Abstract
The human vagina is colonized by a variety of microbes. Lactobacilli are the most common, mainly in healthy women; however, the microbiota composition can change rapidly, leading to infection or to a state in which potential pathogenic microorganisms co-exist with other commensals. In premenopausal women, urogenital infections, such as bacterial vaginosis and aerobic vaginitis, remain an important health problem. Treatment of these infections involves different kind of antibiotics; however, the recurrence rate remains high, and it must be also underlined that antibiotics are unable to spontaneously restore normal flora characterized by an abundant community of Lactobacilli. The main limitation is the inability to offer a long-term defensive barrier, thus facilitating relapses and recurrences.
We report here the antimicrobial activities of two commercially existing Lactobacillus strains, Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus GLA-14 strains and their combination (Respecta® probiotic blend) against four different pathogens responsible for both bacterial vaginosis (Gardenerella vaginalis and Atopobium vaginae) and aerobic vaginitis (Staphylococcus aureus and Escherichia coli) by co-culturing assay. The probiotic combination, even if resulting in a different microbicidal activity against the different strains tested, demonstrated the efficacy of combined Lactobacillus strain treatment.
Keywords: aerobic vaginitis, antimicrobial, bacterial vaginosis, Lactobacillus
Introduction
The human vagina is a complex environment colonized by a diverse community of microorganisms known as the vaginal microbiota; among these, Lactobacillus spp. represents the predominant microorganisms in the healthy vaginal ecosystem.1,2 Lactobacillus species are able to colonize and to produce antimicrobial substances acting to prevent the growth of pathogenic microorganisms.3 Alterations in the microbial composition of vaginal ecosystem are linked to several adverse health outcomes such as bacterial vaginosis (BV) and aerobic vaginitis (AV). BV is the most common vaginal infection worldwide, affecting women of all age groups, and is characterized by a vaginal pH of > 4.5, absence of inflammation, and by an overgrowth of anaerobic bacteria with Gardnerella vaginalis, Atopobium vaginae, Bacteroides spp., Mycoplasma hominis, Peptostreptococcus, and Prevotella being typically prevalent.4 AV is determined on the following criteria: enhanced yellow secretion; pH value ⩾ 5; negative amino-odor test; increased number of leukocytes; absence of Lactobacillus spp.; and microbiologically isolated microorganisms: mainly Escherichia coli, Staphylococcus aureus, group B streptococcus, and enterococci.5 Antibiotics are typically prescribed to treat BV whereas AV frequently requires combined local treatment with: antibiotic (infectious component); steroids (inflammatory component); and/or estrogens (atrophy component). Antimicrobial treatment is usually not fully effective due to antibiotic-resistant bacteria, or for the occurrence of re-infection. As antimicrobial therapy is often partially effective and antibiotics can also cause side effects,6,7 researches on alternative or complementary approaches represents a medical priority. Even if there are different studies demonstrating a significant improvement in treating bacterial vaginal infections with probiotics versus traditional treatments,6 results are often bacterial strain-specific suggesting that only certain probiotic bacteria seem to have effects against defined vaginal infections. In this study, we have analyzed the antimicrobial activity of two commercially existing probiotic strains, L. rhamnosus HN001 and L. acidophilus GLA-14, alone or in combination (Respecta® probiotic blend), against four different pathogens responsible for BV (G. vaginalis and A. vaginae) or AV (S. aureus and E. coli). Our results from mixed cultures with AV and BV pathogens strongly suggest that L. acidophilus GLA-14, alone or combined with L. rhamnosus HN001, can be used in probiotic products to prevent aerobic or anaerobic bacterial infections of the urogenital tract.
Materials and methods
Lactobacillus strains (L. acidophilus GLA-14®, L. rhamnosus HN001™) were stored in milk yeast extract (MYE) at −80°C. Before the experiments, each strain was transferred from the frozen stock culture to MRS (De Man Rugosa Sharpe) broth8 incubated at 37°C under non-agitated aerobic conditions. G. vaginalis and A. vaginae, obtained from University of Göteborg (Sweden) were cultivated anaerobically using the GasPak anaerobic envelope system (Becton Dickinson, Erembodegem, Belgium) at 37°C on Trypticase Soy Agar (TSA) + 5% sheep blood (Becton Dickinson). UPEC E. coli CFT073 (O6:K2:H1, ATCC700928)9 and S. aureus (ATCC29213) were cultured in Luria Bertani (LB) and Tryptone Soy (TSB) broths, respectively.
The capability of L. acidophilus GLA-14 and L. rhamnosus HN001 to interfere with the growth of the different pathogens was evaluated by a liquid co-culture assay in anaerobiosis or in aerobiosis, depending on the particular bacterial strain used.
The co-culture test was performed by incubating in Defined Medium Simulating Genital Tract Secretions (DMSGTS)10 (capable of sustaining the growth of both probiotics and pathogens) different concentrations of the probiotic strains (107 and 108 cfu/mL), alone or in combination, with different concentrations (106 and 107 cfu/mL) of the target pathogen. Controls were carried out by inoculating DMSGTS with the different strains alone.
Incubation was carried out for different lengths of time (range, 6–48 h). To check whether the pathogens were inhibited or killed, 0.05 mL of co-culture suspensions were diluted and seeded on specific agar medium. After an incubation period at 37°C for 24–48 h, bacterial growth was evaluated. No growth was interpreted as microbicidal activity (100% inhibition).
Statistical analysis was performed by Student’s t-test for unpaired data. Data were expressed as the mean and SD and P values of < 0.05 were considered significant.
Results
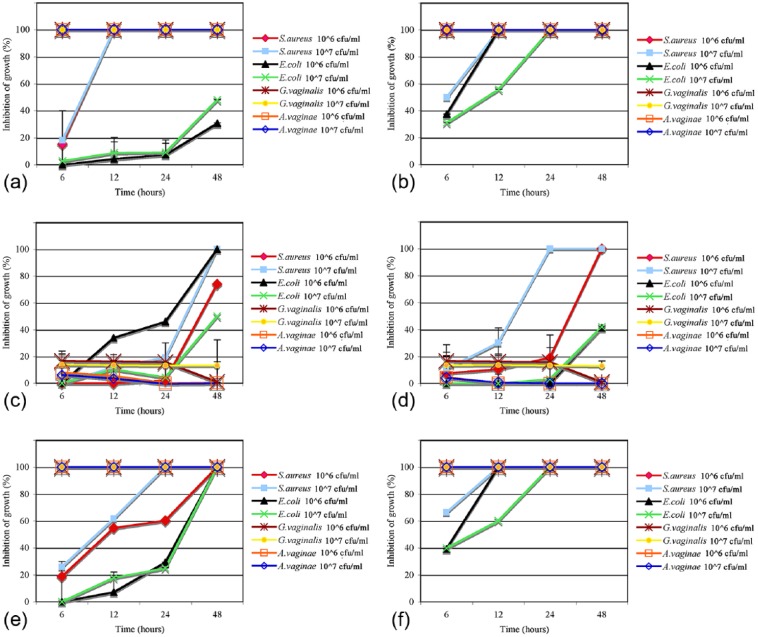
Results of co-culture assay have shown that the AV and BV pathogens were differently sensitive to the probiotics (Figure 1).
Figure 1.
Effect of probiotic strains on AV and BV pathogens in co-culture assay. Percentage of growth inhibition was calculated as the recovered pathogen bacteria at the different time points after incubation with probiotics, alone (a–d) or in combination (e, f), compared with the control cultures (pathogens alone) taken as 100%. (a) L. acidophilus 107 cfu/mL; (b) L. acidophilus 108 cfu/mL; (c) L. rhamnosus 107 cfu/mL; (d) L. rhamnosus 108 cfu/mL; (e) L. acidophilus 107 cfu/mL and L. rhamnosus 107 cfu/mL; (f) L. acidophilus 108 cfu/mL and L. rhamnosus 108 cfu/mL.
L. acidophilus GLA-14 was able to inhibit S. aureus growth after 6 h (Figure 1b) or 12 h (Figure 1a, b), whereas inhibition with L. rhamnosus HN001 was observed at 24 h (Figure 1d) and after 48 h (Figure 1c, d). The combination of both Lactobacilli (107 cfu/mL) with S. aureus inoculum (106 cfu/mL) caused complete inhibition of pathogen growth after 48 h (Figure 1e), whereas when the inoculum of S. aureus was higher (107 cfu/mL), complete inhibition of pathogen growth was observed after 24 h (Figure 1e). The combination of both Lactobacilli (108 cfu/mL) with S. aureus inoculum (106 cfu/mL) caused complete inhibition of pathogen growth since 6 h (Figure 1f), and when the inoculum of S. aureus was 107 cfu/mL, complete inhibition of pathogen growth was observed after 12 h (Figure 1f). L. acidophilus GLA-14 was more active than L. rhamnosus HN001.
L. acidophilus GLA-14 and L. rhamnosus HN001were differently active against E. coli (Figure 1a–d). L. rhamnosus (107 cfu/mL) was more effective than L. acidophilus (107 cfu/mL) against E. coli at 106 cfu/mL (Figure 1a, c) and their combination was synergic against E. coli (107 cfu/mL), inducing a complete inhibition of growth after 48 h (Figure 1a, c, e). A probiotic combination of 108 cfu/mL and an E. coli inoculum of 106 or 107 cfu/mL resulted in a complete inhibition of pathogen growth after 12 h and 24 h, respectively (Figure 1f). This probiotic combination of 108 cfu/mL seems have some slight effects after 6 h incubation with both aerobic pathogens (inoculum of 107 cfu/mL) (Figure 1b, f).
L. acidophilus GLA-14 alone (107 cfu/mL or 108 cfu/mL) was able to inhibit both concentrations of G. vaginalis and A. vaginae after 6 h (Figure 1a, b), whereas L. rhamnosus HN001 had little inhibitory activity (Figure 1c, d). As expected, the combination of both Lactobacilli showed the same inhibition degree of L. acidophilus GLA-14 alone on both anaerobic pathogens.
Taken together, the results obtained showed that L. acidophilus GLA-14 and L. rhamnosus HN001 (Respecta® probiotic blend) were able to inhibit the growth of all tested pathogens at different incubation time, depending on the initial inoculum of pathogen and, for anaerobic strains, from Lactobacillus strain concentrations.
Discussion
The vaginal microbiota is a dynamic ecosystem that in healthy individuals is usually colonized by the Lactobacillus genus but it can rapidly lead to microbiota dysbiosis where a range of microorganisms (such as G. vaginalis and A. vaginae or E. coli, S. aureus, and group B Streptococcus) become predominant and cause polymicrobial BV or AV, respectively.1,11 Incompetent diagnosis and antibiotic resistance, together with the elimination of some helpful bacteria7, are the main causes of the unsatisfactory results of conventional antimicrobic treatments of BV and AV. Evidence of decreased levels of Lactobacillus species in BV and AV has given rise to the concept of their replacement to restore the natural vaginal flora by utilizing probiotic strains. Probiotics, according to the World Health Organization definition, are “live microorganisms which when administered in adequate amounts confer a health benefit on the host.”12 Even though the use of probiotics to colonize the vagina and prevent or treat infection has been considered for some time, only recently their efficacy has been proven, and, different from that observed for antibiotics, no adverse effects have been reported.3 Here we studied the antimicrobial activity of two commercially probiotic strains, L. rhamnosus HN001 and L. acidophilus GLA-14, alone or in combination (Respecta® probiotic blend), against four different pathogens responsible for both BV (G. vaginalis and A. vaginae) and AV (S. aureus and E. coli). The tested probiotic bacteria showed that they possess inhibitory activity towards BV and, mainly, AV pathogenic bacteria, L. acidophilus GLA-14 having, in general, the highest antagonistic effect against anaerobic strains. Such an effect could be due to several mechanisms including the production of toxic compounds such as lactic acid, hydrogen peroxide, and bacteriocins that are enhanced in L. acidophilus rather than L. rhamnosus.13
Our results demonstrate that the Lactobacilli combination was synergic against E. coli, demonstrating that the association of two probiotic strains can be helpful to treat bacterial vaginal infections.
One promising lead towards the treatment of BV and AV is also the vagina colonization by Lactobacilli which forms a barrier against infection.14 In fact, in a recent pilot study it was demonstrated that oral consumption by healthy volunteers of the combination of the same probiotic strains utilized in the present research (L. acidophilus GLA-14 and L. rhamnosus HN001, together with bovine lactoferrin: Respecta® complex) leads to Lactobacillus spp. vaginal colonization.15
In conclusion, commercial probiotics, such as the ones examined here, represent very promising tools to provide protection from BV and AV.
Footnotes
Declaration of conflicting interests: The authors declared the following conflicts of interest with respect to the research, authorship, and/or publication of this article: RR is an employee of Giellepi S.p.A.
Funding: This work was supported by a grant from the “Women’s health: in vitro study of probiotic and prebiotic effect on aerobic vaginitis” ISS/ Giellepi S.p.A. agreement.
References
- 1. Ma B, Forney LJ, Ravel J. (2012) Vaginal microbiome: Rethinking health and disease. Annual Review in Microbiology 66: 371–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravel J, Gajer P, Abdo Z, et al. , et al. (2011) Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America 108: 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mastromarino P, Vitali B, Mosca L. (2013) Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiologica 36: 229–238. [PubMed] [Google Scholar]
- 4. Oakley BB, Fiedler TL, Marrazzo JM, et al. (2008) Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Applied and Environmental Microbiology 74: 4898–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donders G, Van Kalsteren K, Bellen G, et al. (2009) Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. International Journal of Obstetrics and Gynecology 116: 1315–1324. [DOI] [PubMed] [Google Scholar]
- 6. Menard JP. (2011) Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. International Journal of Women’s Health 3: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eade CR, Diaz C, Wood MP, et al. (2012) Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 7: e50106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Man JC, Rogosa M, Sharpe ME. (1960) A medium for the cultivation of lactobacilli. Journal of Applied Bacteriology 23: 130–135. [Google Scholar]
- 9. Mobley HL, Green DM, Trifillis AL, et al. (1990) Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: Role of hemolysin in some strains. Infection and Immunity 58: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geshnizgani AM, Onderdonk AB. (1992) Defined medium simulating genital tract secretions for growth of vaginal microflora. Journal of Clinical Microbiology 30: 1323–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang ZL, Fu LY, Xiong ZA, et al. (2016) Diagnosis and microecological characteristics of aerobic vaginitis in outpatients based on preformed enzymes. Taiwan Journal of Obstetrics and Gynecology 55: 40–44. [DOI] [PubMed] [Google Scholar]
- 12. Food and Agriculture Organization and World Health Organization Expert Consultation. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Córdoba, Argentina: Food and Agriculture Organization of the United Nations and World Health Organization; 2001. Available at: ftp://ftp.fao.org/es/esn/food/probio_report_en.pdf.
- 13. Todorov SD, Furtado DN, Saad SMY, et al. (2011) Bacteriocin production and resistance to drugs are advantageous features for Lactobacillus acidophilus La-14, a potential probiotic strain. New Microbiologica 34: 357–370. [PubMed] [Google Scholar]
- 14. Cadieux PA, Burton J, Devillard E, et al. (2009) Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. Journal of Physiology and Pharmacology 60:13–18. [PubMed] [Google Scholar]
- 15. De Alberti D, Russo R, Terruzzi F, et al. (2015) Lactobacilli vaginal colonisation after oral consumption of Respecta(®) complex: a randomised controlled pilot study. Archives of Gynecology and Obstetrics 292:861–867. [DOI] [PubMed] [Google Scholar]