Abstract
Finding new strategies to counteract periprosthetic infection and implant failure is a main target in orthopedics. Staphylococcus aureus, the leading etiologic agent of orthopedic implant infections, is able to enter and kill osteoblasts, to stimulate pro-inflammatory chemokine secretion, to recruit osteoclasts, and to cause inflammatory osteolysis. Moreover, by entering eukaryotic cells, staphylococci hide from the host immune defenses and shelter from the extracellular antibiotics. Thus, infection persists, inflammation thrives, and a highly destructive osteomyelitis occurs around the implant. The ability of serratiopeptidase (SPEP), a metalloprotease by Serratia marcescens, to control S. aureus invasion of osteoblastic MG-63 cells and pro-inflammatory chemokine MCP-1 secretion was evaluated. Human osteoblast cells were infected with staphylococcal strains in the presence and in the absence of SPEP. Cell proliferation and cell viability were also evaluated. The release of pro-inflammatory chemokine MCP-1 was evaluated after the exposure of the osteoblast cells to staphylococcal strains. The significance of the differences in the results of each test and the relative control values was determined with Student’s t-test. SPEP impairs their invasiveness into osteoblasts, without affecting the viability and proliferation of bone cells, and tones down their production of MCP-1. We recognize SPEP as a potential tool against S. aureus bone infection and destruction.
Keywords: implant infection, inflammation, osteoblast, serratiopeptidase, Staphylococcus
Introduction
Staphylococcus aureus is the first etiological agent of osteomyelitis, a severe infection characterized by progressive inflammation, bone tissue damage, and osteolysis.1,2 Although not considered as a typical intracellular pathogen, S. aureus was found capable to adhere to and to invade cultured osteoblasts, as well as osteoblasts and to osteocytes in vivo.3
The ability of S. aureus to infect bone is tightly correlated to its ability to bind bone extracellular matrix components, notably via multiple adhesins named microbial surface components. Through attachment, S. aureus can be internalized and survive within the cell switching notably to a small-colony variant (SCV) phenotype. The presence of SCV has been highly associated with the development of chronic and therapy-refractory infections.4 Then, S. aureus or its components may potentially modulate the production of cytokines and chemokines, through binding to extracellular and/or intracellular receptors. Infection with S. aureus eventually induces inflammatory cell recruitment, which, combined with increased osteoclastogenesis and osteoblast death, leads to a massive bone loss.5
The internalization within host cells acts protecting bacteria from the host immune response as well as from the medical treatments, as eukaryotic cells are often impermeable to conventional extracellular antibiotics.6 Furthermore, it was demonstrated that cultured osteoblasts infected with bacteria secrete immune modulators of the inflammatory response, namely, chemokine and cytokine molecules, which trigger and feed bone inflammation and destruction.7,8
In this study, we investigate new approaches that might be used to counteract internalization of osteoblasts by S. aureus and the consequent inflammatory stimulation of the osteoclastogenesis process. We focus our attention on serratiopeptidase (SPEP), an extracellular metalloprotease already used as an anti-inflammatory agent.9 Moreover, it has also been shown to modulate adhesin expression and to enhance antibiotic efficacy toward biofilm-forming bacteria, and to interfere with S. aureus adhesion to both abiotic surfaces and epithelial cells.10,11
Methods
Bacterial strains and culture conditions
Bacterial strains used in this work were the following: S. aureus ATCC 6538P (DSMZ 346), reference strain for antimicrobial testing; S. aureus ATCC 25923 (DSM 1104), clinical isolate and reference strain for antimicrobial testing; S. aureus ATCC 12598 (DSM 20372), clinical isolate from septic arthritis and reference strain belonging to ATCC collection; and S. aureus pi99, clinical isolate from orthopedic implant infection. Strains used were already characterized for genes coding for surface proteins relevant for adhesion and internalization to eukaryotic cells.10 Brain heart infusion broth (BHI; Oxoid, UK) was used for the planktonic cultures of S. aureus strains, which were grown under vigorous agitation at 37°C.
Eukaryotic cells
The human osteoblast cell line MG-6312 was cultured in minimal essential medium with Earle’s balanced salt solution (MEM/EBSS), supplemented with 10% fetal calf serum (FCS), 1% glutamine and 1% penicillin–streptomycin in an atmosphere of 95% air, and 5% CO2 at 37°C. All media were from Euroclone, Italy. Monolayers were used 48 h after seeding.
Chemicals
SPEP (2540 U mg−1), obtained from Takeda Italia Farmaceutici (Italy), was dissolved in phosphate-buffered saline (PBS; pH 7.2) at a stock concentration of 20,000 U mL−1 and stored at −20°C.
Cellular infections in vitro
Staphylococcal strains from 18 h cultures in BHI broth, were grown in the absence of SPEP. Subsequently, bacteria were diluted at 1:100 in BHI and sub-cultured up to OD600 = 1.0 at 37°C with or without 200 U mL−1 SPEP (SPEP-pretreated and SPEP-untreated bacteria, respectively). Before infection, human osteoblast cells were maintained in basal medium containing 10% FCS without antibiotic (penicillin–streptomycin) for 24 h at 37°C and 5% CO2. Subsequently, human cells cultured in 24-well plates (BD Falcon, USA) to obtain semi-confluent monolayers (1.75 × 105 cells/well) were separately infected with 0.05 mL of SPEP-pretreated and SPEP-untreated bacterial suspension at a multiplicity of infection (MOI) of about 30 bacteria per cell (MOI 30:1) for 2 h at 37°C and 5% CO2.
After incubation, osteoblast cell monolayers were washed with PBS, and 0.5 mL of fresh medium containing 50 µg/mL of gentamicin was added to each well and incubated for 1 h at 37°C and 5% CO2 to kill extracellular bacteria. First, the sensitivity of bacteria to gentamicin was verified, as well as the non-toxicity toward MG-63 cells. Cells were then lysed by the addition of 0.025% Triton X-100 and the collected supernatants were plated on tryptic soy agar (TSA; Oxoid, UK) followed by an overnight incubation at 37°C to count viable intracellular bacteria. The internalization efficiency is expressed as the percentage of the inoculated bacteria. Data represent the mean of three independent experiments.
Cell proliferation and cell viability
For cell proliferation experiments, osteoblasts were incubated with staphylococcal strains. The infection was performed as previously described. After incubation, the number of living cells was determined by colorimetric 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma-Aldrich, Italy). After removing the supernatant, the resulting intracellular purple formazan was solubilized with dimethyl sulfoxide (DMSO) and spectrophotometrically quantified at 560 nm. Uninfected osteoblasts were used as a control. All experiments were performed with or without SPEP. The results were expressed as the mean percentage in comparison with no infected cells. The cell viability was also assessed by Trypan Blue exclusion dye.13
Measurement of cytokine concentration
The release of pro-inflammatory chemokine MCP-1 was evaluated after the exposure of the osteoblast cells to staphylococcal strains. Following the incubation, the concentration of the chemokine was determined in the culture supernatants by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s recommended procedure (Thermo Fisher, Life Technologies Italia Fil, Italy). As positive control, cells stimulated with lipopolysaccharide (LPS) from Escherichia coli 055:B5 (Sigma-Aldrich) were used.
Statistical analysis
The significance of the differences in the results of each test and the relative control values was determined with Student’s t-test. Values of p < 0.05 were considered statistically significant. The data are the means of three independent experiments.
Results
SPEP action on staphylococcal internalization of osteoblasts
Preliminary experiments were addressed to assess the cytotoxicity of SPEP on MG-63 cells using the same SPEP concentration adopted in the internalization assays (200 U mL−1). Cell morphology, viability, and proliferation remained unaffected by SPEP treatments.
At first, the ability of S. aureus strains to invade MG-63 cells was evaluated. The four strains of S. aureus turned out endowed with a moderate capability to internalize. The internalization efficiency of untreated S. aureus strains is reported in Figure 1. The internalization efficiency of S. aureus was slightly differing depending on the strain (0.23% ± 0.00% for S. aureus 6538P; 0.40% ± 0.17% for S. aureus 25923; 0.45% ± 0.00% for S. aureus 20372; and 0.79% ± 0.29% for S. aureus pi99, see Figure 1).
Figure 1.

Effect of SPEP on cell internalization. MG-63 cells were infected with S. aureus strains at an MOI of 30:1 pretreated and untreated with SPEP, respectively. Internalization efficiency is expressed as the percentage of the initial inoculum of internalized bacteria that were gentamicin resistant 1 h post-infection. Data represent the mean of three independent experiments (*p < 0.05).
In the subsequent experiments, the internalization efficiency of SPEP-pretreated bacteria was compared with that of SPEP-untreated bacteria. Conversely, when bacteria were pretreated with SPEP, they showed a different behavior. In particular, as shown in Figure 1, the internalization efficiency was neatly reduced for all SPEP-pretreated S. aureus strains, and with the only exception of the S. aureus 20372 strain, the reduction by the SPEP treatment turned out statistically significant (Figure 1).
SPEP did not interfere with the internalization process of S. aureus in MG-63 cells when it was added on the cells during the infection (data not shown). In a previous work, we performed a genetic analysis of these bacterial strains in order to highlight the presence of genes coding for surface proteins affecting the adherence capability. Despite results obtained here, S. aureus 20372 strain showed the same protein pattern in terms of Atl, FnbA, ClfA, and SdrC of other tested strains.10
Results obtained seemed to stress the hypothesis the effect of SPEP is on bacterial invasive features rather than on eukaryotic cells permeability to the infection.
SPEP action on osteoblast proliferation
At first, an assay was performed to evaluate the effect of SPEP on the proliferation of MG-63 cells in the absence of bacteria in order to exclude a conditioning of osteoblast proliferation due to the protease (data not shown). The proliferation remained unaffected following SPEP treatment. Then, the effect of bacterial strains pretreated or untreated with SPEP on proliferation of MG-63 was assessed. Figure 2 shows MG-63 proliferation in the presence of S. aureus strains reported as percentage in comparison with untreated cells (100%). Conversely to data reported in literature, after 24 h of incubation, a significant increase in osteoblasts proliferation, compared to that of the cells alone, was observed for S. aureus 6538P and S. aureus 20372. This experiment was performed in triplicate and results obtained have been statistically validated. We could tempt to explain this result, considering that nowadays the relationship between osteoblast response to S. aureus is not completely defined. More efforts are necessary to decipher which components are able to whether decrease or increase osteoblastic activity.5
Figure 2.
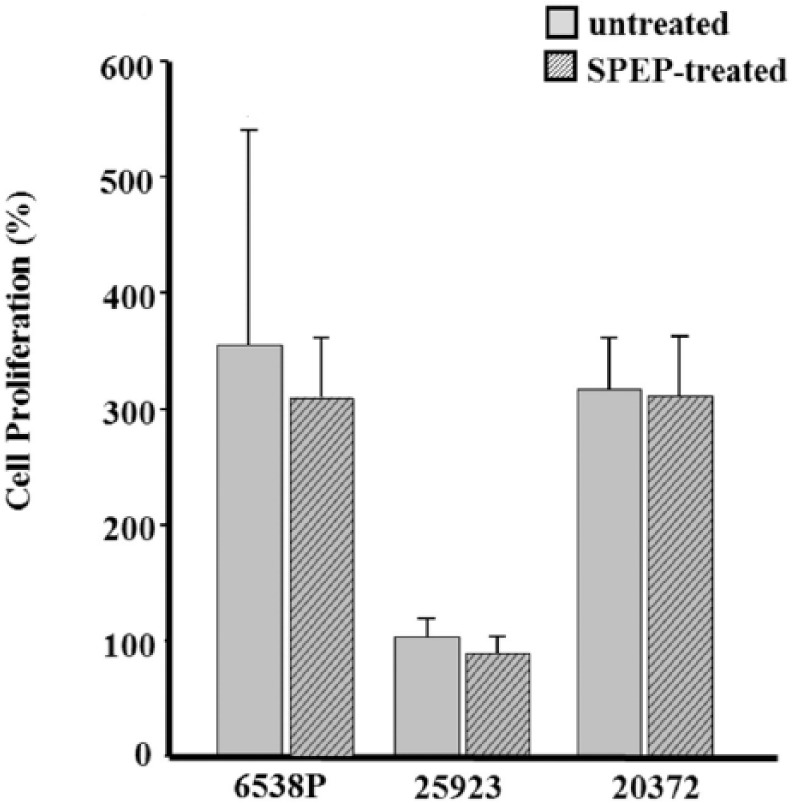
Effect of SPEP on cell proliferation. Osteoblasts were incubated with S. aureus strains pretreated or untreated with SPEP. Proliferation was determined by a colorimetric MTT assay (OD at 560 nm) after 24 h of culture of infected and uninfected cells. Results were expressed as percentages of values normalized with control (100%). The control represents the cells incubated with medium alone. Data are represented as the means and standard deviations of three independent experiments.
When bacteria were pretreated with SPEP, a slight, statistically non-significant, decrease of proliferation was observed for all S. aureus strains.
Effect of SPEP on chemokine release
In order to evaluate the levels of expression for chemokine MCP-1 after staphylococcal infection, a specific ELISA test was performed. Figure 3 shows MCP-1 levels in supernatants from MG-63 cells infected with S. aureus strains after 24 h of incubation. As expected, a significant increase in MCP-1 released compared to those of the cells cultured alone (basal level) was observed. In the basal level, in fact, the MCP-1 levels were 155.7 ± 55.2 pg mL−1. Cells stimulated with LPS and uninfected and unstimulated cells were used as controls. Furthermore, the levels of MCP-1 in MG-63 cells cultured in the presence of LPS and SPEP were also evaluated. This latter represents a control of the responsiveness of the cells and the anti-inflammatory potential of SPEP. As expected, following the treatment with the anti-inflammatory molecule, the production of MCP-1 was significantly diminished level decrease (230.8 ± 140.3 vs 1465.1 ± 196.5 pg mL−1).
Figure 3.
Effect of SPEP on MCP-1 release. MCP-1 secretion in MG-63 cells stimulated with S. aureus strains. After 24 h infection, culture supernatants were collected and MCP-1 was measured by ELISA (*p < 0.05).
Afterwards, the content of chemokine MCP-1 in osteoblasts infected with SPEP-pretreated and SPEP-untreated S. aureus was also evaluated, respectively. Results obtained are reported in Figure 3. MCP-1 levels in supernatants derived from MG-63 cells infected with SPEP-pretreated S. aureus strains were slightly lower than supernatants derived from MG-63 cells infected with SPEP-untreated bacteria. This difference was shared by all four S. aureus strains and turned out statistically significant for two of them (namely, S. aureus 25923 and S. aureus pi99; Figure 3). This result is a consequence of the reduced internalization of SPEP-pretreated bacteria: lower internalization leads less stimulation and lower production of MCP-1.
Discussion
Several studies have also demonstrated that S. aureus can invade bone cells and so evade antimicrobial therapy.4–6 The intracellular persistence of S. aureus in bone cells may facilitate chronic bone infection. Therefore, the discovery of new molecules able to impair internalization of osteoblasts and to control immune response is an important step toward the infection control.
In this article, the capacity of SPEP to affect the staphylococcal internalization of bone cells and to regulate the proliferation and the production of a pro-inflammatory molecule by infected osteoblasts was investigated. Although the main roles of osteoblasts are the synthesis of bone components and the regulation of osteoclasts activity, it was demonstrated that osteoblasts infected with bacteria secrete inflammatory cytokines and chemokines that contribute to feed inflammation and promote bone destruction.7 For this reason, we have chosen to monitor MCP-1 release because this chemokine is involved in the recruitment of osteoclasts and monocytes/macrophages (but also of neutrophils and lymphocytes), when eukaryotic cells are exposed to bacteria.
Our data show that when S. aureus actively invades and persists within human osteoblastic cells, there is an increase in MCP-1 levels. SPEP pre-treatment of the bacteria significantly reduced their internalization for three out of four of the investigated S. aureus strains. Furthermore, the levels of MCP-1 released by osteoblasts infected with SPEP-pretreated bacteria were lower than those observed in cells infected with untreated bacteria.
The use of SPEP in synergy with traditional antibiotics may be suggested as a good strategy to reduce the progression of infection in bone infection and control the progression of inflammatory diseases. Further in vitro and in vivo studies should support this view.
In conclusion, our research demonstrates that SPEP affects the ability of S. aureus to invade bone cells. Furthermore, it could modulate the inflammatory response by reducing the production of chemokine MCP-1 notoriously involved in bone disease that contribute to bone destruction.
Acknowledgments
Carla Renata Arciola would like to acknowledge financial support from “5 per mille” contribution for Health Research to the Rizzoli Orthopaedic Institute of Bologna.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Montanaro L, Testoni F, Poggi A, et al. (2011) Emerging pathogenetic mechanisms of the implant-related osteomyelitis by Staphylococcus aureus. International Journal of Artificial Organs 34: 781–788. [DOI] [PubMed] [Google Scholar]
- 2. Arciola CR, Hänsch GM, Visai L, et al. (2012) Interactions of staphylococci with osteoblasts and phagocytes in the pathogenesis of implant-associated osteomyelitis. International Journal of Artificial Organs 35: 713–726. [DOI] [PubMed] [Google Scholar]
- 3. Khalil H, Williams RJ, Stenbeck G, et al. (2007) Invasion of bone cells by Staphylococcus epidermidis. Microbes and Infection 9: 460–465. [DOI] [PubMed] [Google Scholar]
- 4. Tuchscherr L, Kreis CA, Hoerr V, et al. (2016) Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. Journal of Antimicrobial Chemotherapy 71: 438–448. [DOI] [PubMed] [Google Scholar]
- 5. Josse J, Velard F, Gangloff SC. (2015) Staphylococcus aureus vs. osteoblast: Relationship and consequences in osteomyelitis. Frontiers in Cell and Infection Microbiology 5: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valour F, Trouillet-Assant S, Riffard N, et al. (2015) Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 59: 2029–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyamoto K, Ninomiya K, Sonoda KH, et al. (2009) MCP-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner. Biochemical and Biophysical Research Communications 383: 373–377. [DOI] [PubMed] [Google Scholar]
- 8. Bost KL, Bento JL, Petty CC, et al. (2001) Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staphylococcus aureus or Salmonella. Journal of Interferon & Cytokine Research 21: 297–304. [DOI] [PubMed] [Google Scholar]
- 9. Bhagat S, Agarwal M, Roy V. (2013) Serratiopeptidase: A systematic review of the existing evidence. International Journal of Surgery 11: 209–217. [DOI] [PubMed] [Google Scholar]
- 10. Papa R, Artini M, Cellini A, et al. (2013) A new anti-infective strategy to reduce the spreading of antibiotic resistance by the action on adhesion-mediated virulence factors in Staphylococcus aureus. Microbial Pathogenesis 63: 44–53. [DOI] [PubMed] [Google Scholar]
- 11. Selan L, Papa R, Tilotta M, et al. (2015) Serratiopeptidase: A well-known metalloprotease with a new non-proteolytic activity against S. aureus biofilm. BMC Microbiology 15: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pautke C, Schieker M, Tischer T, et al. (2004) Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Research 24: 3743–3748. [PubMed] [Google Scholar]
- 13. Strober W. (2015) Trypan blue exclusion test of cell viability. Current Protocols in Immunology 111: A3.B.1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]



