Abstract
Liver disease remains a significant global health problem. Increased caffeine consumption has been associated with a lower prevalence of chronic liver disease. This study aimed to investigate the modifying effects of caffeine on liver injury induced by thioacetamide (TAA) administration in male rats and the possible underlying mechanisms. Forty adult male rats were equally classified into four groups: control group, received only tap water; caffeine-treated group, received caffeine (37.5 mg/kg per day); TAA-treated group, received intraperitoneal (i.p.) TAA (200 mg/kg b.w.) twice a week; and caffeine + TAA-treated group, received combined TAA and caffeine in the same previous doses. After eight weeks of treatment, blood samples were collected for biochemical analysis and liver specimens were prepared for histological and immunohistochemical studies and for assessment of oxidative stress. TAA induced liver toxicity with elevated liver enzymes and histological alterations, fatty changes, apoptosis, and fibrosis evidenced by increased immunohistochemical reaction to matrix metalloproteinase-9 (MMP-9) and collagen type IV in hepatocytes. Also, the levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in serum were significantly elevated. Co-treatment with caffeine and TAA restored normal liver structure and function. Caffeine provided an anti-fibrogenic, anti-inflammatory, and antioxidant effect that was associated with recovery of hepatic histological and functional alterations from TAA-induced hepatotoxicity.
Keywords: caffeine, fibrosis, liver, MMP-9, TNF-α
Background
The liver is an important organ in the regulation of metabolic balance in the body and detoxification of harmful chemicals and drugs.1 Liver disease remains a significant global health problem that may result from variety of causes inducing oxidative stress, inflammation, and necrosis of liver cells.2 Thioacetamide (TAA), originally used as a fungicide, was reported to induce liver fibrosis and cirrhosis in experimental animals.3–6 Despite great advances in modern medicine, there is no effective drug available that protects the liver from damage or regenerates it.7
Caffeine is a liposoluble substance absorbed quickly and efficiently through the gastrointestinal tract with 100% bioavailability.8 It is present in many types of foods such as chocolate, teas, energetic drinks, and coffee. It is considered the most used psychoactive substance in the world and about 80% of the population makes daily use.9,10
The potential beneficial health effects of caffeine are controversial. Despite a common perception that coffee consumption may have negative health consequences, Lopez-Garcia et al.11 found that increasing coffee intake led to a reduced rate of cardiovascular death. Benoit et al.12 illustrated that moderate consumption of three to four cups of instant or roasted coffee per day, assuming an average caffeine concentration of 60–85 mg per cup, is good for human health. However, most of the previous studies on the beneficial properties of caffeine on humans have been observational. Scientists at the National Institutes of Health have reported that increased coffee consumption is associated with reduced incidence of hepatocellular carcinoma.13,14
Liver diseases are common heath problems that can lead to fibrosis and cirrhosis and different population surveys detected a possible beneficial association between coffee and liver disease with no adequate studying of liver structure and function. This study therefore aimed to investigate the possible effect of caffeine on TAA-induced liver disease in male rats and the possible underlying mechanisms.
Materials and methods
Drugs and chemicals
TAA and caffeine were purchased from Sigma Chemical Co, (St. Louis, MO, USA).
Animals
This study had been conducted in the Animal House of the Faculty of Medicine, Zagazig University, Egypt, in accordance with the guidance of Ethical Committee for Animal Handling at Zagazig University. Male albino rats (200–250 g) were used after two weeks for proper acclimatization to the standard housing conditions (25 ± 2°C temperature and 12-h light/dark cycle) and were supplied with standard rodent chow and tap water ad libitum.
Experimental design
Forty adult male rats were equally classified into four groups: group 1 (control group): received only tap water in the same amount given to the other groups; group 2 (caffeine-treated group): received caffeine 37.5 mg/kg per day by catheter;15 group 3 (TAA-treated group): received intraperitoneal (i.p.) TAA at a dose of 200 mg/kg b.w., twice a week;16 and group 4 (caffeine + TAA-treated group): received combined TAA and caffeine in the same previous doses.
The chosen caffeine dose in this study (37.5 mg/kg per day) is equivalent to 6 mg/kg per day in humans. It is estimated that an adult human (mean weight, 70 kg) with a moderate coffee intake consumes ~400 mg of caffeine per day, which is 6 mg/kg, per day.17 Furthermore, 6 mg/kg of caffeine is equivalent to three cups of coffee.18
All solutions were prepared daily and offered to the rats ad libitum in aluminum foil-wrapped bottles to avoid light decomposition. Body weight and food/liquids intake were measured daily throughout the experimental period.
After eight weeks of treatment, rats were sacrificed and blood samples were collected from the retro-orbital plexuses for biochemical analysis. Liver was carefully excised and cut into two specimens; one was fixed in 4% paraformaldehyde for histological and immunohistochemical studies and the other was stored in liquid nitrogen until analysis.
Biochemical assay
Blood samples were collected and centrifuged at 4000 rpm for 15 min. The serum was stored at −80°C for biochemical estimation and enzyme-linked immunosorbent assay (ELISA) analysis.
Assessment of liver function
For estimation of liver function, serum levels of transaminases (alanine transaminase [ALT], aspartate transaminase [AST]), gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), and total bilirubin were measured using a biochemistry autoanalyzer (Olympus AU2700, Japan).
Determination of inflammatory cytokines
To determine whether caffeine can suppress inflammation caused by TAA, the serum levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) were measured by ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Assessment of oxidative stress
Liver samples from all groups were homogenized with cold phosphate buffer (pH 7.4) and centrifuged at 4000 rpm for 30 min at 4°C. The supernatant was used to assess liver tissue malondialdehyde (MDA) and protein carbonyl (PCO), an indicator of lipid peroxidations and the activity of glutathione peroxidase (GSH-Px), and reduced glutathione (GSH). Also, thiobarbituric acid reactive substances (TBARS) enzyme levels were measured in tissue homogenate. Measurements were performed as described previously19,20 to determine the oxidative status in the liver specimens.
Histological analysis
Specimens for a light microscope were fixed in a 10% formalin solution and processed to prepare 5-μm thick paraffin sections for hematoxylin and eosin (H&E), Mallory trichrome, and immunohistochemical stains21 for detection of matrix metalloproteinase (MMP-9), collagen type IV, and antioxidant enzyme super oxide dismutase (SOD).
Immunohistochemical analysis was carried out using the streptavidin–biotin–peroxidase complex method. Paraffin sections were deparaffinized, rehydrated in descending grades of alcohol, and incubated overnight with the primary antibody. Sections were rinsed three times with phosphate buffered saline (PBS), then incubated for 1 h with peroxidase-conjugated secondary antibody, and washed three times with PBS. The reaction was developed with 0.05% diaminobenzidine (Dakopatts, Glostrup, Denmark) as the substrate for peroxidase, and finally, the slides were counterstained with Mayer’s hematoxylin. Negative control slides were prepared by replacing the primary antiserum with PBS. The primary antibodies used were: anti MMP-9 Antibody AV33090-100UG; Monoclonal anti-Collagen IV, Cat N≠ SAB4200500; and anti SOD; HPA001401-100UL. All were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Morphometric analysis
The image analyzer computer system Leica Qwin 500 (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK) was used to evaluate the area percentage of blue colored collagen fibers in Mallory trichrome stained sections and the area percentage of brown colored immune reaction of MMP and collage type IV. However, optical density of SOD immune reaction was detected in all studied groups. The area percentage and standard measuring frame of a standard area equal to 118. 476.6 were chosen to record ten readings from five sections/rat of randomly chosen five rats/group.
Statistical analysis
Results were expressed as means ± SEM. Statistical significant difference was determined by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. Probability values (P) less than 0.05 were considered to be statistically significant.
Results
General condition
As shown in Table 1, there was a significant reduction in body weight gain and food and liquid intake in TAA-treated groups, either alone or in combination with caffeine, throughout the experimental period, when compared with the control group. However, a significant difference was detected between the TAA-treated and caffeine + TAA-treated groups (P <0.05).
Table 1.
Effect of caffeine, TAA alone, or in combination on body weight (BW), food, and water consumption (n = 10).
| Control | Caffeine | TAA | Caffeine + TAA | |
|---|---|---|---|---|
| Initial BW (g) | 230 ± 16.7 | 228.5 ± 16.4 | 228 ± 17.1 | 230.7 ± 15.2 |
| Final BW (g) Reduction |
398 ± 27.1 0 |
376 ± 37.3 0 |
200 ± 18.2*
28 |
214 ± 9.2*†
17.7 |
| Food consumption (g/rat/day) | 22.4 ± 2.3 | 23.1 ± 1.9 | 15.2 ± 4.6* | 20.2 ± 1.5*† |
| Liquid consumption (mL/rat/day) | 25.44 ± 2.5 | 25.2 ± 2.1 | 20.2 ± 5.7* | 16.7 ± 2.4*† |
Data are presented as means ± SEM; n = number of rats in each group. Statistical analysis was done using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons.
P <0.05 vs. control and caffeine groups.
P <0.05 vs. control, caffeine, and TAA groups.
Biochemical results
Effect of caffeine on liver function
Statistical comparison among all studied groups as regards liver function tests revealed a non-significant difference between caffeine and control groups. In the TAA-treated group, serum levels of liver enzymes (ALT, AST, GGT, ALP) and total bilirubin were significantly increased, compared to controls (P <0.001), whereas in the caffeine + TAA co-treatment group, the enzyme levels were much lower than the TAA group (P <0.001) (Table 2).
Table 2.
Effect of caffeine, TAA alone, and in combination on liver enzymes in albino rats (n = 10).
| Control | Caffeine | TAA | Caffeine + TAA | |
|---|---|---|---|---|
| ALT (µ/L) | 48.90 ± 2.86 | 47.8 ± 2.43 | 782.27 ± 30.8* | 200.7 ± 25.59*† |
| AST (µ/L) | 98.54 ± 6.1 | 94.27 ± 9.2 | 987.54 ± 158.45* | 217.95 ± 20.57*† |
| GGT (µ/L) | 4.10 ± 0.19 | 3.89 ± 1.01 | 19.25 ± 1.01* | 6.98 ± 1.51*† |
| ALP (µ/L) | 319.80 ± 18.92 | 298.12 ± 25.3 | 2180 ± 135.2* | 306.05 ± 12.32*† |
| Total bilirubin (µm mol/L) | 6.31 ± 0.62 | 6.30 ± 0.56 | 19.68 ± 5.01* | 11.12 ± 0.85*† |
Data are presented as means ± SEM; n = number of rats in each group. Statistical analysis was done using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons.
P <0.001 vs. control and caffeine groups.
P <0.001 vs. TAA groups.
Effect of caffeine on pro-inflammatory cytokine response
As shown in Table 3, the levels of TNF-α, IL-1β, and IL-6 in serum were significantly elevated in TAA-treated rats compared with that of the control group (P <0.05). In contrast, co-treatment of caffeine with TAA decreased these pro-inflammatory cytokines levels, which suggested that caffeine inhibited the inflammatory response induced by TAA administration. However, caffeine per se had no effect on pro-inflammatory cytokines levels in serum.
Table 3.
Effect of caffeine, TAA alone, and in combination on pro inflammatory cytokines in albino rats (n = 10).
| Control | Caffeine | TAA | Caffeine + TAA | |
|---|---|---|---|---|
| TNFα (pg/mL) | 10.15 ± 5.15 | 9.05 ± 2.8 | 120 ± 16.7* | 40.4 ± 10.1*† |
| IL-6 (pg/mL) | 40.8 ± 12.3 | 32.9 ± 19.8 | 420 ± 15.8* | 201.5 ± 20.5*† |
| IL-1B | 20.8 ± 11.9 | 15.8 ± 5.4 | 460.05 ± 60.96* | 272.20 ± 84.32*† |
Data are presented as means ± SEM; n = number of rats in each group. Statistical analysis was done using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons.
P <0.001 vs. control and caffeine groups.
P <0.001 vs. TAA groups.
Effect of caffeine on oxidative stress biomarkers in liver tissue
TAA caused a substantial increase in liver MDA content with a concomitant significant fall in liver GSH content and GSH-Px compared to control group (Table 4). Administration of caffeine alone showed a non-significant decrease in liver MDA content while exhibited a significant increase in GSH content and significant increase in GSH-Px compared to control group (Table 4). Combined administration of caffeine and TAA resulted in a significant reduction in the liver MDA content with significant increase in liver GSH content and GHS-Px content compared to TAA-treated group (Table 4). There was also a statistically significant increase in the hepatic tissues levels of PCO in TAA-treated rats when compared to the control group (P <0.001). However, a significant decrease was found in the caffeine + TAA-treated group compared with the TAA-treated group.
Table 4.
Effect of caffeine, TAA alone, and in combination on lipid perioxidation and antioxidant enzyme activities in liver tissue (n = 10).
| Control | Caffeine | TAA | Caffeine + TAA | |
|---|---|---|---|---|
| MDA (nmol/mg protein) | 4.65 ± 0.15 | 4.57 ± 0.09 | 17.8 ± 0.5*,† | 7.9 ± 0.75*†‡ |
| GSH (umol/mg protein) | 130 ± 5.2 | 159 ± 3.4* | 67 ± 5.2*,† | 118 ± 4.4*†‡ |
| GSH-Px (U/mg protein) | 14.7 ± 0.5 | 19.12 ± 0.3* | 7.52 ± 0.6*,† | 12.34 ± 0.9*†‡ |
| PCO (nmol/g) | 25.45 ± 2.23 | 23.26 ± 3.16 | 33.62 ± 3.24*,† | 24.74 ± 5. 18‡ |
| TBARS (nmol/100 mg) | 1.28 ± 0.6 | 1.1 ± 0.7 | 2.31 ± 0.2*,† | 1.98 ± 0.4*†‡ |
Data are presented as means ±SEM; n = number of rats in each group. Statistical analysis was done using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons.
P <0.05 vs. control.
P <0.05 vs. caffeine groups.
P <0.05 vs. TAA groups.
Moreover, there was significant increase in the hepatic tissues levels of TBARS in TAA-treated rats in comparison to the control rats. However, a significant decrease was observed in the caffeine + TAA-treated group when compared with the TAA-treated rats.
Histological and morphometric results
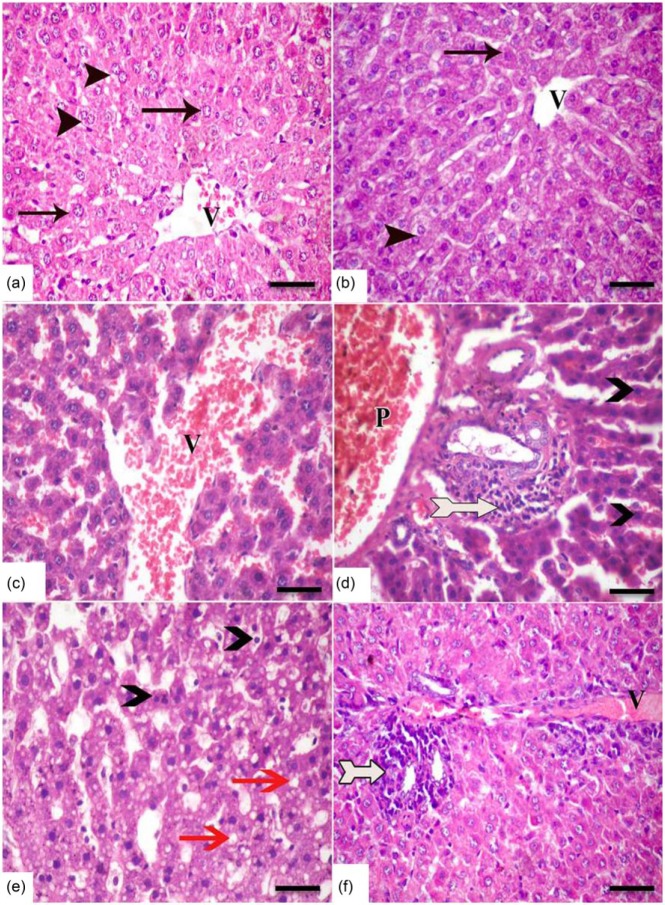
H&E-stained sections in rat liver from both the control and caffeine groups demonstrated normal hepatic architecture with hepatic cords radiating from clear central veins, and are separated by sinusoids, without inflammation or necrosis (Figure 1a, b). In contrast, the TAA-treated group showed significant changes in liver structure with vascular congestion of both central and portal veins and blood sinusoids. Also, infiltration of inflammatory cells in centrilobular regions was obvious (Figure 1c, d). Fatty changes and apoptosis were manifested in most hepatocyte parenchyma. Conversely, treatment with caffeine + TAA restored normal liver histology, although showing slight inflammatory cell infiltration and vascular congestion (Figure 1f).
Figure 1.
A photomicrograph of H&E-stained sections in rat liver of all studied groups. The (a) control group and (b) caffeine group showing cords of normal hepatocytes (arrows) with acidophilic stippled cytoplasm and vesicular nuclei radiating from central veins (v), and separated by sinusoids (s). Some hepatocytes (arrow head) are binucleated. (c–e) In the TAA-treated group, congestion of both central (v) and portal (p) veins and blood sinusoids was seen. Also, inflammatory cells (bifid arrow) heavily infiltrate the portal area. Well circumscribed cytoplasmic vacuoles indicating fatty changes (red arrow) and apoptotic darkly stained nuclei (arrow head) are manifested in most hepatocyte parenchyma. Conversely, co-treatment with caffeine and TAA restored normal liver histology, although the portal area still shows cellular infiltration (bifid arrow) and vascular congestion (v) (f) (scale bar = 50 µm).
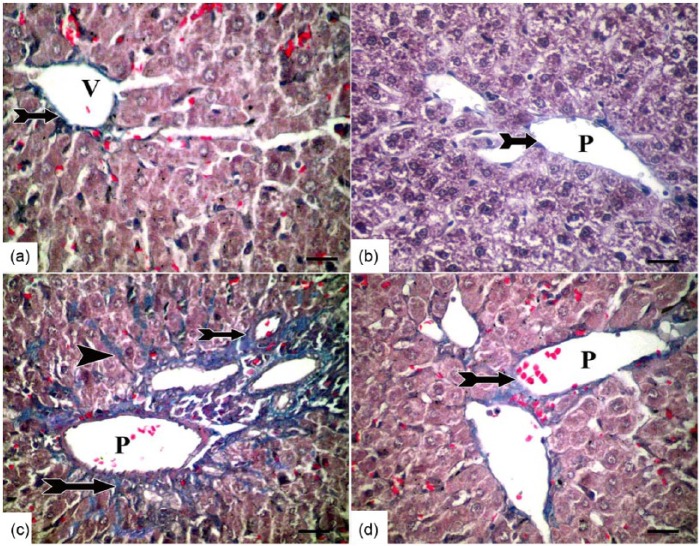
Examination of Mallory trichrome-stained sections (Figure 2) revealed few collagen fibers around the central and portal veins in both the control (Figure 2a) and caffeine (Figure 2b) groups. In the TAA group, the collagen fibers were markedly increased around portal tract and within liver parenchyma (Figure 2c). However, co-treatment with TAA and caffeine restored collagen distribution nearly similar to that of control group (Figure 2d).
Figure 2.
A photomicrograph of Mallory trichrome-stained sections in rat liver of all studied groups. Few collagen fibers (bifid arrow) are seen around the central (v) and portal (p) veins in both the (a) control and (b) caffeine groups. (c) In the TAA group, the collagen fibers are markedly increased around the portal tract (bifid arrow) and within the liver parenchyma (arrow head). (d) In the caffeine + TAA group, collagen distribution is nearly similar to the control group (Mallory trichrome, scale bar = 50 µm).
The previous results were confirmed morphometrically. The area percentage of blue stained collagen fibers was increased significantly in the TAA group compared with both the control and caffeine-treated groups. Also, caffeine administration in the caffeine + TAA group caused a significant decrease in the area percentage of collagen when compared with the TAA group (mean ± SEM, 0.24 ± 0.14 and 2.41 ± 0.52 in the caffeine + TAA and TAA groups, respectively; P <0.05) (Table 5).
Table 5.
Effect of caffeine, TAA alone, and in combination on histomorphometric results of all studied groups.
| Control | Caffeine | TAA | Caffeine + TAA | |
|---|---|---|---|---|
| Area % collagen MT | 0.21 ± 0.18 | 0.2 ± 0.15 | 2.41 ± 0.52* | 0.24 ± 0.14† |
| Area % MMP-9 immunoreactions | 1.9 ± 0.03 | 1.89 ±0.02 | 4.52 ± 1.5* | 2.3 ± 1.02† |
| Area % collagen IV immunoreactions | 0.02 ± 0.01 | 0.01 ± 0.09 | 1.29 ± 0.69* | 0.4 ± 0.01† |
| Optical density SOD immunoreactions | 0.43 ± 0.2 | 0.45 ± 0.3 | 0.1 ± 0.04* | 0.54 ± 0.3† |
P <0.05 vs. control and caffeine groups.
P <0.05 vs. control, caffeine, and TAA groups.
Immunohistochemical analysis revealed similar results in both the control and caffeine groups. The control group was therefore used to be compared with the other studied groups. Also, statistical analysis of the area percentage of brown stained immunoreaction of both MMP-9 and collagen type IV revealed non-significant changes between both groups (P >0.05) (Table 5).
Immunolocalization of MMP-9 in liver specimens revealed a positive cytoplasmic reaction in hepatocytes around the central veins in the control group (Figure 3a). The reaction was markedly increased after administration of TAA to involve all liver parenchyma (Figure 3b). In the caffeine + TAA group, the reaction was decreased compared with the TAA group, to be restricted to pericentral hepatocytes (Figure 3c). A significant increase in the area percentage of MMP-9 immunoreactions was confirmed by morphometric analysis in the TAA group when compared with the control and caffeine + TAA groups (mean ± SEM, 4.52 ± 1.5, 1.9 ± 0.03, 2.3 ± 1.02 for TAA, control, and caffeine + TAA, respectively) (Table 5).
Figure 3.
(a–c) Immunolocalization of MMP-9 (arrow) in liver specimens of all studied groups. (a) Control group showing cytoplasmic reaction in hepatocytes around central veins (v). The reaction is markedly increased after administration of TAA to involve all liver parenchyma (b). In the caffeine + TAA group (c), the reaction decreases to be restricted to pericentral hepatocytes (Immunoperoxidase; scale bar = 50 µm). (d–f) Immunolocalization of collagen type IV in liver specimens of all studied groups. No reaction is detected in hepatocytes of the control group (d). However, a strong reaction is seen in basement membrane of hepatocytes (arrow head) of the TAA-treated group (e, inset). Co-treatment with caffeine and TAA decreases the reaction as compared to TAA alone, to be restricted to pericentral hepatocytes (arrow head) (f) (Immunoperoxidase; scale bar = 50 µm). (g–i) Immunolocalization of superoxide dismutase (arrow) in liver specimens all studied groups. Cytoplasmic reaction is seen in the hepatocytes of the control group (g). In the TAA-treated group, no reaction is detected in liver parenchyma (h) while after co-administration of caffeine with TAA (i), hepatocytes reveal a strong cytoplasmic reaction (Immunoperoxidase; scale bar = 50 µm).
As regards immunolocalization of collagen type IV in liver specimens, no reaction was detected in the hepatocytes of the control group (Figure 3d). However, a strong reaction was seen in the hepatocytes’ basement membrane of the TAA-treated group (Figure 3e). Co-administration of caffeine with TAA decreased the reaction compared with TAA alone, to be restricted to pericentral hepatocytes (Figure 3f). Statistical analysis of morphometric study of this immune reaction revealed a significant increase in the TAA group when compared with the control and caffeine + TAA groups (mean ± SEM, 1.29 ± 0.69, 0.02 ± 0.01, and 0.4 ± 0.01, respectively).
Concerning immunolocalization of superoxide dismutase in liver sections, there was a cytoplasmic reaction in the hepatocytes of the control group (Figure 3g). In the TAA-treated group, no reaction was detected in liver parenchyma (Figure 3h), while after co-administration of caffeine with TAA, hepatocytes revealed a strong cytoplasmic reaction (Figure 3i). Statistical analysis of optical density of cytoplasmic reaction of SOD showed a significant decrease in the TAA group when compared with the control group (mean ± SEM, 0.1 ± 0.04, 0.43 ± 0.2, respectively; P <0.05). Administration of caffeine with TAA caused significant increase in the optical density of SOD immunoreaction (mean ± SD, 0.54 ± 0.3, P <0.05).
Discussion
This study was aimed at the evaluation of the protective utility of caffeine, the world’s most popular psychoactive substance, against TAA-induced liver injury in rats. TAA proves highly useful as an experimental liver injury model. It has been used for years as the lesions caused by this hepatotoxic drug are similar to most cases of liver diseases in human, which makes it a good model to study the mechanism in vivo.22
Per our results, TAA-induced liver toxicity in rats was evidenced by elevated liver enzymes and total bilirubin along with histological disturbance of liver tissue. The previous results were considered by Bahashwan et al.23 as an indication of structural and functional defects in liver cells.
Administration of caffeine with TAA in the present work restored normal liver architecture as well as decreased serum levels of liver enzymes and total bilirubin, compared with the TAA group. In agreement, Ruhl and Everhart24 reported that caffeine consumption (>2 cups per day) was associated with a lower risk of elevated ALT levels and a lower risk of chronic liver disease, compared with non-coffee drinkers. In addition, previous studies illustrated that coffee and some of its major components (caffeine) alter expression and activity of enzymes involved in xenobiotic metabolism in mice and rats, as well as human hepatoma cell lines.25,26
Rezaie et al.27 indicated that caffeine tended to prevent liver damage by maintaining the integrity of the plasma membrane, thereby suppressing the leakage of enzymes through membranes, exhibiting hepato-protective activity. This might be the reason for the restoration of the activities of the marker enzymes during administration of caffeine.
The biochemical findings in our work were supported by histopathological findings in liver specimens. Microscopic results in the TAA group showed different lesions including apoptosis, fatty degeneration, vascular congestion, and apparent fibrosis. TAA is proved by other research to induce fibrosis/cirrhosis associated with hepatocyte death and activation of Kupffer cells (KC) and hepatic stellate cells (HSCs).28–30 Fibrotic liver injury is an active process characterized by abnormal extracellular matrix (ECM) deposition and remodeling.31 Advanced liver fibrosis disrupts the liver’s normal architecture; hepatocytes are replaced with abundant ECM causing hepatocellular dysfunction and portal hypertension.32 Liver fibrosis is the result of extensive tissue remodeling with a net increase in ECM.28
Our study also indicated that TAA-induced liver toxicity is possibly mediated through generation of free radical and oxidative stress, indicated by elevated MDA, PCO, and TBARS. Also, decreased reduced glutathione and GSH-Px were detected in liver tissue and immuno-histochemical expression of SOD was apparently decreased in TAA-treated liver tissue.
TAA is biotransformed by CYP2E1 enzymes located in the microsomes of liver cells that convert it to a highly reactive toxic intermediate, known as thioacetamide sulphur dioxide through oxidation,33 inducing hepatotoxicity in experimental animals and different grades of liver damage including nodular cirrhosis and necrosis of parenchyma cells.34 Toxins target metabolically effective hepatic cells causing dysfunction of hepatocytes and discharge of inflammation-related cytokines.35 This was confirmed in our study by elevation of inflammatory cytokines IL-6, IL-1B, and TNF-α in blood samples of TAA-treated group.
Administration of TAA caused a significant increase in collagen fibers around the portal tract and within liver parenchyma associated with significant increase in expression of MMP-9 and collagen IV in the hepatocytes basement membrane. Similarly, Park et al.30 found that TAA caused an increase in the expression of MMPs and their endogenous inhibitors which were reported to be the key enzymes responsible for degradation and deposition of all the protein components of ECM and basement membrane.36,37 HSCs and hepatocytes produce, remodel, and turn over abnormal ECMs of fibrosis via MMPs.38 MMP-9 is critically involved in the degradation of collagen IV and fibronectin of basement membrane.39
Collagen IV is a major constituent of basement membranes in tissues.40 It is generated during liver fibrogenesis by the activated HSCs in the liver.41 Moreover, a close association was found between increased MMP level and increased collagen fibers in hepatocytes.42,43
In contrast, co-treatment with caffeine and TAA restored liver collagen distribution nearly similar to that of the control group, associated with a significant decrease in area percentage of matrix MMP-9 and collagen type IV immunohistochemical reactions in liver specimens, as compared with the TAA group. The assumption has been that reduced fibrosis was due to a reduction in disease activity as reflected by serum aminotransferase levels. However, most studies were population surveys, with no evaluation of liver histology. So, the possible antifibrotic effects of coffee/caffeine on liver were indirectly assessed. The distinction between anti-fibrogenic effects and protection against decompensation is important in understanding the underlying beneficial mechanism.44 With respect to caffeine specifically, Gressner et al. reported that caffeine interferes with transforming growth factor beta (TGF-β) thus inhibiting expression of connective tissue growth factor (CTGF).45 Caffeine was also found to upregulate peroxisome proliferator-activated receptor gamma (PPARγ) levels, which further reduce CTGF expression.44
Evidence has also shown that MMPs expression by HSCs is regulated in a cytokine-specific pattern.46 Our work supported this theory as the levels of MMP-9 were decreased together with decreased serum levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 in combined caffeine and TAA-treated rats as compared with that of the TAA alone group. Previous research reported marked stimulation of MMPs caused by TNF-α.39,46 Thus, the reduced MMPs activity may be linked to attenuation of the inflammatory and fibrotic processes.47
In this work, the antioxidant properties of caffeine were evidenced by increased liver content of reduced glutathione, GH-Px activity, and immunohistochemical expression of SOD together with decreased MDA, PCO, and TBARS levels, thus showing a reduction of membrane oxidative stress. It was reported that liver fibrosis/cirrhosis resulted from different factors that are associated with increased reactive oxygen species (ROS) production. Further, ROS are involved in the development of liver fibrosis/cirrhosis under these pathologic conditions.48,49 Supporting our results, previous research confirmed the antioxidant effects of coffee, especially its caffeine content, that prevent free radical tissue damage by reducing or eliminating ROS.47,50 Caffeine is rich in phytochemical derivatives such as triterpenes, flavonoids, or polyphenols. The preventive effects of caffeine could be attributed to their protective activity as kahweol and cafestol phenolic diterpenes of caffeine inhibit lipid peroxidation.51,52
In the current study, a significant increase in reduced glutathione and GH-Px in the liver tissue of the caffeine-treated group was detected without significant changes regarding MDA and SOD in this group in comparison to control group. Overall, the results obtained from previous studies about the role of coffee in the modulation of antioxidant enzymes in healthy individuals are conflicting. After coffee intake, healthy individuals reported an increase in GST activity in erythrocytes53 but not in saliva.54 Different types of coffee, doses, bioactive compounds, and duration of the study together with the chosen biomarker and the methods used for their evaluation could partially explain the observed variability among findings. More studies are necessary to understand the effect of coffee on oxidative stress markers in healthy individuals.55
In conclusion, the results presented here provide basic information that may explain the beneficial effects of caffeine on liver diseases observed in clinical trials. Caffeine provided an anti-fibrogenic, anti-inflammatory, and antioxidant effect that was associated with the recovery of hepatic histological and functional alterations from TAA-induced hepatotoxicity.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Koblihová E, Lukšan O, Mrázová I, et al. (2015) Hepatocyte transplantation attenuates the course of acute liver failure induced by thioacetamide in Lewis rats. Physiological Research 64: 689–700. [DOI] [PubMed] [Google Scholar]
- 2. Bishayee A, Darvesh AS, Politis T, et al. (2010) Resveratrol and liver disease: From bench to bedside and community. Liver International 30: 1103–1114. [DOI] [PubMed] [Google Scholar]
- 3. Al-Attar AM. (2011) Hepatoprotective influence of vitamin C on thioacetamide-induced liver cirrhosis in Wistar male rats. Journal of Pharmacology and Toxicology 6: 218–233. [Google Scholar]
- 4. Al-Attar AM. (2012) Attenuating effect of Ginkgo biloba leaves extract on liver fibrosis induced by thioacetamide in mice. Journal of Biomedicine & Biotechnology 2012: 761450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fatima SN, Mahboob T. (2013) Role of selenium in protection of liver cirrhosis. Pakistan Journal of Pharmaceutical Sciences 26: 1097–1102. [PubMed] [Google Scholar]
- 6. Shao CH, Chen SL, Dong TF, et al. (2014) Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. Journal of Surgical Research 186: 408–416. [DOI] [PubMed] [Google Scholar]
- 7. Chattopadhyay RR. (2003) Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. Journal of Ethnopharmacology 89: 217–219. [DOI] [PubMed] [Google Scholar]
- 8. Paluska SA. (2003) Caffeine and exercise. Current Sports Medicine Reports 2: 213–219. [DOI] [PubMed] [Google Scholar]
- 9. Clausson B, Granath F, Ekbom A, et al. (2002) Effect of caffeine exposure during pregnancy on birth weight and gestational age. American Journal of Epidemiology 155: 429–436. [DOI] [PubMed] [Google Scholar]
- 10. Sinha RA, Farah BL, Singh BK, et al. (2014) Caffeine stimulates hepatic lipid metabolism via autophagy-lysosomal pathway. Hepatology 59: 1366–1380. [DOI] [PubMed] [Google Scholar]
- 11. Lopez-Garcia E, Van Dam RM, Li TY, et al. (2008) The relationship of coffee consumption with mortality. Annals of Internal Medicine 148: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benoit S, Christophe C, Angelika T, et al. (2001) Health effects and safety considerations. In: Clarke RJ, Vitzthum OG. (eds) Coffee Recent Developments, Agricultural Series. Oxford: Blackwell Publishing Ltd, pp. 165–183. [Google Scholar]
- 13. Tverdal A, Skurtveit S. (2003) Coffee intake and mortality from liver cirrhosis. Annals of Epidemiology 13: 419–423. [DOI] [PubMed] [Google Scholar]
- 14. Gelatti U, Covolo L, Franceschini M, et al. (2005) Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: A case-control study. Journal of Hepatology 42: 528–534. [DOI] [PubMed] [Google Scholar]
- 15. Urzúa Z, Trujillo X, Huerta M, et al. (2012) Effects of chronic caffeine administration on blood glucose levels and on glucose tolerance in healthy and diabetic rats. Journal of International Medical Research 40: 2220–2230. [DOI] [PubMed] [Google Scholar]
- 16. Hasegawa R, Ogiso T, Imaida K, et al. (1995) Analysis of the potential carcinogenicity of coffee and its related compounds in a medium-term liver bioassay of rats. Food and Chemical Toxicology 33: 15–20. [DOI] [PubMed] [Google Scholar]
- 17. Nawrot P, Jordan S, Eastwood J, et al. (2003) Effects of caffeine on human health. Food Additives and Contaminants 20: 1 – 30. [DOI] [PubMed] [Google Scholar]
- 18. Donovan JL, DeVane CL. (2001) A primer on caffeine pharmacology and its drug interactions in clinical psychopharmacology. Psychopharmacology Bulletin 35: 30–48. [PubMed] [Google Scholar]
- 19. Ohkawa H, Ohishi N, Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95: 351e358. [DOI] [PubMed] [Google Scholar]
- 20. Moron MS, Despierre JW, Minnervik B. (1979) Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta 582: 67e78. [DOI] [PubMed] [Google Scholar]
- 21. Bancroft JD, Gamble M. (2002) Theory and practice of histological techniques. 5th ed. Philadelphia, PA: Churchill Livingstone, pp. 125–138. [Google Scholar]
- 22. Abul H, Mathew TC, Dashti HM, et al. (2002) Level of super-oxide dismutase, glutathione peroxidase and uric acid in thioacetamide-induced cirrhotic rats. Anatomia, Histologia, Embryologia 31: 66–71. [DOI] [PubMed] [Google Scholar]
- 23. Bahashwan S, Memy H, Hassan HA, et al. (2015) Crocin mitigates carbon tetrachloride-induced liver toxicity in rats. Journal of Taibah University Medical Sciences 10: 140–149. [Google Scholar]
- 24. Ruhl CE, Everhart JE. (2005) Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology 128: 24–32. [DOI] [PubMed] [Google Scholar]
- 25. Majer BJ, Hofer E, Cavin C, et al. (2005) Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2). Food and Chemical Toxicology 43: 433–441. [DOI] [PubMed] [Google Scholar]
- 26. Higgins LG, Cavin C, Itoh K, et al. (2008) Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicology and Applied Pharmacology 226: 328–337. [DOI] [PubMed] [Google Scholar]
- 27. Rezaie A, Pashmforosh M, Haghi M, et al. (2014) Hepatoprotective effect of caffeine on diethylnitrosamine-induced liver injury in rats. Bulgarian Journal of Veterinary Medicine 17: 183–190. [Google Scholar]
- 28. Hung KS, Lee TH, Chou WY, et al. (2005) Interleukin-10 gene therapy reverses thioacetamide-induced liver fibrosis in mice. Biochemical and Biophysical Research Communications 14: 324–331. [DOI] [PubMed] [Google Scholar]
- 29. Natarajan SK, Thomas S, Ramamoorthy P, et al. (2006) Oxidative stress in the development of liver cirrhosis: A comparison of two different experimental models. Journal of Gastroenterology and Hepatology 21: 947–957. [DOI] [PubMed] [Google Scholar]
- 30. Park SY, Shin HW, Lee KB, et al. (2010) Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in thioacetamide-induced chronic liver injury. Journal of Korean Medical Science 25: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bataller R, Brenner DA. (2005) Liver fibrosis. Journal of Clinical Investigation 115: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bataller R, Brenner DA. (2001) Hepatic stellate cells as a target for the treatment of liver fibrosis. Seminars in Liver Disease 21: 437–451. [DOI] [PubMed] [Google Scholar]
- 33. Kim KH, Bae JH, Cha SW, et al. (2000) Role of metabolic activation by cytochrome P450 in thioacetamide-induced suppression of antibody response in male BALB/c mice. Toxicology Letters 114: 225–235. [DOI] [PubMed] [Google Scholar]
- 34. Sadasivan S, Latha PG, Sasikumar JM, et al. (2006) Hepatoprotective studies on Hedyotis corymbosa (L.) Lam. Journal of Ethnopharmacology 106: 245–249. [DOI] [PubMed] [Google Scholar]
- 35. Mehendale HM. (2005) Tissue repair: An important determinant of final outcome of toxicant-induced injury Toxicologic Pathology 33: 41–51. [DOI] [PubMed] [Google Scholar]
- 36. Overall CM, Lopez-Otin C. (2002) Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nature Reviews. Cancer 2: 657–672. [DOI] [PubMed] [Google Scholar]
- 37. Consolo M, Amoroso A, Spandidos DA, et al. (2009) Matrix metalloproteinases and their inhibitors as markers of inflammation and fibrosis in chronic liver disease (Review). International Journal of Molecular Medicine 24: 143–152. [DOI] [PubMed] [Google Scholar]
- 38. Palladini G, Ferrigno A, Richelmi P, et al. (2015) Role of matrix metalloproteinases in cholestasis and hepatic ischemia/reperfusion injury: A review. World Journal of Gastroenterology 21: 12114–12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ram M, Sherer Y, Shoenfeld Y. (2006) Matrix metalloproteinase-9 and autoimmune diseases. Journal of Clinical Immunology 26: 299–307. [DOI] [PubMed] [Google Scholar]
- 40. Rowe RG, Weiss SJ. (2008) Breaching the basement membrane: Who, when and how? Trends in Cell Biology 18: 560–574. [DOI] [PubMed] [Google Scholar]
- 41. Gressner OA, Weiskirchen R, Gressner AM. (2007) Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. Journal of Cellular and Molecular Medicine 11: 1031–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friedman SL. (2003) Liver fibrosis: From bench to bedside. Journal of Hepatology 38: S38–S53. [DOI] [PubMed] [Google Scholar]
- 43. Hsu CC, Lai SC. (2007) Matrix metalloproteinase-2, -9 and -13 are involved in fibronectin degradation of rat lung granulomatous fibrosis caused by Angiostrongylus cantonensis. International Journal of Experimental Pathology 88: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Modi AA, Feld JJ, Park Y, et al. (2010) Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology 51: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gressner OA, Lahme B, Rehbein K, et al. (2008) Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPAR gamma and SMAD2/3-dependent pathways. Journal of Hepatology 49: 758–767. [DOI] [PubMed] [Google Scholar]
- 46. Knittel T, Mehde M, Grundmann A, et al. (2000) Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochemistry and Cell Biology 113: 443–453. [DOI] [PubMed] [Google Scholar]
- 47. Arauz J, Marina Galicia M, Pedro Cortés R, et al. (2013) Coffee attenuates fibrosis by decreasing the expression of TGF-b and CTGF in a murine model of liver damage. Journal of Applied Toxicology 33: 970–979. [DOI] [PubMed] [Google Scholar]
- 48. De Minicis S, Brenner D. (2008) Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. Journal of Gastroenterology and Hepatology 23: S98–S103. [DOI] [PubMed] [Google Scholar]
- 49. Perlemuter G, Letteron P, Carnot F, et al. (2003) Alcohol and hepatitis C virus core protein additively increase lipid peroxidation and synergistically trigger hepatic cytokine expression in a transgenic mouse model. Journal of Hepatology 39: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 50. Sanchez-Gonzalez I, Jimenez-Escrig F, Saura-Calixto F. (2005) In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter). Food Chemistry 90: 133–139. [Google Scholar]
- 51. Cavin C, Mace K, Offord EA, et al. (2001) Protective effects of coffee diterpenes against aflatoxin B1-induced genotoxicity: Mechanisms in rat and human cells. Food and Chemical Toxicology 39: 549–556. [DOI] [PubMed] [Google Scholar]
- 52. Huber W, Scharf G, Rossmanith W, et al. (2002) The coffee components kahweol and cafestol induce γ-glutamyl cysteine synthetase, the rate limiting enzyme of chemoprotective glutathione synthesis, in several organs of the rat. Archives of Toxicology 75: 685–694. [DOI] [PubMed] [Google Scholar]
- 53. Corrêa TAF, Monteiro MP, Mendes TMN, et al. (2012) Medium light and medium roast paper-filtered coffee increased antioxidant capacity in healthy volunteers: Results of a randomized trial. Plant Foods for Human Nutrition 67: 277–282. [DOI] [PubMed] [Google Scholar]
- 54. Steinkellner H, Hoelzl C, Uhl M, et al. (2005) Coffee consumption induces GSTP in plasma and protects lymphocytes against (+/-)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: Results of controlled human intervention trials. Mutation Research 591: 264–275. [DOI] [PubMed] [Google Scholar]
- 55. Martini D, Del Bo’ C, Tassotti M, et al. (2016) Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 21: E979. [DOI] [PMC free article] [PubMed] [Google Scholar]