Abstract
The combined use of low and high molecular weight hyaluronic acid (HA) has never been reported in the treatment of osteoarthritis (OA). The aim of this paper was to evaluate the efficacy of a new hybrid association of both preparations in patients suffering from hip OA and to compare the results with those obtained retrospectively from a cohort of patients treated with high molecular weight HA.
Twenty patients with moderate-severe hip OA (grade II–IV according to Kellgren-Lawrence score) were enrolled in the study group. After clinical and functional evaluation (Visual Analogue Scale [VAS] for pain, Lequesne Index, Harris Hip Score), each participant received an intra-articular ultrasound-guided injection of the new HA compound at baseline and after 40 days. The measures were repeated at three and six months. The data collected were retrospectively compared with those obtained in a cohort of 20 patients, matched for sex, age, and severity of hip OA, treated with high molecular weight hyaluronic acid.
The intra-group comparison showed a significant improvement in clinical and functional outcomes at three and six months in both cohorts, while the infra-group comparison showed better results in the patients treated with the study compound at six months (VAS at rest, P <0.04; VAS during activities, P <0.02; Harris Hip Score, P <0.001).
The present study is the first which demonstrates that a combination of low and high molecular weight HA is effective and safe in the management of patients suffering from hip OA and provides better therapeutic results in comparison to high molecular weight HA. We may infer that both HA preparations work synergically, enhancing their positive activities.
Keywords: high molecular weight, hip, low molecular weight, osteoarthritis, safety profile
Introduction
Osteoarthritis (OA) is a very common joint disease characterized by pain and functional impairment, which, particularly in the elderly, may compromise overall health and quality of life.1 Recently, intra-articular injections of hyaluronic acid (HA) have gained consensus in the treatment of this pathologic condition because their positive outcomes (pain reduction and joint function improvement), without relevant side effects.2–6 The short-term HA therapeutic activity has been attributed to its high viscosity, which has a shielding effect on the articular surface (viscosupplementation),7 whereas the long-term efficacy is better explained by normalization of endogenous HA synthesis and chondroprotection (biosupplementation).1,7 At present, HA compounds with different molecular weights (MWs) are present in the market. These compounds differ for some characteristics. Indeed, the enhanced diffusion of low MW (LMW) preparations through the extracellular matrix of the synovium is thought to maximize the concentration and to facilitate the interaction with target synovial cells, thereby modulating better the synovial inflammation,1,7–9 whereas high MW (HMW) HA is more effective on the articular visco-elastic properties.1,7,8,10,11 In the normal synovial fluid, hyaluronans with different MW are present, suggesting that they cooperate in maintaining the articular homeostasis. Therefore, the administration of both LMW and HMW HA in the same preparation seems to be more physiologic and could provide better results. The technical problems, which, so far have made this approach impossible, have been solved linking HMW HA (1100–1400 KDalton) to LMW HA (80–100 KDalton) by weak hydrogen bonds in a dynamic hybrid complex, which favors a cooperative action between these HA preparations (chemically non-modified HA of bio-fermentative origin). This new patented compound could provide better anti-inflammatory and cell proliferation stimulating activities. Therefore, the aim of this paper was twofold: first, to report preliminary results about the efficacy and safety profile of this compound in patients suffering from moderate–severe hip OA; and second, to compare these results with those obtained retrospectively from a group of patients treated with HMW HA which is widely used in the treatment of this joint disease.
Participants and methods
The study was performed according to the Declaration of Helsinki and informed written consent was obtained from each patient prior to the inclusion.
Patients with moderate–severe hip OA (grade II–IV according to Kellgren-Lawrence [K-L] score on radiographic examination performed at least three months before), symptomatic for more than three months, were enrolled. Exclusion criteria were the following: recent hip trauma; intra-articular injection with steroids, HA, or platelet-rich plasma and/or treatments with steroids or non-steroidal anti-inflammatory drugs (NSAIDs) within the previous three months (acetaminophen was allowed); rheumatic pathologies (rheumatoid, psoriatic and reactive arthritis, arthritis associated with inflammatory bowel diseases, and spondiloarthritis), and severe systemic diseases (renal, hepatic, cardiac, infections, endocrinopathies, malignancies).
At baseline, demographic and anthropometric data were collected. In each participant, height and weight were measured and body mass index (BMI) was then calculated. Clinical and functional measures included pain at rest and during activities during the previous week (visual analogue scale [VAS]), Lequesne Index (LI), Harris Hip Score (HHS), and monthly consumption of NSAIDs (number of patients and tablets).
Afterwards, patients received an ultrasound-guided intra-articular injection of 2 mL HA (3.2%, 32 mg HWM and 32 mg LMW, not cross-linked) at baseline and after 40 days. Briefly, in sterile condition, using the antero-inferior approach, with the patient in the supine position and the hip in the neutral position, a 20-Gauge spinal needle was inserted at the base of the femoral neck. HA was then injected and its intra-articular placement verified by the direct visualization of the hyperechoic flow.
After each injection, the patients were asked to restrict the use of the leg for at least 24 h and to limit or stop heavy and painful hip activities; rest, ice packs, and acetaminophen (max 4 g/day) were allowed. However, NSAID intake was allowed as rescue medication when acetaminophen was ineffective. Moreover, patients were suggested to follow non-pharmacological recommendations such as weight loss and a rehabilitation protocol in order to recover and/or maintain hip range of motions and muscles strength. Adverse events (pain, swelling, redness, etc.) were registered.
The clinical and functional assessment was repeated after three months and the last injection at six months. Patient satisfaction was registered by means of a five-point Likert scale (1, not at all satisfied; 2, slightly satisfied; 3, somewhat satisfied; 4, very satisfied; 5, extremely satisfied).
The results obtained in this group were compared with those collected from a cohort of patients suffering from moderate–severe hip OA who had been treated beforehand with 2.5 mL HMW HA (2%, 50 mg; not cross-linked, MW 800–1200 KDalton). These patients were matched for sex, age, and K-L grade (II–IV), had followed the same administration schedule (first injection at baseline and second injection after 40 days), and had been evaluated with the same standard protocol.
Data are reported as mean ± standard deviation for continuous variables, whereas categorical and dichotomous variables are reported as frequencies and percentage. The significance level was determined at P <0.05. The two-sample Student’s t-test was used to compare continuous variables, when the distribution of data was normal; the Wilcoxon’s rank sum test was used otherwise. The χ2 test was used to evaluate associations between categorical data.
The values of clinical parameters registered at three and six months were compared with baseline values, in the study group and in the group of participants treated with HMW HA. Therefore, an infra-groups comparison was performed.
Results
Twenty patients were enrolled in both groups. No significant difference was observed in the demographic and clinical data at baseline (age, 63.6 ± 5.6 versus 63.3 ± 6.2; BMI, 24.6 ± 1.7 versus 24.4 ± 1.7; symptoms duration, 10.3 ± 3.8 versus 11.5 ± 4.2; VAS at rest, 2.2 ± 1 versus 2.4 ± 0.8; VAS after exercise, 4.5 ± 1 versus 4.4 ± 1.5; LI, 5.5 ± 3.1 versus 5.4 ± 3.3; HHS, 66.7 ± 16.5 versus 65 ± 14).
At three and six months, VAS scores, both at rest and during activities, decreased significantly in both groups; disability (LI) was also reduced and the HHS improved accordingly (Table 1). In the comparison between groups (study group versus controls), better results were observed in the patients treated with the new hybrid compound, both in terms of pain reduction (VAS at rest, P <0.04; VAS during activities, P <0.02) and improved function (HHS, P <0.001) at six months (Figures 1 and 2).
Table 1.
Clinical evaluation at three and six months in both groups.
| HI–LOW Hybrid HA | HMW HA | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 months | P * | 6 months | P * | 3 months | P * | 6 months | P * | |
| VAS rest | 1.4 ± 0.8 | 0.000 | 0.8 ± 0.8 | 0.000 | 1.6 ± 0.6 | 0.000 | 1.3 ± 0.5 | 0.000 |
| VAS activities | 3.3 ± 0.8 | 0.000 | 2.5 ± 0.8 | 0.000 | 3.5 ± 1.3 | 0.000 | 3.2 ± 1.2 | 0.000 |
| LI | 4.6 ± 2.6 | 0.000 | 3.9 ± 2.5 | 0.000 | 5.1 ± 3 | 0.002 | 4.9 ± 2.9 | 0.002 |
| HHS | 72.3 ± 12.1 | 0.001 | 81.3 ± 8.6 | 0.001 | 69.5 ± 12.5 | 0.0003 | 72.5 ± 12.1 | 0.001 |
Compared to baseline values.
Significant differences were also observed in the comparison between the three- and six-month values in both groups in all the clinical evaluations.
Figure 1.
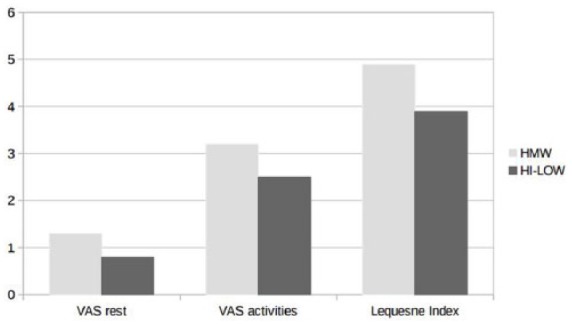
At six months, better results were observed in the HI–LOW Hybrid HA group.
Figure 2.
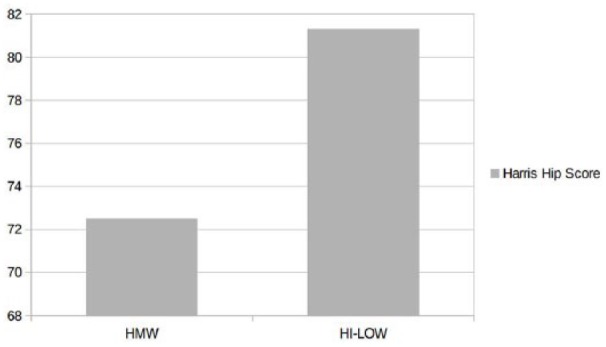
HHS at six-month follow-up in both groups.
At the final follow-up, compared to baseline, monthly NSAID consumption was reduced from five to three patients (tablets 1.4 ± 0.5 to 1 ± 0) and from four to three patients (tablets 1.5 ± 0.5 to 1 ± 0) in the study group and controls, respectively. Eleven (55%) and nine (45%) patients were extremely/very satisfied in the study group and controls, respectively.
In all patients, HA was always correctly placed into the articular space and adverse events were never observed (only slight discomfort after injection).
Discussion
The present study shows that the new HA compound (a combination of LMW and HMW HA) is safe and effective in the treatment of moderate–severe hip OA and provides better results in comparison with HMW HA.
The increased efficacy of the new preparation can be explained by the synergistic effects of different hyaluronans. Indeed, HMW HA retains, by means of its hydrophilic properties and the increased residence time into the joint, higher amounts of fluid in the articular space, thus improving viscoelastic properties (viscosupplementation);8–13 on the other hand, the enhanced penetration of LMW HA into the extracellular matrix of the synovium influences positively several articular biological activities, exploiting its immunosuppressive, anti-inflammatory, anti-apoptotic, anti-angiogenic, anti-fibrotic, and analgesic properties.10–14 Moreover, this combination mimics the physiologic composition of synovial fluid, where HA of different MW are present.
Some limitations of the study must be acknowledged. First, it is an observational trial including a limited number of patients with a short follow-up period (six months). Second, the results were retrospectively compared with HMW HA and not with LMW HA. Third, a placebo effect, which is usually in the range of 15–30%, cannot be ruled out. Moreover, preclinical studies regarding the effect of the new preparation in experimental models, such as those performed by Iannitti et al.15 in a rabbit model of collagenase-induced knee osteoarthritis, are desirable.
Another bias may be related to the conservative therapies (patient education, weight loss, exercise, etc.) we provided and, in a few cases, to the concomitant use of NSAIDs for short periods as rescue medication. Despite these limitations, our preliminary observations provide a reasonable basis for continuing on to perform a randomized controlled trial comparing this new compound with other treatments options.
In conclusion, this new HA compound is effective and safe in the management of patients suffering from mild–moderate hip OA. However, these findings need confirmation by controlled trials, with longer follow-up periods, and enrolling a larger number of patients, in order to analyze the effects in representative cohorts of different ages, BMI, and degree of hip joint pathology. Given that it is unknown which of the two components plays a major role, future studies should include three patient groups, treated with LMW HA, HMW HA, and this new compound, with comparable volume and injection schedules. Moreover, the efficacy of the new therapeutic agent on other OA joints should be evaluated.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Abate M, Pelotti P, De Amicis D, et al. (2008) Viscosupplementation with hyaluronic acid in hip osteoarthritis (a review). Upsala Journal of Medical Science 113: 261–277. [DOI] [PubMed] [Google Scholar]
- 2. Iannitti T, Rottigni V, Palmieri B. (2012) A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. International Journal of Immunopathology and Pharmacology 25: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 3. Henrotin Y, Raman R, Richette P, et al. (2015) Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Seminars in Arthritis and Rheumatism 45: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. Jevsevar D, Donnelly P, Brown GA, et al. (2015) Viscosupplementation for osteoarthritis of the knee: A systematic review of the evidence. Journal of Bone and Joint Surgery. American Volume 97: 2047–2060. [DOI] [PubMed] [Google Scholar]
- 5. Abate M, Scuccimarra T, Vanni D, et al. (2014) Femoroacetabular impingement: Is hyaluronic acid effective? Knee Surgery, Sports Traumatology, Arthroscopy 22: 889–892. [DOI] [PubMed] [Google Scholar]
- 6. Maheu E, Rannou F, Reginster JY. (2016) Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Seminars in Arthritis and Rheumatism 45(Suppl. 4): S28–33. [DOI] [PubMed] [Google Scholar]
- 7. Abate M, Pulcini D, Di Iorio A, et al. (2010) Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Current Pharmaceutical Design 16: 631–640. [DOI] [PubMed] [Google Scholar]
- 8. Rayahin JE, Buhrman JS, Zhang Y, et al. (2015) High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomaterials Science & Engineering 1: 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao N, Wang X, Qin L, et al. (2015) Effect of molecular weight and concentration of hyaluronan on cell proliferation and osteogenic differentiation in vitro. Biochemical and Biophysical Research Communications 465: 569–574. [DOI] [PubMed] [Google Scholar]
- 10. Euppayo T, Siengdee P, Buddhachat K, et al. (2015) Effects of low molecular weight hyaluronan combined with carprofen on canine osteoarthritis articular chondrocytes and cartilage explants in vitro. In Vitro Cellular & Developmental Biology. Animal 51: 857–865. [DOI] [PubMed] [Google Scholar]
- 11. Iannitti T, Morales-Medina JC, Coacci A, et al. (2016) Experimental and clinical efficacy of two hyaluronic acid-based compounds of different cross-linkage and composition in the rejuvenation of the skin. Pharmaceutical Research 33: 2879–2890. [DOI] [PubMed] [Google Scholar]
- 12. Abate M, Vanni D, Pantalone A, et al. (2015) Hyaluronic acid in knee osteoarthritis: preliminary results using a four months administration schedule. International Journal of Rheumatic Diseases. DOI: 10.1111/1756-185X.12572. [DOI] [PubMed] [Google Scholar]
- 13. Sato E, Ando T, Ichikawa J, et al. (2014) High molecular weight hyaluronic acid increases the differentiation potential of the murine chondrocytic ATDC5 cell line. Journal of Orthopaedic Research 32: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 14. Altman RD, Manjoo A, Fierlinger A, et al. (2015) The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskeletal Disorders 16: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iannitti T, Elhensheri M, Bingöl AO, et al. (2013) Preliminary histopathological study of intra-articular injection of a novel highly cross-linked hyaluronic acid in a rabbit model of knee osteoarthritis. Journal of Molecular Histology 44: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


