Abstract
Thymic peptides are immune regulators produced mainly in the thymus. However, thymic peptides such as thymosin-α and thymopoietin have precursors widely expressed outside the thymus, localized in cell nuclei, and involved in vital nuclear functions. In stress-related conditions, they can relocalize. We hypothesized that another thymic peptide, thymulin, could be similarly produced by non-thymic cells during stress and have a precursor therein. Non-thymic cells, including macrophages and fibroblasts, were exposed to oxidative stress, heat, apoptosis, or necrosis. Extracellular thymulin was identified in media of both cell types 2 h after exposure to stress or lethal signals. Therefore, thymulin is released by non-thymic cells. To examine possible thymulin precursors in non-thymic cells, macrophage lysates were analyzed by western blotting. Bands stained with anti-thymulin antibody were detected in two locations, approximately 60 kDa and 10 kDa, which may be a possible precursor and intermediate. All of the exposures except for heat were effective for induction of the 10 kDa protein. BLAST search using thymulin sequence identified SPATS2L, an intranucleolar stress-response protein with molecular weight of 62 kDa, containing thymulin-like sequence. Comparisons of blots stained with anti-thymulin and anti-SPATS2L antibodies indicate that SPATS2L may be a possible candidate for the precursor of thymulin.
Keywords: extrathymic production, precursor, SPATS2L, thymic peptides, thymulin
Introduction
In the last three decades, a large body of data has accumulated concerning two important aspects of thymic peptide hormones: their effects on immune cell activity and their interactions with the neuroendocrine system. Our analysis of data1 showed that different thymic hormones (with thymulin, thymosin-α, and thymopoietin being the most commonly studied) exert different effects on immune cells and the stress-response system and in particular on the hypothalamic–pituitary–adrenal axis. For example, thymulin provides mostly inhibitory effects on inflammatory immune responses2,3 and stimulates the neuroendocrine system, which is often suppressed during inflammation. On the other hand, thymosin-α demonstrates distinct stimulatory effects on immune cells and inhibitory effects on the neuroendocrine system.1,4 Thymopoietin stimulates immune cells in healthy animals and humans but inhibits their inflammation activity without a considerable effect on the neuroendocrine mechanisms.1,5 Based on their immunological activity, thymic peptides are used as immunomodulators in therapy of various inflammatory diseases and immunodeficiencies.
However, there is a third aspect of thymic peptides that has not yet been given much attention. This aspect involves the features of precursors of these compounds. Thymic peptides are secreted mainly by thymic epithelial cells (TECs), but this secretion, probably, is not the only source of these peptides. Thymosin-α and its precursor prothymosin-α, as well as thymopoietin and its precursor LAP2 protein, if considered as non-secreted intracellular proteins, are widely expressed ubiquitous intranuclear proteins associated with chromatin and cell cycle regulation.6,7 For example, thymopoietin (which is a 49 amino-acid peptide) is a fragment of the ubiquitous intranuclear protein named “lamina-associated polypeptide 2” (LAP2), which is involved in the internal architecture of the nucleus. LAP2 localizes in the nuclear membrane and is associated with nuclear lamina proteins such as lamin B2 as well as with chromatin (review in Dechat et al.8). It is established that LAP2 is expressed not only in the thymus but also in many other tissues, especially those with a high proliferation rate. Thus, there was reason to propose its role in proliferation and cell cycle regulation.7 Interestingly, LAP2 is cleaved by caspases in apoptosis, but not necrosis, and one of its fragments remains associated with chromatin, while another one solubilizes.9 The cleavage of this protein is one of the earliest events in apoptosis.10,11
Prothymosin-α, the precursor of thymosin-α, is also an intracellular protein with wide distribution throughout body tissues. It has been suggested that prothymosin-α might play a role in differentiation and proliferation, regulating structural rearrangements of chromatin.12 Interestingly, prothymosin-α exits from a nucleus into the cytoplasm in response to proapoptotic stimuli, inhibiting formation of apoptosomes.13 Furthermore, it was shown that prothymosin-α is secreted into extracellular space during the early stages of necrosis and has anti-necrotic effects.14 Additionally, in apoptosis, prothymosin-α is cleaved by caspases, but without secretion into extracellular space.15
Therefore, thymosin-α and thymopoietin have the interesting combination of intranuclear, immunomodulating, and neuroendocrine functions, wherein their activity spectra are different.
Based on the aspects of thymosin-α and thymopoietin described above, we put forward a hypothesis that anti-inflammatory thymic peptide thymulin may demonstrate similar properties. To test this hypothesis, we measured thymulin secretion in non-thymic cells, such as macrophages and fibroblasts, in response to some different stress or lethal exposures, in particular oxidative stress, apoptosis, heating, or necrosis. To control the exposure effects, we measured some markers inherent to the exposures, such as levels of the inducible heat-shock protein Hsp72, caspase 3, phosphorylated form of IKK protein (an indicator of NF-kappaB pathway activation that is phosphorylated in response to many stress factors), as well as cell viability and membrane permeability.
The second aim of the present study was to propose candidates for thymulin analogues or precursors. Indeed, a precursor of thymulin has yet to be identified, including thymic or non-thymic cells. In the present study, we determined level of thymulin in the intracellular space to study the mechanisms involved in thymulin synthesis and find candidates for higher molecular weight precursor of thymulin (which itself is a nonapeptide with a molecular weight of approximately 0.8 kDa).
Confirmation of extrathymic sources of thymulin would allow us to support the fundamental conception of interactions between intranuclear, immunomodulating, and neuroendocrine functions of thymic hormones. Additionally, a potential protein candidate for thymulin precursor may be useful as a new target of therapies for inflammatory diseases.
Materials and methods
Cell culture conditions
RAW 264.7 cells (mouse monocyte macrophage cell line) or L929 cells (C3H/An mouse connective tissue cell line) obtained from the American Type Culture Collection were cultured in RPMI medium supplemented with 10% fetal calf serum (FCS), penicillin G (100 unit/mL), gentamycin (100 μg/mL), and streptomycin (100 μg/mL) at 37°C in a 5% CO2 atmosphere. Cells were seeded in a concentration of 1 × 106 cells on 100-mm plates. In each of the experiments, the cells from a separate passage (ranging from the third to seventh passages) were pooled, and the cell’s aliquots were treated separately. After seeding, cells were incubated in the flask overnight for adaptation.
Exposures
Before treatments, cells were washed, re-suspended in serum-free RPMI medium, and adapted for 15 min. Oxidative stress was induced through treatment with hydrogen peroxide 25 µM for 2, 4, or 6 h in ELISA experiments, or hydrogen peroxide 10 μM, 25 μM, or 50 μM for 2 h in western blotting experiments. The heat shock was induced by transferring the culture flasks from a 37°C incubator to a 42°C incubator for 1 h. Apoptosis was induced by treating with 25 μM of etoposide (Sigma, USA) for 2 h. To induce necrosis using an ATP depletion method,16 the cells were initially washed and re-suspended in serum-free RPMI medium without glucose (Sigma, USA) containing 2 mM pyruvate (Sigma, USA). After adaptation to this medium for 15 min, cells were exposed to 2.5 μM oligomycin (Sigma, USA) for 45 min, and then challenged with etoposide (Sigma, USA) for 2 h.
Viability, apoptosis, and membrane permeability assays
Cultures treated with either pro-apoptotic, pro-necrotic, or oxidative agents were stained with a mixture of the membrane permeant dye Hoechst 33342 (500 ng/mL) (Thermo Fisher Scientific, USA) and the membrane impermeant dye propidium iodide (20 μM) (Sigma, USA). Necrotic cells with permeable membrane and non-condensed nuclei, as well as apoptotic cells with impermeable membrane and condensed or fragmented nuclei, were scored. Fluorescence of reaction products was analyzed using microscope Leica TCS SP-5 (Leica Microsystems, Germany). Cell counting was performed using a software package ImageJ v.1.50 (National Institutes of Health, USA).
ELISA
Supernatants were obtained by centrifugation of cell samples at 2000 g and used in the ELISA either as-is or after lyophilization (using freeze drier Labconco, USA) and reconstitution in different amounts of distilled water (from 0.1 to 1.0 of the initial volume). The results were comparable in both cases, accounting for the dilution. The measurements were performed by the ELISA method. The liquid supernatants obtained as described above were plated on 96-well ELISA plates (Cornig, USA) (100 μL per well) and incubated for 3 h at 37°C for protein fixing on the plate surface. Then, plates were washed three times and a blocking buffer (with 5% skim milk, US Biological, USA) was plated (200 μL per well) and incubated overnight. Afterwards, plates were washed three times and a primary antibody solution (100 μL per well) was plated and incubated for 2 h at 37°C. Rabbit anti-thymulin antibody (Abcam, UK) was used as the primary antibody. After washing, a secondary antibody was plated (100 μL per well) and incubated for 1 h at 37°C. The biotinylated goat anti-rabbit IgG antibody (IMTEK, Russia) was used as the secondary antibody. The biotinylated secondary antibody was then bound to peroxidase-conjugated streptavidin (IMTEK, Russia). To visualize binding, 100 μL of ABTS green dye (Sigma, USA) dissolved in 0.05 M citrate buffer (pH 4.0) with 0.01% H2O2 was applied. The optical density was measured at 405 nm with the plate spectrophotometer (Multiscan EX, Thermo Electron Corporation). Within each separate experiment, characteristics of samples were concurrently measured in six repetitions. The average values from four experiments were processed to determine the significance of differences between groups (n = 4).
Western blot analysis
To prepare specimens, cells were lysed in an ultrasonic disintegrator with constant stirring for 2 min. The concentration of the total cellular protein was determined by the Bradford method with Bradford solution (Sigma, USA). Proteins were precipitated in acetone, solubilized, boiled for 5 min, and stored at −70°C. Proteins were determined by 10% PAGE. The specificity of the analysis was tested by immunoblotting. The proteins were transferred from the gel onto a nitrocellulose membrane in a transblot chamber. Upon blocking, the membrane was exposed for 2 h to an antibody to one of the following proteins: thymulin (mouse anti-thymulin antibody, Abcam, UK), SPATS2L (mouse anti-SPS2L, Abcam, UK), mouse Hsp72, inducible form (rabbit anti-mouse HSP 72, StressGen Biotechnologies), and mouse phospho-IKK (rabbit anti-phospho-IKK, Cell Signaling Technology, USA). After washing, the nitrocellulose membranes were incubated for 1 h with the anti-rabbit or anti-mouse biotinylated antibody (Jackson ImmunoResearch, USA), and peroxidase-conjugated streptavidin (IMTEK, Russia) was added for 1 h. The loading control was mouse monoclonal anti-human GAPDH (US Biological, Swampscott, MA, USA). ECL Plus chemiluminescent cocktail (Amersham/GE Healthcare, UK) was used to develop the immunostaining of blots following the manufacturer’s instructions, exposing the blot to Kodak film. A position of proper bands was determined using a molecular weight marker (Spectra Multicolor Broad Range Protein Ladder, Fermentas, USA) during the electrophoresis stage and by the specific binding to antibodies after blotting. Quantitative evaluation of protein bands was performed using the Qapa software (Pushchino, Russia). Obtained numeric data were normalized to corresponding loading control (the GAPDH band) and expressed in relative units as the mean of three independent measurements.
BLAST search
BLAST search of the protein sequence was performed in the NCBI protein database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis
Statistical analysis was performed using Student’s two-sided unpaired t-test. Pearson product-moment correlation coefficients (Pearson-r) were determined using Excel 2010, and corresponding P values were determined using a table of critical values.
Results
Determination of optimal times/dosages for stress and lethal exposure in macrophages
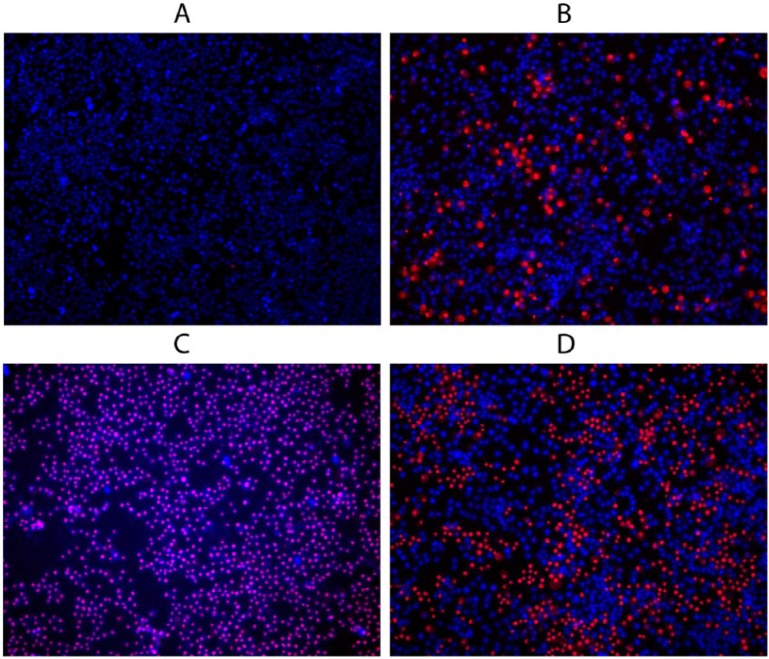
To control stress exposure and select optimal exposure times and dosages of the chemical agents, the RAW 264.7 cells were analyzed with fluorescent microscopy after staining with a mixture of the membrane permeable DNA dye (shown in blue) and the membrane non-permeable DNA dye (shown in red) (Figure 1). We detected normal cells with impermeable membranes and non-condensed nuclei (dim blue spots on the images), apoptotic cells with impermeable membranes and condensed or fragmented nuclei (bright blue spots), and necrotic cells (red or pink spots).
Figure 1.
Fluorescent images of RAW 264.7 cells stained using a mixture of the membrane permeable DNA dye and the membrane non-permeable DNA dye after chemical stress exposures. (a) Control, (b) apoptosis (induced with etoposide), (c) necrosis (ATP depletion protocol (27), as described in the Materials and Methods), (d) oxidative stress (induced with 50 μM hydrogen peroxide). One representative image from a single experiment including three repetitions is shown. Scale bar represents 20 μm.
Based on the amount of the normal, apoptotic, and necrotic cells, we determined the optimal dosages and times of exposure. The criteria were as follows: for the apoptotic cell group, this was a dose/time combination inducing maximal apoptosis with minimal necrosis. Fluorescent imaging of the exemplary apoptotic cell population that underwent the selected exposure is presented in Figure 1b. For the necrotic cell group, this was a dose/time combination inducing maximal necrosis with a minimal dose at a minimal test time. Fluorescent imaging of the exemplary necrotic cell population is presented in Figure 1c. For the oxidative stress group, this was a dose/time combination inducing minimal necrosis with a maximal dose (three doses were finally selected, 10 μM, 25 μM, or 50 μM, duration 2 h). Imaging of the exemplary cells exposed to 50 μM of hydrogen peroxide is presented in Figure 1d.
The selected parameters (Table 1) were used in further experiments. Additionally, the medium dose of the hydrogen peroxide (25 μM) was used with varying durations of exposure (2, 4, and 6 h).
Table 1.
Number of apoptotic and necrotic cells after stress and lethal treatments.
| Time/dose, substance | Apoptotic cells | Necrotic cells | |
|---|---|---|---|
| Control | Saline, 2 h | Single | Single |
| Pro-apoptotic exposure | 25 μM etoposide, 2 h | 34.5 ± 3.3% | 9.9 ± 3.6% |
| Pro-necrotic exposure | 50 μM etoposide, plus 2.5 μM oligomycin, 2 h | 0% | 100% |
| Low oxidative stress | 10 μM of H2O2, 2 h | 2.1 ± 0.5% | 1.2 ± 0.8% |
| Medium oxidative stress | 25 μM of H2O2, 2 h | 8.1 ± 0.5% | 18.1 ± 4.3% |
| High oxidative stress | 50 μM of H2O2, 2 h | 15.6 ± 0.1% | 32.9 ± 3.3% |
Data are percentages of total amounts of cells counted in the microscope’s field of vision, n = 300 or more ± SEM.
Thymulin was released into culture media by mouse RAW 264.7 macrophages and L929 fibroblasts
Concentrations of thymulin in the cell supernatants were determined in RAW 264.7 cells and L929 cells by an indirect ELISA method using the following experimental groups: oxidative stress (25 μM of hydrogen peroxide, duration 2, 4, and 6 h), pro-apoptotic exposure (25 μM of etoposide for 4 h), and control (no additional treatments, duration of 4 h). The results are presented in Table 2.
Table 2.
Thymulin determination in supernatants of cultured macrophages RAW 264.7 and fibroblasts L929.
| RAW 264.7 cells (ng/mL) | L929 cells (ng/mL) | |
|---|---|---|
| Control | 3.58 ± 0.05 | 4.07 ± 0.04 |
| Apoptosis | 3.93 ± 0.07* | 3.94 ± 0.06 |
| 25 μM of H2O2, 2 h | 3.88 ± 0.05* | 3.85 ± 0.06 |
| 25 μM of H2O2, 4 h | 4.05 ± 0.05* | 3.99 ± 0.05 |
| 25 μM of H2O2, 6 h | 4.01 ± 0.07* | 3.91 ± 0.04 |
Extracellular values of thymulin released by RAW 264.7 macrophages and L929 fibroblasts in response to apoptosis (induced with 25 μM etoposide) or oxidative stress (induced with 25 μM of hydrogen peroxide for 2, 4, or 6 h). The values are averaged results obtained by the ELISA method in four independent experiments expressed in ng/mL ± SEM. Within each of the independent experiments, the data were averaged in quadruplicates.
Significant difference from the control group, P <0.01 (Student’s unpaired two-sided t-test). Signal-to-noise ratio 6:1.
Thymulin was found in supernatants of all experimental cell groups, including the control group, in generally similar amounts, 3–4 ng/mL. It should be mentioned that the signal-to-noise ratio for the ELISA plates (blocked with skimmed milk proteins) was approximately 6:1, indicating specificity of the reaction. All stress exposures significantly increased the thymulin content in media by approximately 10% compared to the control group in RAW 264.7 cells, but not in L929 cells. This increase in RAW 264.7 cells was observed after 2 h of oxidative stress and further exposure did not result in any additional increase. The concentrations of thymulin in supernatants from RAW 264.7 cells and L929 were essentially similar. Therefore, the thymulin or thymulin-containing protein was released into cultural medium relatively quickly (in less than 2 h) by both cell types.
Thymulin-containing protein underwent conversions in response to the stress and lethal treatments
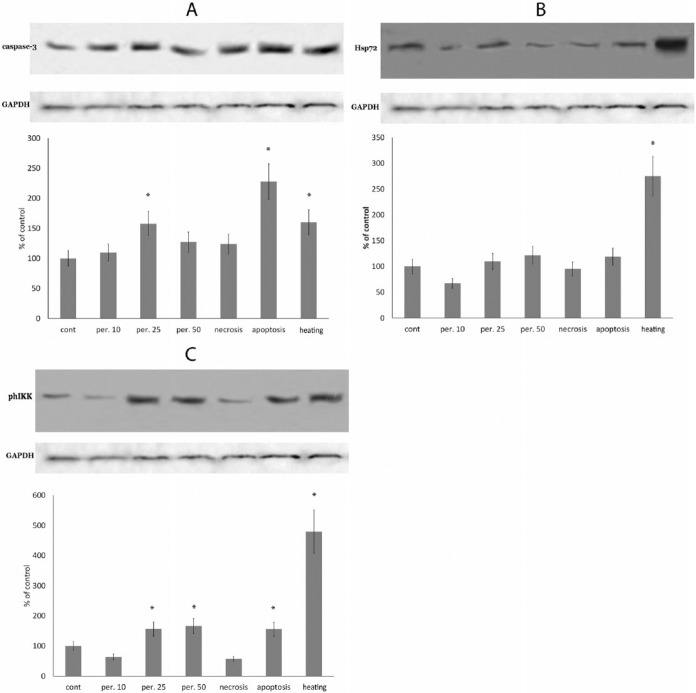
To investigate mechanisms involved in thymulin synthesis during stress and to find candidates for a higher molecular weight precursor of thymulin, RAW 264.7 cells were exposed to oxidative stress (10 μM, 25 μM, and 50 μM of hydrogen peroxide), pro-apoptotic agent, pro-necrotic agent, heating at 42°C for 1 h, or sham treatments. Thymulin was determined by western blotting in cell lysates. To confirm the stress exposure, values of caspase-3, inducible heat-shock protein Hsp72, and ph-IKK protein were also measured in samples. The results are presented in Figure 2.
Figure 2.
Production of caspase-3 (a), heat-shock protein Hsp72 (b), and phosphorylation of IKK protein (c) in RAW 264.7 macrophages in response to oxidative stress (induced with 10, 25, or 50 μM of hydrogen peroxide), proapoptotic exposure (induced with 25 μM etoposide), pronecrotic exposure (ATP depletion), as described in the Materials and Methods, or heating at 42°C for 1 h. Equal amounts of protein were analyzed by western blot analysis using the corresponding antibodies. Blot pictures show a single representative experiment, while the histograms below the protein bands show protein levels. Histograms show the amount of proteins measured by protein blot densitometry software QAPA, normalized to the internal GADPH control (shown below), averaged for four independent experiments and expressed as the percentage of the control group (± SEM). *Denotes a significant difference from the control, P <0.05 (two-sided unpaired Student’s t-test).
As expected, the caspase-3 level was highest in cells that underwent apoptotic exposure, where it was approximately twice as high as in the control cells. Additionally, a significant increase of caspase-3 was also observed in cells exposed to the high dosages (25 μM and 50 μM) of hydrogen peroxide and in the heated cells (Figure 2a). The level of inducible heat-shock protein Hsp72 was significantly increased only in the heated cells group. Additionally, there was a small, but not significant increase of Hsp72 in cells treated with the maximal concentration of hydrogen peroxide (Figure 2b). The level of the phosphorylated IKK protein is an indicator of NF-kappaB pathway activation, which is activated in response to many stresses. We observed a significant increase of the IKK protein level in all stress groups, but not in the necrosis group. The most prominent response was seen in the heating group (more than 450%) (Figure 2c).
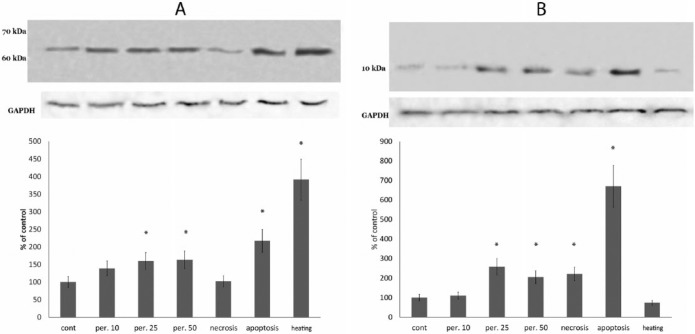
Two bands stained with the anti-thymulin antibody were observed at locations corresponding to molecular weights of approximately 60–65 kDa and approximately 10 kDa (Figure 3a and b). The higher molecular weight band was increased in all of the stress groups except the necrosis group, in correlation to the phosphorylated IKK protein level. The lower molecular weight band was increased under oxidative stress, apoptosis, and necrosis, but not in the heated cells. Therefore, the 10 kDa protein correlated with the caspase-3 level rather than with the phosphorylated IKK protein level. Additionally, this protein was the only protein tested that increased significantly in the necrosis group.
Figure 3.
Protein bands at regions corresponding 60 kDa (a) and 10 kDa (b) that are stained by thymulin antibody in lysates of RAW 264.7 macrophages exposed to oxidative stress induced with 10, 25, or 50 μM of hydrogen peroxide, proapoptotic exposure (induced with 25 μM etoposide), pronecrotic exposure (ATP depletion), as described in the Materials and Methods, and heating at 42°C for 1 h. Equal amounts of protein were analyzed by western blot analysis using the corresponding antibodies. Blot pictures show a single representative experiment, while the histograms below the protein bands show protein levels. Histograms show the amount of proteins measured by protein blot densitometry software QAPA, normalized to the internal GADPH control (shown below), averaged for four independent experiments and expressed as the percentage of the control group (± SEM). *Denotes a significant difference from the control, P <0.05 (two-sided unpaired Student’s t-test).
Therefore, based on ELISA and western blot analyses, we have suggested that these 60 kDa and 10 kDa proteins may be a precursor and intermediate product, respectively, containing a thymulin-like amino-acid sequence. Hence, it appears that during stress exposure and necrosis some unknown 60 kDa precursor of thymulin is cleaved, resulting in a lower molecular weight intermediate (10 kDa) that may undergo further cleavage (intracellularly or extracellularly) resulting in the thymulin formation.
Thymulin-containing protein was similar to a peptide identified with a BLAST search
A BLAST query using a canonical thymulin amino-acid sequence UAKSQGGSN17 indicated a lack of exact alignment matches, but identified a SPATS2-like protein that contains a thymulin-like sequence. In the mouse, this protein (accession number Q91WJ7) consists of 558 amino acids and contains SPATS2L449-457 = PAKSQGGGN. In human, SPATS2-like protein (accession number Q9NUQ6) also consists of 558 amino-acids and contains SPATS2L449-457 = PAKSQGSGN. Thus, there are only two differences: P (proline) in SPATS2L instead of U (5-oxiproline or pyroglutamate) in thymulin, and the difference in amino-acids 7-8 (GS) of thymulin that are interchanged (SG) in the human SPATS2L or changed to GG in the mouse SPATS2L. The SPATS2-like protein is a recently described ubiquitous intranucleolar protein that may migrate to the cytoplasm in response to stress.18
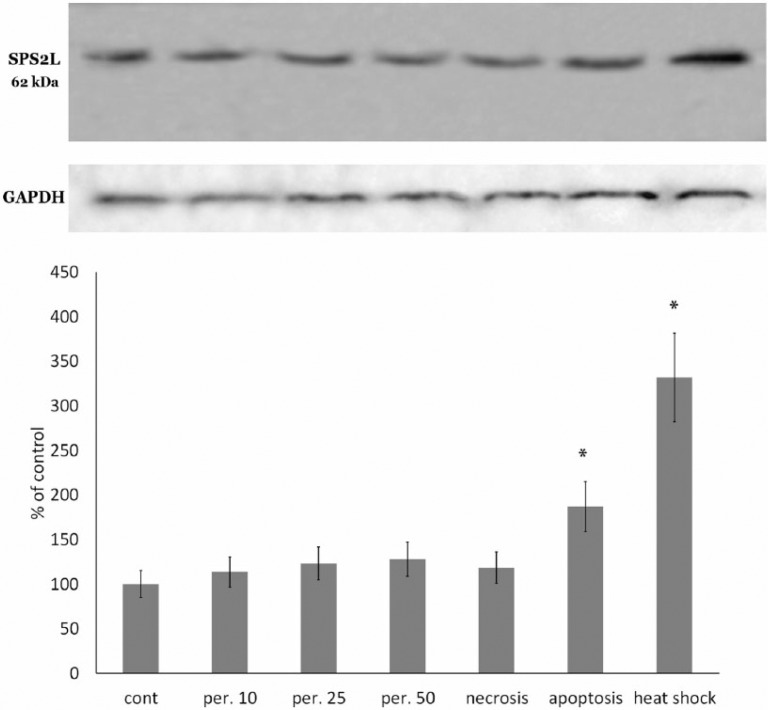
We performed a western blot analysis of the lysates of RAW 264.7 cells exposed to the abovementioned stress exposures, i.e. oxidative stress (10 μM, 25 μM, and 50 μM of hydrogen peroxide), apoptosis, necrosis, heating, or sham treatment, using an antibody for the full-length mouse SPATS2-like protein. The results are provided in Figure 4.
Figure 4.
Protein bands that were stained by SPATS2L antibody in lysates of RAW 264.7 macrophages exposed to oxidative stress (induced with 10, 25, or 50 μM of hydrogen peroxide), proapoptotic exposure (induced with etoposide), pronecrotic exposure (ATP depletion), as described in the Materials and Methods, and heating at 42°C for 1 h. Equal amounts of protein were analyzed by western blot analysis using the corresponding antibodies. Blot pictures show a single representative experiment, while the histograms below the protein bands show protein levels. Histograms show the amount of proteins measured by protein blot densitometry software QAPA, normalized to the internal GADPH control (shown below), averaged for four independent experiments and expressed as the percentage of the control group (± SEM). *Denotes a significant difference from the control, P <0.05 (two-sided unpaired Student’s t-test).
SPATS2-like protein was detected in blots of all experimental groups in the location approximately 60 kDa molecular weight (estimated molecular weight = 61.7 kDa), which is very close to the above-mentioned higher molecular weight thymulin-stained band (60–65 kDa). Moreover, the changes in the SPATS2-like band between the experimental cells groups (measured by the blot densitometry) were strongly correlated to the changes in higher molecular weight thymulin-stained band. Calculation of the Pearson correlation coefficient for these two bands among the tested exposures showed Pearson’s r = 0.925 (P <0.01). It should be noted that 10 kDa bands did not stain by the anti-SPATS2L antibody. Therefore, it is reasonable to believe that the SPATS2-like protein may be a precursor of the thymulin.
Discussion
The thymic peptides, thymosin-α, thymopoietin, and thymulin, share some common properties: they are relatively small peptides (9–50 residues) produced mainly in the thymus; they affect T-lymphocyte maturation and proliferation; they influence peripheral immune cells changing their cytokine response; they are stable in saline solution but have a very short half-life in plasma (several minutes); their levels in blood correlate to levels of neuroendocrine stress hormones;1 and finally, the precursors of thymosin-α and thymopoietin are vital ubiquitous intranuclear proteins associated with chromatin that may leave the nucleus in some situations, in particular, those involving stress.6,7 The ubiquitous distribution of precursors for thymosin-α and thymopoietin is generally consistent with a known fact that immune cells remain responsive to the thymic peptides despite thymus involution and a decrease in its secretory functions with age.19 One may suggest that these peptides are secreted by any cells in response to stress or damaging stimuli, similar to danger-associated molecular patterns (DAMPs). Moreover, it is possible their appearance in extracellular space may be one of the earliest (as showed in the references10,11) localized signals for the surrounding immune cells, indicating certain intracellular events, such as apoptosis or necrosis, thereby activating or inhibiting their activity. Before the study, we hypothesized that thymulin may also have a ubiquitously expressed precursor, and therefore may be produced by non-thymic cells. Our study was designed to confirm the hypothesis.
It should be noted that all cells with necrosis induced by the oligomycin/etoposide combination (Figure 1c) had dim (pink) rather than bright (red) staining by propidium iodide, compared to other necrotic cells (died of any other causes). We have interpreted the pink staining as an increase of membrane permeability due to ATP depletion by the oligomycin, with membrane pores formation, and without the membrane disruption. Therefore, the entrance of the propidium iodide was lower than after a membrane disruption. The similar mechanism was described earlier.20
Our experimental data confirmed the abovementioned hypothesis by showing that the thymulin (or some thymulin-containing protein) was released into cultural media by macrophage and fibroblast cells. It should be noted that both lineages produced similar amount of thymulin, although the macrophage cells RAW 264.7 were more responsive to the stress or lethal exposures than fibroblast cells L929. The data may indicate that thymulin may be produced not only by thymic epithelial cells, but also by cells of very different origins. The responsiveness of the macrophages may reflect their increased sensitivity to stress exposures.
Further, based on our data, we suppose that the potential thymulin precursor is the approximately 60 kDa protein that was detected in macrophages’ lysates and that the precursor may be cleaved forming an approximately 10 kDa intermediate, containing the thymulin-like sequence. Interestingly, the induction of the intermediate was observed during necrotic and apoptotic exposures, as well as during oxidative stress, as early as 2 h after the beginning of the exposure. Thus, this is a fairly early event. Changes in production of the “full-length precursor” were consistent to changes in phosphorylation of the IKK protein that is a member of NF-kappaB pathway. This pathway is activated in response to many stresses, such as the oxidative stress, irradiation, high temperature, or ligand-mediated reactions, leading to expression of genes related to stress responses. This is in line with our earlier data that thymulin effects are mediated by NF-kappaB pathway.5,21 However, changes in content of the 10 kDa intermediate were consistent with the caspase-3 activation and necrosis rather than the NF-kappaB activation, indicating the role of the intermediate in the pathological processes. Thus, the potential thymulin precursor is a stress-related protein being cleaved in some stress-related or pathological conditions and released into extracellular space. Considering the fact that thymulin provides mostly inhibitory effects on immune cells,2,3 we may propose that its role in inhibiting the inflammatory response (that often damages surrounding host cells) is based on the feedback from these cells.
There was no exact alignment match for the thymulin sequence in protein databases. However, the BLAST search using the thymulin sequence revealed a SPATS2-like protein (SPATS2L) that contains a thymulin-like sequence. The SPATS2L protein (synonyms: SPS2L, SGNP, DNAPTP6) is a poorly investigated protein that is localized both in the cytoplasm (as a component of 60S ribosomal subunit) and nucleolus. Furthermore, it is ubiquitously expressed.18 Its exact roles are unknown so far, but its involvement in regulation of ribosomes and translation processes has been proposed. Zhu et al.18 showed that under oxidative stress nucleolar localization of SPATS2L decreases, and it rapidly (within 1 h) colocalizes with stress granules. Stress granules are places where the products of halted protein synthesis accumulate, and they contain eIF3, eIF4E, eIF4G, small ribosomal subunits, mRNK transcripts, RNA-binding proteins, etc.22,23 The arrest of protein synthesis is one of the first cell responses to cellular stress.24,25 Zhu et al.18 also showed that knock-down of the SPATS2L gene resulted in an increase of protein synthesis, rather that its arrest, so it may be a critical regulator of the translation in stress responses.
Based on the data from previous literature, we may summarize that SPATS2L is a ubiquitous intranuclear protein closely related to stress responses; it can exit the nucleus in response to stress, and furthermore, it is involved in the earliest responses to stress. Therefore, it shares remarkable similarities with other thymic peptides precursors, such as prothymosin-α and LAP2 (see Introduction). Additionally, it corresponds with our present study that showed changes in the level of the 60 kDa thymulin precursor in response to various stresses. Indeed, expressions of both SPATS2L protein and 60 kDa protein are increased during the exposures to stress. Moreover, the comparison of anti-SPATS2L-stained bands and anti-thymulin-stained bands showed a strong correlation both in molecular weight and exposure-induced changes. Thus, there is a good chance that SPATS2L and the thymulin precursor may be identical.
On the other hand, there are some differences in the amino-acid sequences between thymulin and SPATS2L. SPATS2L protein contains (among others) the following sequence: PAKSQGGGN (mouse) or PAKSQGSGN (human). Thus, there are only two differences from thymulin (UAKSQGGSN): (1) the difference in amino-acids 7-8 (GS) of thymulin that are interchanged (SG) in the human SPATS2L or are GG in the mouse SPATS2L; and (2) P (proline) in SPATS2L instead of U (5-oxiproline or pyroglutamate) in thymulin. Thus, the former difference in residues 7-8 may reflect species specificity. As to the latter difference, there is room for speculation. For example, it was demonstrated that some factors such as ionizing radiation,26 UV irradiation,27 or exposure to a combination of copper, ascorbate, and oxygen28 led to oxidative proteolysis at proline residues. This proteolysis resulted in a protein fragment with some N-terminal oxidized form of proline.29 Dean et al. suggested that the oxidized proline form is a pyroglutamate (or 5-oxiproline), though they did not provide a clear confirmation. Similar oxidative proteolysis at proline residues has been demonstrated for several proteins such as the serum albumin, thyroid-stimulating hormone (TSH), and collagen.26,28,29
Thus, we can suggest that thymulin is a product of oxidative proteolysis of SPATS2L protein during exposure to oxidative stress, though any other mechanisms cannot be readily excluded at this time. Indirect support for this suggestion in the present study is that thymulin-containing intermediate production increased in oxidative stress, but remained unchanged in response to heat shock.
Therefore, our work has confirmed interesting relationships between immunomodulating, neuroendocrine, and intracellular roles of thymic peptides that may play an important role in the central regulation of immunity.
Acknowledgments
The authors thank Elsevier WebShop Language Service for help with language editing.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The work was supported by Program of Russian Academy of Sciences “Molecular and Cellular Biology” and by Russian Foundation for Basic Research (grant nos. 14-44-03558 and 16-34-00231).
References
- 1. Lunin SM, Novoselova EG. (2010) Thymus hormones as prospective anti-inflammatory agents. Expert Opinion on Therapeutic Targets 14: 775–786. [DOI] [PubMed] [Google Scholar]
- 2. Safieh-Garabedian B, Dardenne M, Pleau JM, et al. (2002) Potent analgesic and anti-inflammatory actions of a novel thymulin-related peptide in the rat. British Journal of Pharmacology 136: 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lunin SM, Khrenov MO, Novoselova TV, et al. (2008) Thymulin, a thymic peptide, prevents the overproduction of pro-inflammatory cytokines and heat shock protein Hsp70 in inflammation-bearing mice. Immunological Investigations 37: 858–870. [DOI] [PubMed] [Google Scholar]
- 4. Chen C, Li M, Yang H, et al. (2005) Roles of thymosins in cancers and other organ systems. World Journal of Surgery 29: 264–270. [DOI] [PubMed] [Google Scholar]
- 5. Novoselova EG, Lunin SM, Khrenov MO, et al. (2009) Involvement of NF-kappaB transcription factor in the antiinflammatory activity of thymic peptides. Doklady Biological Sciences 428: 484–486. [DOI] [PubMed] [Google Scholar]
- 6. Smith MR. (1994) Prothymosin alpha: In search of a function. Leukemia & Lymphoma 18: 209–214. [DOI] [PubMed] [Google Scholar]
- 7. Theodor L, Shoham J, Berger R, et al. (1997) Ubiquitous expression of a cloned murine thymopoietin cDNA. Acta Haematologica 97: 153–163. [DOI] [PubMed] [Google Scholar]
- 8. Dechat T, Vlcek S, Foisner R. (2000) Review: Lamina-associated polypeptide 2 isoforms and related proteins in cell cycle-dependent nuclear structure dynamics Journal of Structural Biology 129: 335–345. [DOI] [PubMed] [Google Scholar]
- 9. Gotzmann J, Vlcek S, Foisner R. (2000) Caspase-mediated cleavage of the chromosome-binding domain of lamina-associated polypeptide 2 alpha. Journal of Cell Science 113: 3769–3780. [DOI] [PubMed] [Google Scholar]
- 10. Buendia B, Santa-Maria A, Courvalin JC. (1999) Caspase dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. Journal of Cell Science 112: 1743–1753. [DOI] [PubMed] [Google Scholar]
- 11. Cohen GM. (1997) Caspases: The executioners of apoptosis. Biochemical Journal 326: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomez-Marquez J. (2007) Function of prothymosin alpha in chromatin decondensation and expression of thymosin beta-4 linked to angiogenesis and synaptic plasticity. Annals of the New York Academy of Sciences 1112: 201–209. [DOI] [PubMed] [Google Scholar]
- 13. Jiang X, Kim HE, Shu H, et al. (2003) Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science 299: 223–226. [DOI] [PubMed] [Google Scholar]
- 14. Ueda H, Fujita R, Yoshida A, et al. (2007) Identification of prothymosin-alpha1, the necrosis-apoptosis switch molecule in cortical neuronal cultures. Journal of Cell Biology 176: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsunaga H, Ueda H. (2010) Stress-induced non-vesicular release of prothymosin-α initiated by an interaction with S100A13, and its blockade by caspase-3 cleavage. Cell Death and Differentiation 17: 1760–1772. [DOI] [PubMed] [Google Scholar]
- 16. Leist M, Single B, Castoldi AF, et al. (1997) Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. Journal of Experimental Medicine 185: 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bach JF, Dardenne M, Pleau JM, et al. (1976) [Biochemical characterization of a circulating thymic factor] Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences. Serie D: Sciences Naturelles 283: 1605–1607. [PubMed] [Google Scholar]
- 18. Zhu CH, Kim J, Shay JW, et al. (2008) SGNP: An essential Stress Granule/Nucleolar Protein potentially involved in 5.8s rRNA processing/transport. PLoS One 3: e3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zatz MM, Goldstein AL. (1985) Thymosins, lymphokines, and the immunology of aging. Gerontology 31: 263–277. [DOI] [PubMed] [Google Scholar]
- 20. Dong Z, Patel Y, Saikumar P, et al. (1998) Development of porous defects in plasma membranes of adenosine triphosphate-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Laboratory Investigation 78: 657–668. [PubMed] [Google Scholar]
- 21. Lunin SM, Glushkova OV, Khrenov MO, et al. (2013) Thymic peptides restrain the inflammatory response in mice with experimental autoimmune encephalomyelitis. Immunobiology 218: 402–407. [DOI] [PubMed] [Google Scholar]
- 22. Mangel WF, McGrath WJ, Toledo DL, et al. (1993) Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature 361: 274–275. [DOI] [PubMed] [Google Scholar]
- 23. Kedersha N, Chen S, Gilks N, et al. (2002) Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Molecular Biology of the Cell 13: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bertolotti A, Zhang Y, Hendershot LM, et al. (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biology 2: 326–332. [DOI] [PubMed] [Google Scholar]
- 25. Williams BR. (2001) Signal integration via PKR. Science’s STKE: Signal Transduction Knowledge Environment 2001: re2. [DOI] [PubMed] [Google Scholar]
- 26. Schuessler H, Schilling K. (1984) Oxygen effect in the radiolysis of proteins. Part 2. Bovine serum albumin. International Journal of Radiation Biology and Related Studies in Physics, Chemistry, and Medicine 45: 267–281. [DOI] [PubMed] [Google Scholar]
- 27. Kato Y, Uchida K, Kawakishi S. (1992) Oxidative fragmentation of collagen and prolyl peptide by Cu(II)/H2O2. Conversion of proline residue to 2-pyrrolidone. Journal of Biological Chemistry 267: 23646–23651. [PubMed] [Google Scholar]
- 28. Bateman RC, Youngblood WW, Busby WH, et al. (1985) Nonenzymatic peptide alpha-amidation. Implications for a novel enzyme mechanism. Journal of Biological Chemistry 260: 9088–9091. [PubMed] [Google Scholar]
- 29. Dean RT, Wolff SP, McElligott MA. (1989) Histidine and proline are important sites of free radical damage to proteins. Free Radical Research Communications 7: 97–103. [DOI] [PubMed] [Google Scholar]