaria nigripes mitigates spatial memory impairment induced by rapid eye movement sleep deprivation. International Journal of Clinical and Experimental Medicine 7: 356–62. [PMC free article] [PubMed] [Google Scholar]
- 9. Peng W-F, Wang X, Hong Z, et al. (2015) The anti-depression effect of Xylaria nigripes in patients with epilepsy: A multicenter randomized double-blind study. Seizure 29: 26–33. [DOI] [PubMed] [Google Scholar]
- 10. Chen YI, Tzeng CY, Cheng YW, et al. (2015) The involvement of serotonin in the hypoglycemic effects produced by administration of the aqueous extract of Xylaria nigripes with steroid-induced insulin-resistant rats. Phytotherapy Research 29: 770–776. [DOI] [PubMed] [Google Scholar]
- 11. Xiong J, Huang Y, Wu X-Y, et al. (2016) Chemical constituents from the fermented mycelia of the medicinal fungus Xylaria nigripes. Helvetica Chimica Acta 99: 83–89. [Google Scholar]
- 12. Chen J, Lo H, Lin F, et al. (2014) Effects of medium components and culture conditions on mycelial biomass and the production of bioactive ingredients in submerged culture of Xylaria nigripes (Ascomycetes), a Chinese medicinal fungus. International Journal of Medicinal Mushrooms 16: 431–447. [DOI] [PubMed] [Google Scholar]
- 13. Stankovic MS. (2011) Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujev Journal of Science 33: 63–72. [Google Scholar]
- 14. Gyori BM, Venkatachalam G, Thiagarajan PS, et al. (2014) OpenComet: An automated tool for comet assay image analysis. Redox Biology 2: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martins N, Barros L, Ferreira ICFR. (2016) In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends in Food Science & Technology 48: 1–12. [Google Scholar]