Abstract
Sea buckthorn (Hippophae rhamnoides L.) has multifarious medicinal properties including immunoregulatory effect. The total flavonoids of Hippophae rhamnoides L. (TFH) are the main active components isolated from berries of sea buckthorn. The aim of this study was to evaluate the effects of TFH on the cytotoxicity of NK92-MI cells and its possible mechanisms. NK92-MI cells were treated with TFH (2.5 or 5.0 mg/L) or phosphate-buffered saline (PBS) for 24 h, the cytotoxicity against K562 was detected by measuring the release of lactate dehydrogenase (LDH), expression levels of NCRs (NKp30, NKp44, NKp46) and NKG2D were detected by flow cytometry, and expression levels of perforin and granzyme B were detected by western blot. Cytokine Antibody Arrays with 80 cytokine proteins were used to profile the effect of TFH on cytokines. Western blot was adopted to detect the effects of TFH on STAT1, STAT4, and STAT5 signal pathway. Compared with the normal control group, TFH could significantly enhance NK92-MI cell cytotoxicity against K562 cells, upregulate expressions of NKp44, NKp46, perforin, and granzyme B. TFH could upregulate expressions of IL-1α, IL-2, IL-7, IL-15, CSF-2, CSF-3, MCP-1, MIG, IFN-γ, TNF-α, and TNF-β and downregulate expressions of IL-16, MIP-1β, CX3CL-1, and MIF. TFH could increase expressions of phospho-STAT1 and phospho-STAT5. The results suggest that TFH stimulated NK92-MI cells to activate and enhance cytotoxicity of NK92-MI cells.
Keywords: cytotoxicity, flavonoids, Hippophae, natural killer cell
Introduction
Natural killer (NK) cells are members of the innate immune system which are known to mediate major histocompatibility complex (MHC) unrestricted cytotoxicity against virally infected and neoplastic cells.1 Besides their ability to kill target cells directly, NK cells are also known to be potent immune modulators with the ability to produce abundant cytokines capable of modulating immune responses.2 The NK92-MI cell line displays functional characteristics of activated NK cells, so it is a useful model for the study of NK-cell biology.3
Sea buckthorn (SBT; Hippophae rhamnoides L.) is a thorny nitrogen fixing deciduous shrub, native to both Europe and Asia. Berries of SBT have been used in Tibetan, Mongolian, and Chinese traditional medicines for the treatment of different diseases for more than 1000 years.4 In recent studies, we found that SBT oil obtained from the SBT berries could protect against chronic stress-induced inhibitory function of NK cells in rats,5 and the mechanisms need further researches.
Flavonoids belong to a group of low-molecular-weight phenylbenzopyrones which have various pharmacological properties including antioxidant activity, anticancer, and immunomodulatory effects.6,7 SBT fruit is a rich source of flavonoids (0.6% in dry fruits).8 The total flavonoids of Hippophae rhamnoides L. (TFH) are the main active components isolated from berries of SBT.8
In this study, we investigated the effects of TFH on the cytotoxicity of NK92-MI cells and its molecular mechanisms.
Materials and methods
TFH
The crude extract of TFH was provided by Liaoning Dongning Pharmaceutical Co., Ltd (Fuxin, China). The content of the TFH in the crude extract was determined to be 82.5%. The main chemical components of TFH were identified by ultraviolet spectrum, nuclear magnetic resonance, and mass spectra. It was defined that crude extract contained four main flavonoid components including isorhamnetin (45.23%), quercetin (24.56%), kaempferol (6.83%), and myricetin (3.39%).
Cell culture
NK92-MI cells were obtained from ATCC and passaged several times in our laboratory. Cells were cultured in alpha modification of Eagle’s minimum essential medium (a-MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM l-glutamine, 0.2 mM inositol, 0.02 mM folic acid, 0.01 mM 2-mercaptoethanol, 12.5% fetal bovine serum (FBS), and 12.5% horse serum (Sigma-Aldrich Corporation, St. Louis, MO, USA). The target cell line K562 was grown in1640 medium supplemented with 10% FBS.
Cytotoxicity assay
NK92-MI cell cytotoxicity was determined using a colorimetric, non-radioactive, assay that quantitatively measures the release of lactate dehydrogenase (LDH) after cell lysis. To detect the effects of TFH on cytotoxicity of NK92-MI cells, NK92-MI cells (effector) were treated with TFH (2.5 or 5.0 mg/L) or phosphate-buffered saline (PBS) for 24 h and finally co-cultured with K562 (target) cells at an effectors-to-targets (E:T) ratio of 4:1 for 4 h. The supernatants were collected, and LDH release in the supernatants was evaluated using a colorimetric reaction (absorbance at 490 nm). The spontaneous and maximum LDH release was measured by adding100 μL of a-MEM medium or 1% NP-40 to the effector cells or target cells. TFH showed no direct cytotoxic effect on K562 cells or NK92-MI cells alone. The percentage-specific lysis was calculated as follows
Flow cytometric analysis
Flow cytometry was used for analysis of surface NCRs and NKG2D stained with allophycocyanin (APC)-conjugated anti-NKp46 monoclonal antibody (MoAb), phycoerythrin (PE)-conjugated anti-NKp30 MoAb, PerCP-conjugated anti-NKp44 MoAb, fluorescein isothiocyanate (FITC)-conjugated anti-NKG2D monoclonal antibody (MoAb; R&D Systems Inc., Minneapolis, MN, USA). After incubation with TFH (2.5 or 5.0 mg/L) or PBS for 24 h, NK92-MI cells were washed, resuspended to 5 × 105 cells/mL in ice-cold PBS supplemented with 0.5% bovine serum albumin (BSA), incubated with 10 μL of conjugated antibodies for 40 min at 4°C, washed twice in PBS/0.5% BSA, and analyzed directly using a FACScan flow cytometer (FACS Calibur; BD Biosciences, San Jose, CA, USA). We counted 10,000 events per sample, and the percentages of cells with NCRs and NKG2D were obtained by single-color analysis.
Western blot analysis
To detect the effect of TFH on protein expression of perforin, granzyme B, and STAT1, STAT3 and STAT5 signal pathway, NK92-MI cells were incubated with TFH (2.5 or 5.0 mg/L) or PBS for 24 h. Proteins were then extracted using a Total Protein Extraction Kit. Western blot analysis was performed as previously described (Diandong et al.3). Antibodies for perforin, granzyme B, phospho-STAT1, STAT1, phospho-STAT4, STAT4, phospho-STAT5, and STAT5 and the secondary antibodies were obtained from Abcam (Cambridge, UK). The membranes were visualized by enhanced chemiluminescence (ECL) detection system (Bio-Rad Laboratories, USA).
Cytokine antibody arrays
NK92-MI cells were incubated with TFH (2.5 or 5.0 mg/L) or PBS for 24 h. The supernatants were collected for the secreted cytokine analysis. RayBiotech Cytokine Antibody Arrays (AAH-CYT-G5-8) were used to study the effect of TFH on 80 cytokine proteins released by NK92-MI cells. Each experiment was performed in duplicate and according to the manufacturer’s instructions. Briefly, antibody-coated array membranes were first incubated for 30 min with 100 μL of blocking buffer. After 30 min, blocking buffer was decanted and replaced with 100 μL supernatant. Membranes were incubated overnight at 4°C with mild shaking. The next day, the media were decanted and membranes were washed and subsequently incubated with 70 μL biotin-conjugated antibodies for 2 h. After that, biotin-conjugated antibodies were removed, and membranes were incubated with horseradish peroxidase (HRP)-conjugated streptavidin for 2 h.
Detection of spots using chemiluminescence was acquired with semiquantitative analysis of signal intensities from Quantity One software (Bio-Rad Laboratories). Intensities were normalized as a percentage of positive controls on each membrane.
Verification of antibody array results by enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were performed using IFN-γ, IL-2, and IL-16 ELISA kits (CUSABIO, Wuhan, China) following manufacturer’s instructions. Briefly, 100 μL of supernatants from samples and standards were added to 96-well plates pre-coated with capture antibody. After incubation, 100 μL of prepared biotinylated antibody mixture was added to each well. After 1 h, the mixture was decanted and 100 μL streptavidin solution was placed in each well and incubated. Substrate reagent (100 μL) was then added to each well for 30 min followed by the addition of 50 μL of stop solution. Data were quantified by optical density at 450 nm.
Statistical analysis
The results are presented as mean ± standard deviation. Data were assessed using a Shapiro–Wilk test to determine the normal distribution. All distributions were normal, either with 95% or 99% confidence interval. A one-way analysis of variance (ANOVA) was used to detect statistical significance followed by Tukey’s post hoc multiple comparisons using SPSS 16.0 software; p < 0.05 was considered to indicate a statistically significant result.
Results
Effects of TFH on cytotoxicity of NK92-MI cells
The LDH-release cytotoxicity assay was used to determine cytotoxicity of NK92-MI cells against K562 cells. As shown in Figure 1, TFH (2.5 and 5.0 mg/L) could significantly enhance NK92-MI cell cytotoxicity against K562 cells (p < 0.05).
Figure 1.
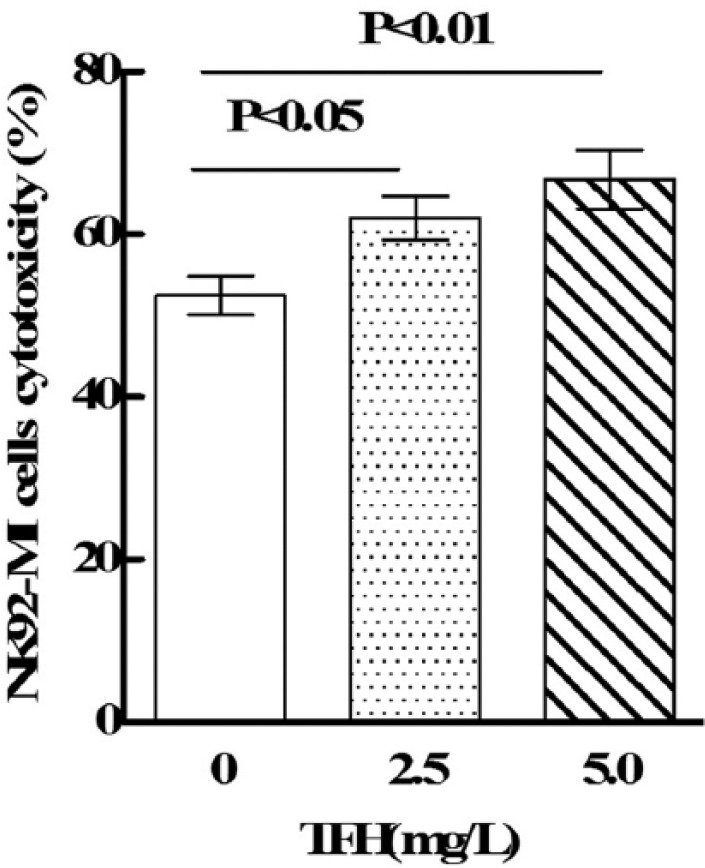
The effects of TFH on NK92-MI cell cytotoxicity.
Effects of TFH on expressions of NCRs and NKG2D in NK92-MI cells
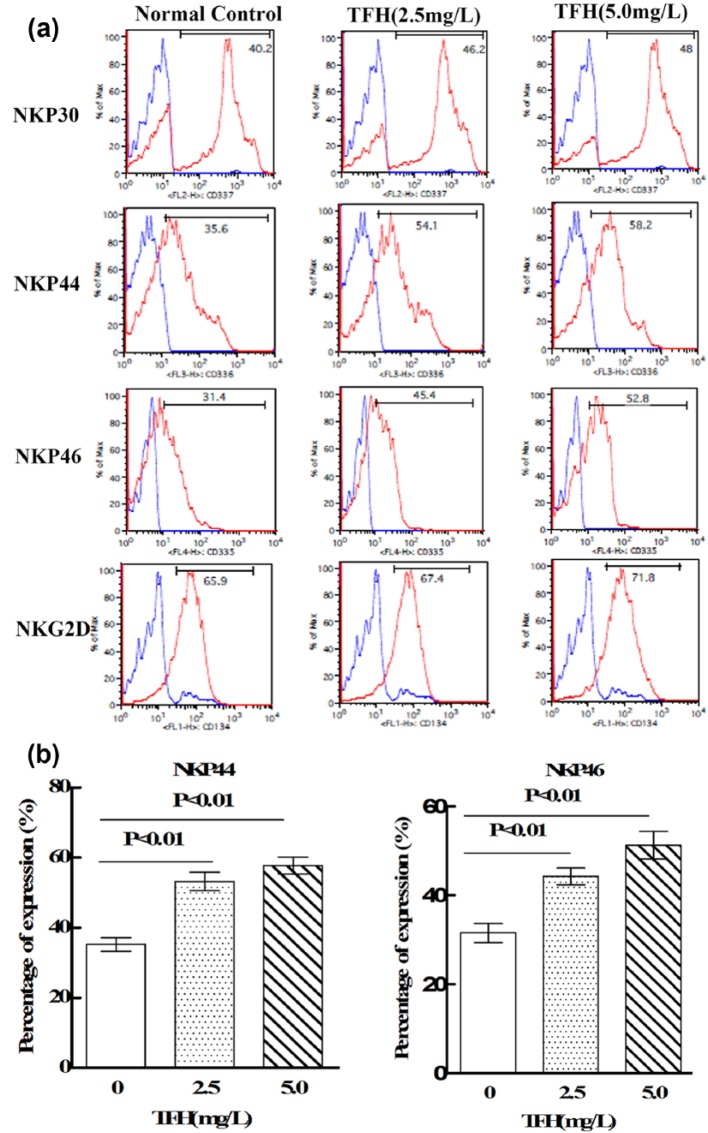
Flow cytometry was performed to determine the effects of TFH on expressions of NCRs and NKG2D in NK-92MI cells. As shown in Figure 2, TFH (2.5 and 5.0 mg/L) could significantly increase expressions of NKp44 and NKp46 in NK92-MI cells (p < 0.01).
Figure 2.
Effects of TFH on expressions of NK92-MI cells’ NCRs and NKG2D: (a) flow cytometry was performed to analyze NKp30, NKp44, NKp46, and NKG2D expression in NK92-MI cells. (b) Results of statistical analysis.
Effects of TFH on expressions of perforin and granzyme B in NK92-MI cells
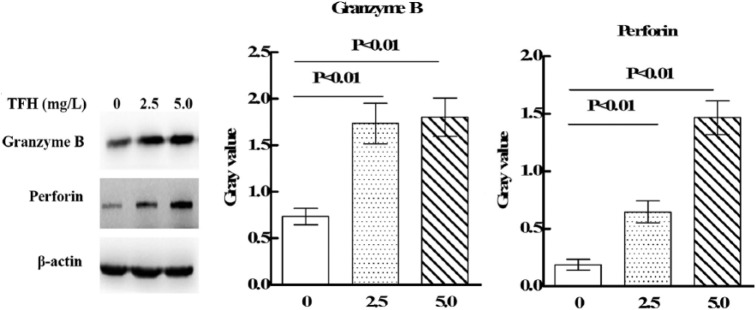
Western blot was performed to determine the effects of TFH on expressions of perforin and granzyme B in NK-92MI cells. As shown in Figure 3, TFH (2.5 and 5.0 mg/L) could significantly upregulate expressions of perforin and granzyme B in NK92-MI cells (p < 0.01).
Figure 3.
Effect of TFH on expressions of NK92-MI cells’ perforin and granzyme B.
Effects of TFH on cytokine secretion of NK92-MI cells
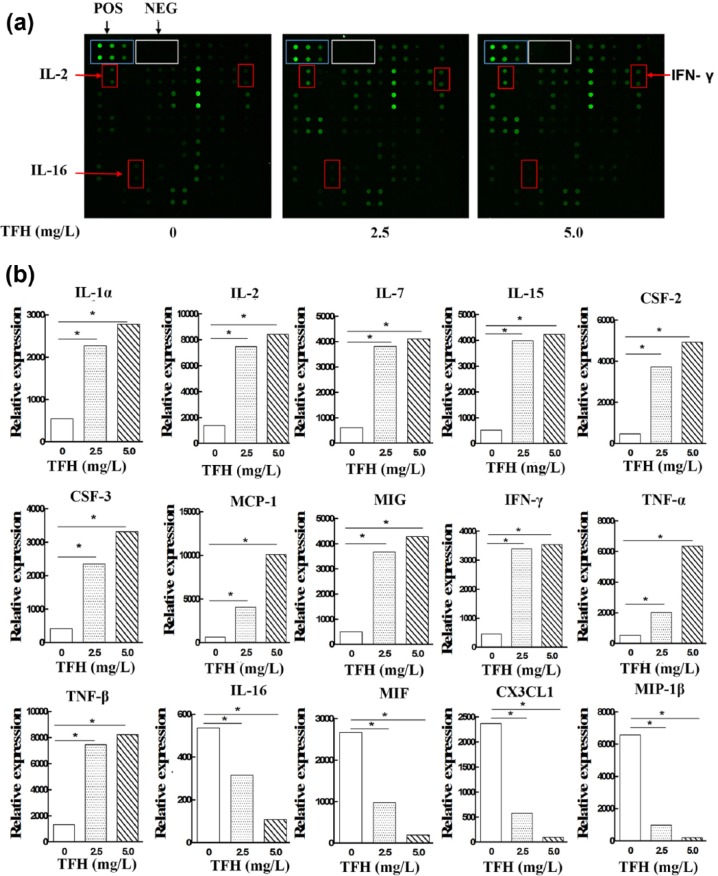
To profile the effect of TFH on cytokines, Cytokine Antibody Arrays with 80 cytokine proteins were used (Table 1). As shown in Figure 4, the dot blot intensity analysis of the supernatants derived from NK92-MI cells showed that TFH could upregulate expressions of IL-1α, IL-2,IL-7, IL-15, CSF-2, CSF-3, MCP-1, MIG, IFN-γ, TNF-α, and TNF-β. While compared with the control group, IL-16, MIP-1β, CX3CL-1, and MIF were downregulated in the TFH-treated groups.
Table 1.
Microarray layout.
| A | B | C | D | E | F | G | H | I | J | K | L | M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pos 1 | Pos 2 | Pos 3 | Neg | Neg | Neg | ENA-78 | CSF-3 | CSF-2 | GRO | GRO-a | I-309 | IL-1α |
| 2 | |||||||||||||
| 3 | IL-1β | IL-2 | IL-3 | IL-4 | IL-5 | IL-6 | IL-7 | IL-8 | IL-10 | IL-12 p70 | IL-13 | IL-15 | IFN-γ |
| 4 | |||||||||||||
| 5 | MCP-1 | MCP-2 | MCP-3 | CSF1 | MDC | MIG | MIP-1β | MIP-1d | RANTES | SCF | SDF-1 | TARC | TGF-β1 |
| 6 | |||||||||||||
| 7 | TNF-α | TNF-β | EGF | IGF-I | ANG | OSM | THPO | VEGF | PDGF-BB | Leptin | BDNF | BLC | Ck b 8-1 |
| 8 | |||||||||||||
| 9 | CCL11 | CCL24 | CCL26 | FGF-4 | FGF-6 | FGF-7 | FGF-9 | Flt-3LG | CX3CL-1 | GCP-2 | GDNF | HGF | IGFBP-1 |
| 10 | |||||||||||||
| 11 | IGFBP-2 | IGFBP-3 | IGFBP-4 | IL-16 | IP-10 | LIF | LIGHT | MCP-4 | MIF | MIP-3a | NAP-2 | NT-3 | NT-4 |
| 12 | |||||||||||||
| 13 | OPN | OPG | PARC | PIGF | TGF-β2 | TGF-β3 | TIMP-1 | TIMP-2 | Neg | Neg | Neg | Neg | Neg |
Figure 4.
Antibody arrays. Culture supernatants from NK92-MI cells were analyzed by antibody arrays. (a) The differentially expressed cytokines IL-2, IFN-γ, and IL-16 were marked in the microarray. (b) The differentially expressed cytokines were all listed. Shown in the graphs (*) is the expression that expressed a significant change.
Verification of antibody array results by ELISA
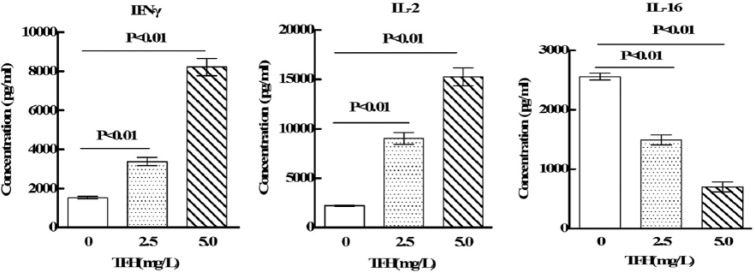
Among the differentially expressed cytokines analyzed by antibody arrays, IFN-γ, MCP-1, and TNF-α were selected randomly and subjected to ELISA for corroborating the results of antibody arrays. As shown in Figure 5, the results confirmed that TFH induces upregulation of IFN-γ, MCP-1, and TNF-α in NK92-MI cells. These expression profiles were completely consistent with antibody arrays. Therefore, ELISA detection of the differentially expressed proteins confirmed the reliability and validity of the antibody arrays.
Figure 5.
Results of antibody arrays were verified by ELISA.
TFH activated STAT3 and STAT5 of NK cells
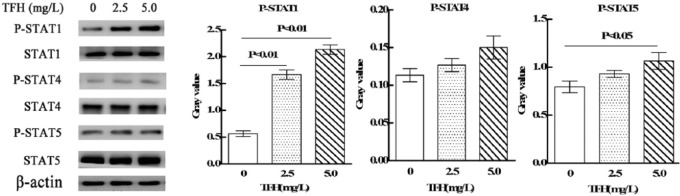
In order to determine the intracellular signaling mechanisms of TFH, western blot was adopted to detect the effects of TFH on STAT1, STAT4, and STAT5 signal pathway. As shown in Figure 6, whole-cell extracts from TFH-treated NK92-MI cells demonstrated strong activation of STAT1 and relative weak activation of STAT5, and there is no significant effects on STAT4 signal pathway.
Figure 6.
Effect of TFH on STAT1, 3, and 5 signal pathway in NK92-MI cells.
Discussion
SBT has drawn the attention of scientists because of its multifarious medicinal properties including immunoregulatory effects.9,10 Flavonoids are one of the active ingredients extracted from SBT. In this study, we investigated the effect of TFH on NK-cell functions using NK92-MI cell line.
NK cell–mediated lysis of target cells is determined by the balance of signaling between activating and inhibitory receptors on NK cells.11 Activating receptors, including NCRs (NKp46, NKp44, and NKp30) and NKG2D, bind ligands induced by cellular stress, infection, or tumor transformation.12 Activating signals are transmitted through immunoreceptor tyrosine–based activating motifs (ITAMs) located in the cytoplasmic tail of the receptor or through ITAMs in adaptor molecules, which associate with activating receptors at the cell surface.13 When a target cell lacks or underexpresses HLAI and/or overexpresses activating ligands, NK cells eliminate that target by releasing preformed cytotoxic granzymes and perforin stored as granules or activate apoptosis pathways in the target cell.14 In this study, we detected expressions of activating receptors (NKp30, NKp44, NKp46, and NKG2D) in NK92-MI cells by flow cytometry analysis. The results showed that TFH significantly enhanced expressions of NKp44 and NKp46 in NK92-MI cell. These findings suggest that TFH may activate NK92-MI cells by upregulating the expression levels of activating receptors NKp44 and NKp46.
Perforin and granzymes are stored in cytoplasmic granules and function after direct interaction between NK and target cells.15 Perforin, a membrane pore-forming molecule, permeabilizes cells.16 Granzymes, a family of serine proteases, disrupt cell-cycle progression, inflict DNA damage, and dissolve the nucleus upon entrance into the cell.17 Granzyme B is the most abundant serine protease stored in the secretory granules of CTLs and NK cells.18 Results showed that TFH could increase the expressions of both perforin and granzymes B. These findings suggest that TFH may increase cytotoxicity of NK92-MI cells against K562 cells by upregulating the expressions of perforin and granzymes B.
NK cells are important sources of various cytokines and chemokines.19 Results showed that TFH could upregulate expressions of IL-1α, IL-2, IL-7, IL-15, CSF-2, CSF-3, MCP-1, MIG, IFN-γ, TNF-α, and TNF-β and downregulate expressions of MIP-1β, CX3CL-1, IL-16, and MIF. IFN-γ and TNF-α productions by NK cells have been identified as an integral part of NK-cell cytotoxic activity.20 Activated NK cells are the main source of IFN-γ.21 IL-2 and IL-15 have been shown to enhance perforin expression in NK cells.22 IL-15 is an important regulator of NK-cell development, homeostasis, and activation.23
Researches have showed that JAK-STAT signal pathway was involved in regulating cytolysis activity of NK cell. STAT1, 4, and 5 were related to the cytotoxicity of NK cells.24–26 Results showed that TFH strongly upregulated expression of phospho-STAT1 and relatively weakly upregulated phospho-STAT5. STAT1 is a crucial regulator of IFN-γ production and NK-cell cytotoxicity.24,27,28 Stat1-deficient mice are highly susceptible to bacterial and viral infections.29 STAT5 transmits signals downstream of IL-2 and IL-15, and its expression is indispensable for the survival of peripheral NK cells.30 There is evidence that the loss of STAT5 reduces NK-cell numbers and impairs cytolytic responses.31 Our findings suggest that TFH may increase cytotoxicity of NK92-MI cells against K562 cells through STAT1 and STAT5 signal pathway.
In conclusion, the results suggest that TFH stimulated NK92-MI cells to activate and enhance cytotoxicity of NK92-MI cells. Therefore, TFH has the potential to be an immuno-potentiating agent, which can potentially be used as functional food and pharmaceutical therapy ingredients. In future studies, it will be worthwhile to further study the effects of TFH on the regulation of immune responses in vivo.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the Program for Liaoning Excellent Talents in University (No. LJQ2015072) and the National Natural Science Foundation of China (grant no. 81303079).
References
- 1. Oh SJ, Yang JI, Kim O, et al. (2017) Human U87 glioblastoma cells with stemness features display enhanced sensitivity to natural killer cell cytotoxicity through altered expression of NKG2D ligand. Cancer Cell International 17: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koller A, Bianchini R, Schlager S, et al. (2016) The neuropeptide galanin modulates natural killer cell function. Neuropeptides 64: 109–115. [DOI] [PubMed] [Google Scholar]
- 3. Diandong H, Kefeng S, Weixin F, et al. (2013) The role of Gαs in activation of NK92-MI cells by neuropeptide substance P. Neuropeptides 48: 1–5. [DOI] [PubMed] [Google Scholar]
- 4. Yang B, Kalimo KO, Mattila LM, et al. (1999) Effects of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on atopic dermatitis. The Journal of nutritional biochemistry 10: 622–630. [DOI] [PubMed] [Google Scholar]
- 5. Diandong H, Feng G, Zaifu L, et al. (2016) Sea buckthorn (Hippophae rhamnoides L.) oil protects against chronic stress-induced inhibitory function of natural killer cells in rats. International Journal of Immunopathology and Pharmacol 29: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyu SY, Park WB. (2005) Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Archives of Pharmacal Research 28: 573–581. [DOI] [PubMed] [Google Scholar]
- 7. Burkard M, Leischner C, Lauer UM, et al. (2017) Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. The Journal of Nutritional Biochemistry 46: 1–12. [DOI] [PubMed] [Google Scholar]
- 8. Mishra KP, Chanda S, Karan D, et al. (2008) Effect of seabuckthorn (Hippophae rhamnoides) flavone on immune system: An in-vitro approach. Phytotherapy Research 22: 1490–1495. [DOI] [PubMed] [Google Scholar]
- 9. Geetha S, Sai Ram M, Singh V, et al. (2002) Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides): an in vitro study. Journal of Ethnopharmacology 79: 373–378. [DOI] [PubMed] [Google Scholar]
- 10. Geetha S, Singh V, Ram MS, et al. (2005) Immunomodulatory effects of seabuckthorn (Hippophae rhamnoides L.) against chromium (VI) induced immunosuppression. Molecular and Cellular Biochemistry 278: 101–109. [DOI] [PubMed] [Google Scholar]
- 11. Ni L, Wang L, Yao C, et al. (2017) The histone deacetylase inhibitor valproic acid inhibits NKG2D expression in natural killer cells through suppression of STAT3 and HDAC3. Scientific Reports 7: 45266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang CJ, Chen YY, Lu CC, et al. (2014) Ganoderma lucidum stimulates NK cell cytotoxicity by inducing NKG2D/NCR activation and secretion of perforin and granulysin. Innate Immunity 20: 301–311. [DOI] [PubMed] [Google Scholar]
- 13. Kwon HJ, Kwon SJ, Lee H, et al. (2015) NK cell function triggered by multiple activating receptors is negatively regulated by glycogen synthase kinase-3beta. Cellular Signalling 27: 1731–1741. [DOI] [PubMed] [Google Scholar]
- 14. Noone C, Kihm A, English K, et al. (2013) IFN-gamma stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells and Development 22: 3003–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pessina S, Cantini G, Kapetis D, et al. (2016) The multidrug-resistance transporter Abcc3 protects NK cells from chemotherapy in a murine model of malignant glioma. Oncoimmunology 5: e1108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thiery J, Lieberman J. (2014) Perforin: A key pore-forming protein for immune control of viruses and cancer. Sub-cellular Biochemistry 80: 197–220. [DOI] [PubMed] [Google Scholar]
- 17. Dotiwala F, Mulik S, Polidoro RB. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nature Medicine 22: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rousalova I, Krepela E. (2010) Granzyme B-induced apoptosis in cancer cells and its regulation (review). International Journal of Oncology 37: 1361–1378. [DOI] [PubMed] [Google Scholar]
- 19. Cheon S, Song SB, Jung M, et al. (2008) Sphingosine kinase inhibitor suppresses IL-18-induced interferon-gamma production through inhibition of p38 MAPK activation in human NK cells. Biochemical and Biophysical Research Communications 374: 74–78. [DOI] [PubMed] [Google Scholar]
- 20. Huth TK, Staines D, Marshall-Gradisnik S. (2016) ERK1/2, MEK1/2 and p38 downstream signalling molecules impaired in CD56 dim CD16+ and CD56 bright CD16 dim/- natural killer cells in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. Journal of Translational Medicine 14: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Z, Frascaroli G, Bayer C, et al. (2015) Interleukin-2 from Adaptive T Cells Enhances Natural Killer Cell Activity against Human Cytomegalovirus-Infected Macrophages. Journal of Virology 89: 6435–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salvucci O, Mami-Chouaib Moreau JL, et al. (1996) Differential regulation of interleukin-12- and interleukin-15-induced natural killer cell activation by interleukin-4. European Journal of Immunology 26: 2736–2741. [DOI] [PubMed] [Google Scholar]
- 23. Huntington ND. (2014) The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunology and Cell Biology 92: 210–213. [DOI] [PubMed] [Google Scholar]
- 24. Liang S, Wei H, Sun R, et al. (2003) IFNalpha regulates NK cell cytotoxicity through STAT1 pathway. Cytokine 23: 190–199. [DOI] [PubMed] [Google Scholar]
- 25. Gotthardt D, Sexl V. (2016) STATs in NK-cells: The good, the bad, and the ugly. Frontiers in Immunology 7: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delconte RB, Kolesnik TB, Dagley LF, et al. (2016) CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nature Immunology 17: 816–824. [DOI] [PubMed] [Google Scholar]
- 27. Au-Yeung N, Mandhana R, Horvath CM. (2013) Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAK-STAT 2: e23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyagi T, Gil MP, Wang X, et al. (2007) High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. The Journal of Experimental Medicine 204: 2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lesinski GB, Anghelina M, Zimmerer J, et al. (2003) The antitumor effects of IFN-α are abrogated in a STAT1-deficient mouse. The Journal of Clinical Investigation 112: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckelhart E, Warsch W, Zebedin E, et al. (2011) A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood 117: 1565–1573. [DOI] [PubMed] [Google Scholar]
- 31. Berger A, Sexl V, Valent P, et al. (2014) Inhibition of STAT5: A therapeutic option in BCR-ABL1-driven leukemia. Oncotarget 5: 9564–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]