Abstract
The aim of this study was to explore the expression and clinical significance of Foxp3 in colorectal tumor cells. An immunohistochemistry assay was used to detect the expression of Foxp3 in 173 cases of colorectal cancer. The relationship between the clinicopathological factors and the prognosis of colorectal cancer was analyzed. The rate of positive Foxp3 expression in tumor cells was 89.7%. There were no significant differences between cases with and without expression of Foxp3 with regard to sex, age, primary cancer sites, and distal metastasis. The expression of Foxp3 was negatively correlated with lymph node metastasis and pathological tumor, node, metastasis (pTNM) stage in tumor cells (P < 0.05), which reflects the depth of invasion. Foxp3 expression also had a positive correlation with the degree of differentiation (P < 0.01). A high level of Foxp3 expression was observed more often in tumor cells compared to tumor-surrounding tissues (P = 0.003). High expression of Foxp3 was also associated with longer overall and disease-free survival (P ⩽ 0.001). Foxp3 expression in colorectal cancer cells correlates with many clinicopathological characteristics; moreover, high expression of Foxp3 may be a promising potential prognostic factor for patients with colorectal cancer.
Keywords: colorectal cancer, Foxp3, prognosis
Introduction
Colorectal cancer is a malignant cancer with a high potential for invasion.1 In China, the incidence and mortality rates of colorectal cancer have been gradually increasing; these rates are higher than those for other cancers worldwide.2 The occurrence and outcomes of colorectal cancer are believed to be related to immune response.
As one member in forkhead family, Foxp3 is specifically expressed in Treg cell, including CD4+ CD25high Treg cells and CD8+ CD25high Treg cells.3 Treg cells are the most important factors for the development of immunological suppression and immune tolerance. Some studies have confirmed that high Foxp3 expression in Treg cells allows tumor cells to escape from immunological surveillance and promotes proliferation and development of tumor cells. Recent studies found that Foxp3, besides being a reliable maker of Treg cells, is also expressed by other kinds of cells, such as tumor cells and normal tissue cells, where it exerts a tumor-suppressing function.4,5
Previous studies indicated that the expression of Foxp3 in Treg cells was a prognostic factor in patients with colorectal cancer.6,7 A study with a small sample size indicated that a higher level of Foxp3 in colon cancer cells was associated with a poor prognosis,8 but our previous experiments showed contradictory results. Therefore, this study aimed to investigate the expression of Foxp3 in tumor specimens and normal tissue samples from patients with colorectal cancer and examine the relationship between Foxp3 expression, and clinicopathological factors and patient prognosis.
Materials and methods
Specimens
Colorectal tumor tissue and normal tissue samples from 174 patients with colorectal cancer confirmed by pathological diagnosis at Harbin Medical University Cancer Hospital from April to December 2009 were evaluated in this study. All specimens were obtained from patients who had not received any chemotherapy before surgery. Use of the tissue specimens was approved by the hospital ethics committee, and the patients provided informed consent. There were 94 men and 80 women, aged from 31 to 87 years. The median and mean ages were 62 and 61.4 ± 11.9 years, respectively. There were 49 cases of colorectal cancer, 116 cases of rectal cancer, and 9 cases of rectosigmoid cancer. There were 128 cases of well-differentiated and moderately differentiated cancer and 46 cases of poorly differentiated cancer. The postoperative pathological tumor, node, metastasis (pTNM) stage was stage I in 42 cases, stage II in 65, stage III in 54, and stage IV in 13. There were 13 and 161 patients with and without metastasis, respectively. The follow-up time from the pathological diagnosis date extended to 3 years later. A total of 30 of the 174 patients were lost to follow-up, leaving 31 patients who died and 113 who survived. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of X Medical University Cancer Hospital. Written informed consent was obtained from all participants.
Immunohistochemistry
Immunohistochemistry kits (HRP system; Beijing XiYa JinQiao Biological Technology Co., Ltd, Beijing, China) were used to detect the expression of Foxp3. All specimens were fixed with 10% neutral formalin and embedded in paraffin, and then 4-µm slices were mounted on slides and exposed to a mouse monoclonal anti-human Foxp3 antibody (ABGENT, Santiago, USA) at a dilution of 1:500. The staining procedure was performed according to the kit instructions. A negative control stained with phosphate-buffered saline instead of anti-Foxp3 and secondary antibody was included separately, and all specimens were observed under the microscope (×400).
Results evaluation
Positive staining for Foxp3 was defined as the observation of brown particles under the microscope (×400). The number of cells with positive nuclear staining was counted on five fields, and the mean value per field was calculated. The number of cells with cytoplasmic staining was obtained from five different fields and graded on a 4-point scale: negative (−), defined as a positive rate from 0% to 10%; weakly positive (+), from 11% to 25%; positive (++), from 26% to 50%; and strongly positive (+++), 50% or more. Pathological diagnosis and classification were conducted by two pathologists using a double-blind method.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software, with P > 0.05 denoting no significant statistical difference. Foxp3 expression according to the age and type of tissue was analyzed using the χ2 test. A t-test was used to compare groups, and a chi-square test was used for analyzing correlations. Survival analysis was analyzed using Kaplan–Meier curve, and difference among groups was assessed using Log-rank test.
Results
Expression of Foxp3 in colorectal tumor cells
As shown in Figure 1, Foxp3 was expressed in the nucleus or cytoplasm of 89.7% (156/174) of the colorectal tumor cases, including weak positivity in 34.0% (53/174), positivity in 39.1% (61/174), and strong positivity in 26.9% (42/174) of the cases.
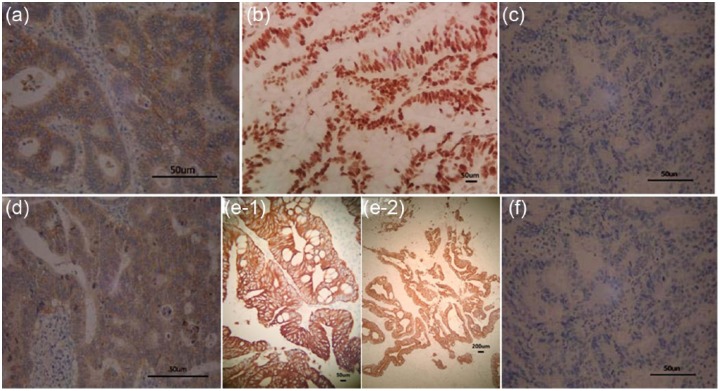
Figure 1.
Foxp3 in colorectal carcinoma by immunohistochemical staining (SP×400). (a) The positive expression of Foxp3 in cytoplasm of colorectal carcinoma cells; (b) the positive expression of Foxp3 in the cell nucleus of colorectal carcinomas; (c) the negative expression of Foxp3 in colorectal carcinoma cells (with phosphate-buffered saline instead of anti-Foxp3); (d) the weak positive expression of Foxp3 in colorectal carcinoma cells; (e) the strong positive expression of Foxp3 in colorectal carcinoma cells (e-1: 40×; e-2: 20×); (f) the negative expression of Foxp3 in colorectal carcinoma cells (with secondary antibody).
Relationship between the expression of Foxp3 in colorectal carcinoma cells and clinicopathological features
As shown in Table 1, Foxp3 expression was not correlated with sex, age, or tumor site of colorectal cancer patients (P > 0.05), but there was a correlation with other clinicopathological features, such as degree of differentiation, infiltrating depth, lymph node metastasis, and pTNM staging. As T stage increased, Foxp3 expression decreased, demonstrating that Foxp3 expression was related to T stage in colorectal cancer patients (P < 0.05); furthermore, Foxp3 expression was negatively correlated with N stage (P = 0.004) and pTNM (P < 0.05). Foxp3 expression was significantly lower in the poorly differentiated tumor group (P < 0.001).
Table 1.
The relationship between the expression of Foxp3 in colorectal carcinoma cells and clinical pathological features.
| Clinical pathological features | Tumor cells Foxp3 |
P | |
|---|---|---|---|
| − | + | ||
| Sex | |||
| Male | 10 | 84 (89.4%) | 0.890 |
| Female | 8 | 72 (90.0%) | |
| Age (years) | |||
| <60 | 10 | 70 (87.5%) | 0.389 |
| ⩾60 | 8 | 86 (91.5%) | |
| Tumor site | |||
| Colorectal | 9 | 39 (81.3%) | |
| Rectum | 8 | 109 (93.2%) | 0.072 |
| Rectosigmoid | 1 | 8 (88.9%) | |
| T stage | |||
| T1 + T2 | 1 | 48 (97.9%) | <0.05 |
| T3 + T4 | 17 | 108 (86.4%) | |
| N stage | |||
| NO | 6 | 106 (94.6%) | <0.01 |
| N+ | 12 | 50 (80.6%) | |
| Remote metastasis | |||
| M0 | 17 | 144 (89.4%) | 0.744 |
| M1 | 1 | 12 (92.3%) | |
| TNM stage | |||
| I + II stage | 6 | 101 (94.4%) | 0.010 |
| III + IV stage | 12 | 55 (82.1%) | |
| Degree of differentiation | |||
| Well/moderate differentiation | 5 | 123 (96.1%) | <0.01 |
| Poor differentiation | 13 | 33 (77.7%) | |
TNM: tumor, node, metastasis.
P < 0.05 represented there was a statistical significance.
Difference in Foxp3+ expression density between tumor cells and normal tissue cells and its influence on the prognosis of colorectal cancer patients
As shown in Figures 1 and 2 and Table 2, when Foxp3 expression was detected using the immunohistochemistry, a positive rate >25% was defined as high expression and <25% was defined as low expression. There were 103 cases of tumor cells with high Foxp3 expression (59.2%) and 75 cases of normal intestinal tissue cells with high Foxp3 expression (43.1%). This result showed that Foxp3 expression tended to be increased in tumor tissues, and this increase was significantly different from that observed in normal intestinal tissues (P = 0.003).
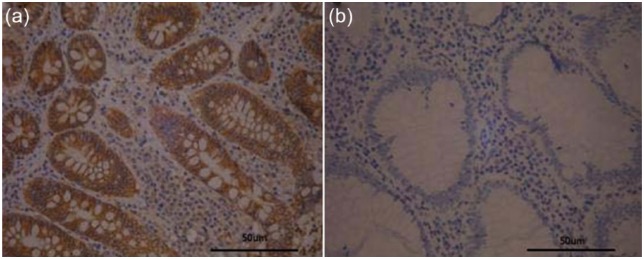
Figure 2.
Foxp3 in normal tissues from patients with colorectal cancer by immunohistochemical staining (SP×400). (a) The positive expression of Foxp3 in normal tissues from patients with colorectal cancer and (b) the negative expression of Foxp3 in normal tissues from patients with colorectal cancer.
Table 2.
Colorectal tumor cells and normal intestinal tissue cell Foxp3+ expression density comparison.
| Tissue | No. | Foxp3+ high expression | P value |
|---|---|---|---|
| Colorectal tumor cells | 174 | 103 (59.2%) | 0.003 |
| Normal intestinal tissue | 174 | 75 (43.1%) |
Relationship between Foxp3 expression and prognosis of colorectal cancer patients
As shown in Figures 3 and 4, all patients (174 cases) were followed up for 3 years, and the survival time ranged from 5 to 36 months. A total of 30 patients were lost to follow-up, which means that there were 144 patients with full data available, including 31 who died during the follow-up period. Foxp3 expression in tumor cells was divided into a high expression group (>25% positive cells per high-power field) and a low expression group (⩽25% positive cells per high-power field). There were 86 and 58 cases with high and low Foxp3 expression, respectively. The Kaplan–Meier method was used to analyze the median survival time, which was 30 and 26 months in the high and low expression Foxp3 groups, respectively. This implies that high Foxp3 expression is associated with a longer median survival time. An increase of Foxp3 expression in tumor cells was clearly associated with improved overall (P = 0.001; Figure 3) and disease-free survival (P < 0.001; Figure 4).
Figure 3.
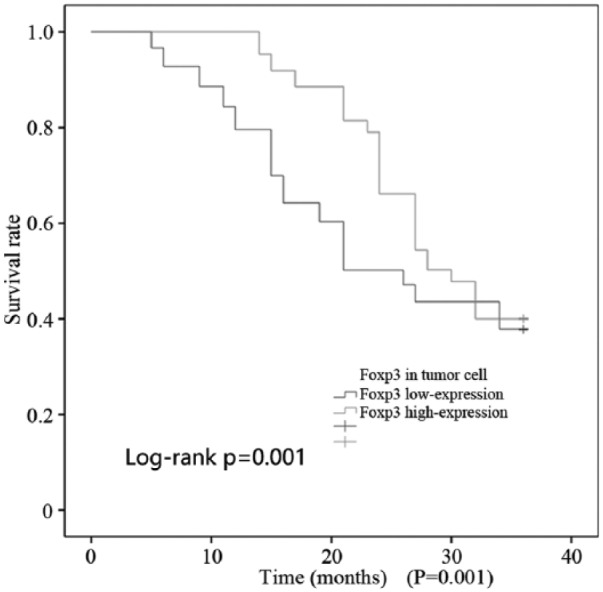
OS of colorectal cancer patients with Foxp3 high expression.
Figure 4.
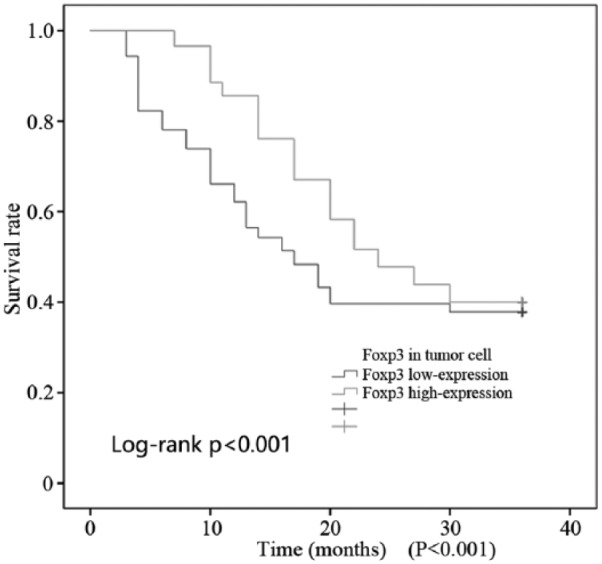
DFS of colorectal cancer patients with Foxp3 high expression.
In tumor tissues, Foxp3+Treg cellular infiltration simultaneous exists, and CD4+CD25+ is characteristic marker in Treg cells. Herein, we found that Foxp3 is expressed in infiltration cells, and we will perform study to mark CD4 and CD25 to future distinguish expression difference of Foxp3 between infiltration cells and tumor cells.
Discussion
The theory of tumor immune escape allows researchers to investigate the development of cancers from a new perspective. This study analyzed the correlation between the clinicopathological features and prognosis of colorectal cancer patients through detection of the expression of Foxp3 in colorectal tumor cells.
Studies showed that Foxp3 expression was different between normal and tumor tissue cells, with different expression levels, subtypes, and density. In this study, the positive expression rate of Foxp3 was 89.7% (156/174) in tumor cells and there was no correlation with sex, age, or tumor sites (P > 0.05); however, there was a significant correlation with the degree of differentiation, infiltrative depth, lymph node metastasis, and pTNM staging. To further explore the influence of Foxp3 expression on the prognosis of colorectal cancer, patients were classified into high (>25%) and low Foxp3 expression groups (⩽25%). Foxp3 expression was increased in tumor tissues relative to the surrounding tissue (P = 0.003).
Previous studies reported that Foxp3 expression in tumor cells has been shown to play an important role in the prognosis of many cancers.9,10 Our results show that higher Foxp3 expression in tumor cells was associated with improved disease-free survival and overall survival (P < 0.05; Figures 3 and 4). This result is in contrast to the results from the study by Kim et al.8 The main reason for this may be differences in sample size, experimental methods, and statistical analysis. The biological mechanism of action of Foxp3 in colorectal cancer tumor tissue is not clear. A previous study showed that Foxp3 can have an anti-tumor immune effect by inhibiting proto-oncogenes and activating the transcription of tumor suppressor genes.11 SKP2 is a proto-oncogene expressed by many tumors12 and can regulate cell division and proliferation in the G2/M phase of the cell cycle via SKP2-p27-CDK1/CDK2. Foxp3 is a transcriptional repressor of SKP2, and can inhibit SKP2 expression through interaction with the promoter of SKP2.4 In many tumors, a lack of Foxp3 expression leads to overexpression of SKP2 and cell cycle disorder, which causes loss of inhibition of cell proliferation and promotes tumorigenesis.13 Similarly, Chew et al.14 also proved that a higher expression of SPARC and Foxp3 have been associated with a good prognosis in stage II colorectal cancer, suggesting that Foxp3 may be a prognostic indicator in cancer. Accordingly, we speculate that these issues are important reasons for the inconsistencies in the conclusions of our study and that of Kim et al. Therefore, our study also provides relevant information for future study. To investigate the difference further, we will perform studies to discuss and explore its mechanism using multiple experimental methods.
To sum up, Foxp3 had an immune suppression effect in colorectal cancer cells, which supports the idea of a single role of Foxp3. Thus, we determined that Foxp3 has an important role in the development of tumor immunology. With further study, new anti-tumor immunotherapies could be developed to help advance the field of anti-tumor therapy.
Acknowledgments
X. S., Z. F., and Y. W. contributed equally to this study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Banskota S, Regmi SC, Kim JA. (2015) NOX1 to NOX2 switch deactivates AMPK and induces invasive phenotype in colon cancer cells through overexpression of MMP-7. Molecular Cancer 14: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Littman DR, Rudensky AY. (2010) Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140: 845–858. [DOI] [PubMed] [Google Scholar]
- 3. Fontenot JD, Rasmussen JP, Williams LM, et al. (2005) Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22: 329–341. [DOI] [PubMed] [Google Scholar]
- 4. Li W, Wang L, Katoh H, et al. (2011) Identification of a tumor suppressor relay between the FOXP3 and the Hippo pathways in breast and prostate cancers. Cancer Research 71: 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zuo T, Liu R, Zhang H, et al. (2007) FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. Journal of Clinical Investigation 117: 3765–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Z, Huang Q, Liu G, et al. (2014) Presence of FOXP3(+) Treg cells is correlated with colorectal cancer progression. International Journal of Clinical and Experimental Medicine 7: 1781–1785. [PMC free article] [PubMed] [Google Scholar]
- 7. Frey DM, Droeser RA, Viehl CT, et al. (2010) High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. International Journal of Cancer 126: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 8. Kim M, Grimmig T, Grimm M, et al. (2013) Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS ONE 8: e53630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winerdal ME, Marits P, Winerdal M, et al. (2011) FOXP3 and survival in urinary bladder cancer. BJU International 108: 1672–1678. [DOI] [PubMed] [Google Scholar]
- 10. Kashimura S, Saze Z, Terashima M, et al. (2012) CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer 15: 144–153. [DOI] [PubMed] [Google Scholar]
- 11. Triulzi T, Tagliabue E, Balsari A, et al. (2013) FOXP3 expression in tumor cells and implications for cancer progression. Journal of Cellular Physiology 228: 30–35. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama KI, Nakayama K. (2006) Ubiquitin ligases: Cell-cycle control and cancer. Nature Reviews Cancer 6: 369–381. [DOI] [PubMed] [Google Scholar]
- 13. Pagano M. (2004) Control of DNA synthesis and mitosis by the Skp2-p27-Cdk1/2 axis. Molecular Cell 14: 414–416. [DOI] [PubMed] [Google Scholar]
- 14. Chew A, Salama P, Robbshaw A, et al. (2011) SPARC, FOXP3, CD8 and CD45 correlation with disease recurrence and long-term disease-free survival in colorectal cancer. PLoS ONE 6: e22047. [DOI] [PMC free article] [PubMed] [Google Scholar]