Abstract
Oral immunotherapy (OIT) has been introduced as a new immune-modulating treatment under investigation for food allergies. The aim of our study was to evaluate the success of OIT in a cohort of children with milk allergy. These children underwent OIT in a clinical practice and were followed for up to ten years. The secondary endpoint was to describe the main adverse events during OIT and compare them to those reported in the literature.
Eighty-two milk-allergic children started OIT. According to the OIT endpoint reached after one year, all of the children enrolled in the study were divided into four groups: complete desensitization; partial desensitization; step down; and stop groups. Any adverse events that occurred during OIT were also recorded.
Of the 82 patients, eight were recruited in the last months of 2010 so they were still ongoing at the end of the study. For that reason, they were excluded from the analysis. The majority (73%) of the 74 children evaluated (51 boys, 23 girls; median age, 7 years; age range, 2–18 years; specific serum IgE for cow’s milk, 36 KUA/L [range, 3–100 KUA/L]; milk SPT wheal diameter, 7 mm [range, 2–15 mm]) reached complete (58.1%) or partial (14.9%) desensitization, 9.4% were subjected to step down. The remaining 17.6% of the children discontinued OIT because of the occurrence of chronic gastroenteric (GE) symptoms (46.1%) or acute asthma (15.3%) following milk intake.
In agreement with the literature, we found that chronic GE symptoms was the main reason for OIT discontinuation. OIT represents a valid tool for the treatment of food allergies in children; however, the risk of potential adverse reactions, both IgE- and non-IgE-mediated, should be discussed with parents prior to the initiation of OIT.
Keywords: asthma, children, desensitization, eosinophilic esophagitis, gastroenteric symptoms, milk allergy, oral immunotherapy
Introduction
Immune-mediated food reactions are classified into IgE-mediated, non-IgE-mediated, or mixed reactions. In IgE-mediated cases, allergen-specific immunoglobulin E (IgE) antibodies induce the activation of mast cells and basophils, which in turn causes the release of histamine. The onset of typical clinical food allergy symptoms (such as pruritus, hives, vomiting, abdominal pain, asthma, or even anaphylaxis) occurs within 30–60 min of ingesting the food product. Milk, peanuts, and eggs are the most frequent foods involved in pediatric cases of food allergies.1 In contrast, a T-cell response is considered responsible for non-IgE-mediated reactions, such as celiac disease, food protein-induced colitis or enterocolitis (FPIES), and eosinophilic esophagitis (EoE). These reactions are characterized by the delayed onset of predominantly gastroenteric (GE) symptoms. Thus far, the elimination diet remains the first line of treatment for these types of food allergies. More recently, oral immunotherapy (OIT) has been introduced as a new immune-modulating treatment aimed at inducing desensitization in IgE-mediated food allergies. OIT raises the tolerance threshold and may reduce the risk of severe IgE-mediated reactions (anaphylaxis) and improve a patient’s quality of life.2,3 In particular, a meta-analysis by Nurmatov et al.4 reported a significant reduction in the likelihood of reaction at a food challenge following the OIT compared to controls (risk ratio: 0.21, 95% confidence interval [CI], 0.21–0.38). However, there are several obstacles for the use of OIT worldwide, including: (1) the possibility of acute reactions in any phase of the desensitization protocol; (2) uncertainty about long-term efficacy; (3) the development of chronic non-IgE-mediated food allergies, such as EoE, once the food is reintroduced into the diet; and (4) compliance issues because a lack of patient compliance could lead to severe life-threatening reactions. For all these reasons OIT is still under investigation for food allergy. Several approaches have been proposed to improve the safety of OIT. These include: research on using alternative routes of exposure, modified hypoallergenic products and adjuvants for immunotherapy as well as on biomarkers and successful OIT to facilitate patient selection.5 The aims of our study were to evaluate the success of OIT in a cohort of 82 children with a severe milk allergy and to describe the main adverse events, both acute and chronic, during OIT comparing them with those reported in the literature.
Patients and methods
Over the course of five years (2005–2010), 82 children with a severe allergy to milk started OIT with milk based on the protocol of Mori et al.6 All of the children underwent skin prick tests (SPT) with fresh cow’s milk (30 mg/L), its progressive dilutions with saline solution (1/10 = 3 mg/mL; 1/100 = 0.3 mg/mL; 1/1000 = 0.03 mg/mL; 1:10,000 = 0.003 mg/mL; 1/100,000 = 0.0003 mg/mL; 1/1,000,000 = 0.00003 mg/mL), and serum-specific IgE detection (Immunocap) at baseline. The skin test is considered to be positive with a wheal skin reaction ⩾ 3 mm. We started OIT with the dilution immediately below the endpoint dilution (positive SPT) and we progressively increased the amount of milk administered every 20 min according to Mori et al.’s protocol.6 If the child had a reaction to the milk, we stopped the procedure for that day and we started again the next day using only half of the dose that provoked the allergic reaction. The end dose was defined as 100–150 mL cow’s milk, and this dose was reached in approximately six months. Typically, we performed the higher increments at the hospital, discharging the patient with half of the dosage reached. During oral desensitization therapy, the children did not receive pre- or co-treatment with antihistamines or corticosteroids.
All of the children had a personal history of anaphylactic reactions to milk within the last 6–12 months5 or a severe allergic reaction to very low doses of milk (< 0.6 mg of cow’s milk protein) and had milk-specific IgE detected in the serum or during a SPT.7
An allergy specialist collected the personal medical history of each patient, and the severity of all clinical reactions described by the parents was evaluated according to the current definition of a food allergy.8
Children were tested with a SPT for common inhalant and food allergens (i.e. pollen, mites, mold, cat and dog epithelia, milk, albumen, soy, wheat, cod fish, peanuts, and latex using commercial extracts at 0.1 mg/mL from Alk Abellò, Milan, Italy). The positive and negative controls for the SPT were obtained using histamine (10 mg/mL, ALK-Abellò, Milan, Italy) and normal saline, respectively.
All of the children who were found to be allergic to inhalants and were affected by asthma had controlled asthma prior to the initiation of OIT.9 In particular, asthmatic children did not require a bronchodilator in the previous six months, and the asthma was independent of the pollen season. Both parents gave written informed consent before their child began milk OIT and were made aware of the risks/benefits related to this type of treatment.
Based on the OIT endpoint reached, all of the enrolled children were divided into four groups: (1) the complete desensitization group: children tolerant to 100–150 mL of cow’s milk; (2) the partial desensitization group: children who tolerated 2–50 mL of cow’s milk and had never reached 100–150 mL of cow’s milk; (3) the step-down group: children who had to reduce their daily milk intake one to two months after reaching complete desensitization due to the occurrence of severe adverse events; and (4) the stop group: children who had to stop OIT because of severe acute or chronic reactions independent of the amount of milk already reached.
GE, respiratory, and skin symptoms during OIT were recorded in a diary and noted as dose-related. Acute symptoms were sometimes associated with treatments such as epi use, beta agonist, and antihistamines use. No endoscopies were performed.
The children were followed for one year (a mean time of six months), starting from OIT initiation. Those children who are still undergoing OIT were recruited in the last few months of 2010.
A 5–10-year follow-up interview by phone was performed.
The data were analyzed using a commercially available statistical software package (SPSS, Chicago, IL, USA). Data were analyzed using the Mann–Whitney–Wilcoxon test for independent samples. A P value < 0.05 was considered statistically significant.
Results
Eighty-two children evaluated (52 boys, 30 girls; age range, 2–18 years; median age, 7 years). The median serum-specific IgE level for cow’s milk in the study population was 36 KUA/L (range, 3–100 KUA/L). The median milk SPT wheal diameter was 7 mm (range, 2–15 mm). The atopic status of the children in the study is summarized in Table 1. Most of our patients (73%) achieved complete (43/74; 58.1%) or partial (11/74; 14.9%) desensitization to milk. After a mean time period of six months, the immunotherapy desensitization protocol had been discontinued in 17.6% of the children after they reached a daily dose in the range of 2–50 mL of cow’s milk. Reasons for discontinuation included chronic GE symptoms (46.1%) and acute asthma after milk intake during the OIT protocol (15.3%). In addition, 23% of the patients discontinued OIT due to poor compliance and 15.3% due to stress (mainly the fear of an adverse reaction) (Table 2). The GE symptoms most commonly described were abdominal pain, nausea, vomiting, reduced appetite, and dysphagia. In addition, 7/74 patients (9.4%) utilized a step-down protocol due to the presence of GE symptoms (71.4%) (Figure 1). We next examined differences in clinical characteristics among patients who achieved complete desensitization, partial desensitization, those who had to do the step-down therapy, and those who discontinued OIT.
Table 1.
Demographic characteristics of the studied population.
| Sex (M:F) | Median age (years) [range] | Wheal size milk P+P (mm) [range] | Milk-specific IgE (KUA/L) [range] | Aeroallergen-positive SPT | Baseline respiratory symptoms (allergic rhinitis or controlled asthma) | |
|---|---|---|---|---|---|---|
| Complete desensitization (n = 43) | 31:12 | 8 [2–18] | 8 [2–15] | 7.29 [3–100] | 62% | 33% |
| Partial desensitization (n = 11) | 6:5 | 9 [3–18] | 10 [3–12] | 31.4 [12–87] | 70% | 100% |
| Step down (n = 7) | 5:2 | 7 [3–12] | 7 [3–12] | 38.8 [3–74] | 62.5% | 12.5% |
| OIT discontinuation (n = 13) | 9:4 | 7 [2–17] | 10 [3–12] | 40.8 [3–87] | 77% | 15.3% |
OIT, oral immunotherapy; P+P, prick by prick; SPT, skin prick test.
Table 2.
Symptoms during OIT and follow-up at 5–10 years based on recall.
| Total (n; %) | Patients with adverse reactions (n; %) | GE vs. asthma (%) | FU at 5–10 years/symptoms (n; %) | |
|---|---|---|---|---|
| Complete desensitization | 43/74 (58.1%) | 20 (46.5%): mild symptoms (oral allergy syndrome, urticaria, rhinitis); 7 (16.2%): GE; 12 (27.9%): asthma; 2 (4.6%): asthma + GE; 2 (4.6%): anaphylaxis | 20.9% vs. 32.5% | 30/43 (69.76%): regular milk intake; 1/43 (2.3%): only baked milk; 12 (28%): no information |
| Partial desensitization | 11/74 (14.9%) | 3 (27.27%): GE; | 36.3% vs. 63.6% | 4 (36.3%): only baked milk; 7 (63.63%): milk avoidance; 3/11 (27.27%): GE symptoms; 1/11 (9%) asthma; 1/11 (9%): fear; 2/11 (18.18%): urticaria |
| 6 (54.54%): asthma; | ||||
| 1 (9%): asthma + GE; | ||||
| 1 (9%): anaphylaxis | ||||
| Step down | 7/74 (9.4%) | 3 (42.8%): GE; | 71.4% vs. 42.8% | 1 (14.2%): regular milk intake; 1 (14.2%): only baked milk; 2 (28.5%): milk products but no milk because of taste preference; 2 (28.5%) few milliliters of milk because of GE symptoms; 1 (14.2%): no information |
| 2 (28.5%): asthma + urticaria + GE; | ||||
| 1 (14.2%): asthma + urticaria; | ||||
| 1 (14.2%): no compliance | ||||
| OIT discontinuation | 13/74 (17.6%) | 4 (30.76%): GE; | 46.1% vs. 15.3% | 3 (23.07%): partial desensitization; 5 (38.46%): milk avoidance; 2 (15.3%): GE symptoms; 1 (7.69%): asthma; 2 (15.3%): anaphylaxis; 3 (23.07%): no compliance; 2 (15.3%): no information |
| 2 (15.3%): asthma + GE; | ||||
| 2 (15.3%): stress (fear of reactions); | ||||
| 2 (15.3%): anaphylaxis; | ||||
| 3 (23.07%): no compliance | ||||
| Ongoing | 8/82 (9.8%) | Not recorded | 3 (37.5%): regular milk intake; 1 (12%): only baked milk; 2 (25%): partial desensitization because of GE symptoms in one case and asthma in the other case; 2 (25%): milk avoidance; 1 (12.5%): anaphylaxis; 1 (12.5%): fear |
GE, gastroenteric; OIT, oral immunotherapy.
Figure 1.
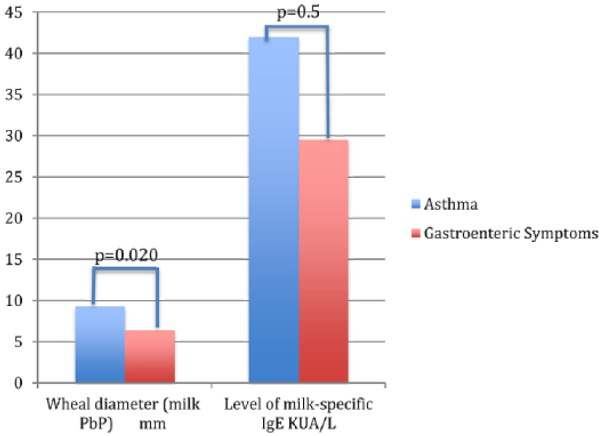
Differences in P values (P < 0.05 by the Mann–Whitney–Wilcoxon test) related to prick by prick (P+P) wheal diameter (mm) and level of milk-specific IgE (KUA/L) among children who develop GE vs. asthmatic symptoms.
The patients who achieved complete desensitization had fewer asthma symptoms at baseline (33% versus 100%, P < 0.001) and lower levels of milk-specific IgE (7.29 KUA/L [3–100] versus 31.4 KUA/L [12–87], P = 0.013) compared to patients with only partial desensitization to milk (Table 1). The patients with complete desensitization to milk had increased asthma symptoms at baseline (33% versus 15.3%, P = 0.16), a lower level of milk-specific IgE, as measured by both SPT wheal size (8 mm [2–15] versus 10 mm [3–12], P = 0.016) and by serum-specific IgE levels (7.29 KUA/L [3–100] versus 40.8 KUA/L [3–87], P = 0.004) compared to those who discontinued OIT (Table 1). Compared with patients who participated in step-down therapy, those who achieved complete desensitization had increased asthma symptoms at baseline (33% versus 12.5%, P < 0.05) and a lower level of milk-specific serum IgE (7.29 KUA/L [3–100] versus 38.8 KUA/L [3–74], P = 0.046) (Table 1). Compared with patients who discontinued OIT, those who achieved partial desensitization had increased asthma symptoms at baseline (100% versus 15.3%, P < 0.001), a similar level of milk-specific IgE, as measured by SPT wheal size (10 mm [3–12] versus 10 mm [3–12], P = 0.878), and lower levels of milk-specific serum IgE (31.4 KUA/L [12–87] versus 40.8 KUA/L [3–87], P = 0.477). The patients also tended to be older (9 years [3–18] versus 7 years [2–17], not significant) (Table 1). Compared with the patients who participated in step-down therapy, those who achieved partial desensitization to milk had increased asthma symptoms at baseline (100% versus 12.5%, P < 0.001), higher levels of milk-specific IgE, as measured by SPT wheal size (10 mm [3–12] versus 7 mm [3–12], P = 0.395), and lower levels of milk-specific IgE, as measured by serum-specific IgE (31.4 KUA/L [12–87] versus 38.8 KUA/L [3–74], P = 0.028) (Table 1). Finally, the patients who participated in step-down therapy had similar clinical characteristics to those who discontinued OIT. Overall, the most common side effects that prevented the completion or continuation of OIT were acute asthma and chronic GE symptoms. Those who became partially desensitized reported acute respiratory symptoms during OIT more frequently (asthma versus GE symptoms: 63.6% versus 36.3%). In contrast, those who completed step-down therapy or had to stop OIT developed chronic GE symptoms (71.4% and 46.1%, respectively) more frequently than those who were partially desensitized to milk at the end of the study (36.3%) (Table 1).
At the 5–10-year follow-up, most of the participants who had completed OIT continued to consume milk regularly (69.76%); 28% were lost at follow-up (Table 2). In contrast, most of the participants who were in the partial desensitization group tended to avoid milk after the 5–10-year period (63.63%). However, even those who had to reduce their daily milk intake after a few years were still drinking milk in small doses. Among those who discontinued OIT, only 23.07% reached a partial desensitization to milk after the 5–10-year period, and most of those children still had a free milk diet (38.46%). Interestingly, those with GE symptoms had lower levels of IgE specific to milk compared to those who developed asthma (Figure 1). Approximately one-third of the patients who were in the ongoing group during the period of recruitment reached complete milk desensitization when investigated after ten years. However, they were excluded from further analysis.
Discussion
Our results here are consistent with data reported in the literature. In fact, previous studies have suggested that children undergoing OIT for food allergies present GE symptoms in approximately 30% of cases10 and specifically in 10% of children treated with oral desensitization to milk.11,12 Also, respiratory symptoms (dyspnea, asthma) have been described due to OIT in approximately 10% of patients.10 These patients usually require a bronchodilator and steroid therapy but rarely stop OIT. In our experience, adverse respiratory reactions were the main cause of partial desensitization to milk. In agreement with the literature, the respiratory symptoms did not cause our patients to stop OIT. In our case study, in agreement with the literature, we found that chronic GE symptoms were the main reason why a patient would stop OIT. Interestingly, those who could not achieve complete oral desensitization had mainly acute reactions (such as anaphylaxis or asthma) as well as higher levels of milk-specific IgE. In contrast, those who achieved complete desensitization but had to step down or stop OIT mainly had GE symptoms and lower levels of milk-specific IgE, suggesting that there are two mechanisms that can lead to OIT failure: one is a failure to induce desensitization and stop the IgE-mediated reaction; and the other occurs when IgE-mediated reactions are successfully stopped but another type of reaction, most likely not mediated by IgE, occurs. Patients who are treated with OIT may develop immediate and delayed GE complications.10 The acute symptoms are fairly easy to explain as part of an IgE-mediated reaction.13 However, chronic GE symptoms are more difficult to understand. Mounting evidence suggests that OIT can induce EoE. In large milk-OIT studies, EoE has been reported in 2.7–10.3% of patients.12,14 The type of symptoms and their chronicity may be compatible with the development of EoE in our case study. This finding suggests that in children, the incidence of EoE following OIT may be higher than 2.7%.15 However, as described by Narisety et al., not all of the children showing symptoms consistent with EoE undergo an esophageal endoscopy, thus leading to an uncertain diagnosis.16 In our case, for example, many of the symptoms were so severe that we needed to step down or stop OIT. In conclusion, compared to other non-life-threatening adverse reactions, GE events appear to have the largest effect on the success of oral desensitization therapy. Several of the children in our study also showed late-onset GE symptoms (i.e. vomiting, abdominal pain) during OIT, with complete clinical remission after the discontinuation of OIT. For technical reasons, we could not perform endoscopies in these patients. Thus, the potential diagnosis of EoE remains a conjecture. To date, OIT represents a valid tool for the treatment of food allergies in children and can improve their quality of life. However, the risks of potential adverse reactions, with both IgE- and non-IgE-mediated food allergies, should be discussed with parents prior to the initiation of OIT. More recently OIT with hypoallergenic products or with adjuvants association could be proposed in high-risk patients.5,17
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Skripak JM, Matsui EC, Mudd K, et al. (2007) The natural history of IgE-mediated cow’s milk allergy. Journal of Allergy and Clinical Immunology 120(5): 1172–1177. [DOI] [PubMed] [Google Scholar]
- 2. Le HU, Burks AW. (2014) Oral and sublingual immunotherapy for food allergy. World Allergy Organization Journal 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pajno GB, Cox L, Caminiti L, et al. (2014) Oral immunotherapy for treatment of immunoglobulin E-mediated food allergy: The transition to clinical practice. Pediatric Allergy, Immunology, Pulmonology 27: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nurmatov U, Devereux G, Worth A, et al. (2014) Effectiveness and safety of orally administered immunotherapy for food allergies: A systematic review and meta-analysis. British Journal of Nutrition 111: 12–22. [DOI] [PubMed] [Google Scholar]
- 5. Vazquez-Ortiz, Turber O. (2016) Improving the safety of oral immunotherapy for food allergy. Pediatric Allergy and Immunology 27: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mori F, Pucci N, Rossi ME, et al. (2010) Oral desensitization to milk: How to choose the starting dose! Pediatric Allergy and Immunology 21: e450–e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Timothy K, Vander Leek. (2006) Diagnostic tests for food allergy: PST, CAP FEIA, and Oral Challenge Session # 2010. AAAAI March 2006. [Google Scholar]
- 8. Taylor SL, Hefle SL, Bindslev-Jensen C, et al. A consensus protocol for the determination of the threshold doses for allergenic foods: how much is too much? Clinical and Experimental Allergy 34: 689–695. [DOI] [PubMed] [Google Scholar]
- 9. Sampson HA, Munoz-Furlong A, Campbell RL, et al. (2006) Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/ Food Allergy and Anaphylaxis Network symposium. Journal of Allergy and Clinical Immunology 117: 391–397. [DOI] [PubMed] [Google Scholar]
- 10. Thomas M, Gruffydd-Jones K, Stonham C, et al. (2009) Assessing asthma control in routine clinical practice: Use of the Royal College of Physicians ‘3 questions’. Primary Care Respiratory Journal 18: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patriarca G, Nucera E, Pollastrini E, et al. (2007) Oral specific desensitization in food-allergic children. Digestive Diseases and Sciences 52: 1662–172. [DOI] [PubMed] [Google Scholar]
- 12. Stain ML, Golberg ML, Levy MB, et al. (2011) Non IgE mediated reactions to milk oral immunotherapy (MOI). Journal of Allergy and Clinical Immunology 127: AB30. [Google Scholar]
- 13. Zapatero L, Alonso E, Fuentes V, et al. Oral desensitization in children with cow’s milk allergy. Journal of Investigational Allergology and Clinical Immunology 18: 389–396. [PubMed] [Google Scholar]
- 14. Cianferoni A, Muraro A. (2012) Food-induced anaphylaxis. Immunology and Allergy Clinics of North America 32: 165–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanchez-Garcia S, Rodriguez del Rio P, Escudero C, et al. (2012) Possible eosinophilic esophagitis induced by milk oral immunotherapy. Journal of Allergy and Clinical Immunology 129: 1155–1157. [DOI] [PubMed] [Google Scholar]
- 16. Narisety SD, Skripak JM, Steele P, et al. (2009) Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. Journal of Allergy and Clinical Immunology 124: 610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vázquez-Ortiz M, Alvaro-Lozano M, Alsina L, et al. (2013) Safety and predictors of adverse events during oral immunotherapy for milk allergy: Severity of reaction at oral challenge, specific IgE and prick test. Clinical and Experimental Allergy 43: 92–102. [DOI] [PubMed] [Google Scholar]


