Abstract
HSP60 has been implicated in chronic inflammatory disease pathogenesis, including chronic obstructive pulmonary disease (COPD), but the mechanisms by which this chaperonin would act are poorly understood. A number of studies suggest a role for extracellular HSP60, since it can be secreted from cells and bind Toll-like receptors; however, the effects of this stimulation have never been extensively studied. We investigated the effects (pro- or anti-inflammatory) of HSP60 in human bronchial epithelial cells (16-HBE) alone and in comparison with oxidative, inflammatory, or bacterial challenges. 16-HBE cells were cultured for 1–4 h in the absence or presence of HSP60, H2O2, lipopolysaccharide (LPS), or cytomix. The cell response was evaluated by measuring the expression of IL-8 and IL-10, respectively, pro- and anti-inflammatory cytokines involved in COPD pathogenesis, as well as of pertinent TLR-4 pathway mediators. Stimulation with HSP60 up-regulated IL-8 at mRNA and protein levels and down-regulated IL-10 mRNA and protein. Likewise, CREB1 mRNA was up-regulated. H2O2 and LPS up-regulated IL-8. Experiments with an inhibitor for p38 showed that this mitogen-activated protein kinase could be involved in the HSP60-mediated pro-inflammatory effects. HSP60 showed pro-inflammatory properties in bronchial epithelial cells mediated by activation of TLR-4-related molecules. The results should prompt further studies on more complex ex-vivo or in-vivo models with the aim to elucidate further the role of those molecules in the pathogenesis of COPD.
Keywords: COPD, CREB1, ERK1, HSP60, IL-8, IL-10, JNK1, MyD88, NF-κB p65 subunit, p38α, TLR-4, 16-HBE
Introduction
The bronchial epithelium plays an active role in airway defense mechanisms by releasing cytoprotective mucus and coordinating local inflammation and immune responses through the generation of cytokines and chemokines.1
Molecular chaperones, many of which are heat shock proteins (HSPs), play key roles in defending cells from stressors and interact with receptors involved in regulation of inflammation. Consequently, HSPs’ malfunction can cause pathological processes, including cancer and chronic inflammatory diseases.2 However, the role of HSPs in chronic obstructive pulmonary disease (COPD) pathogenesis is still poorly understood and debated.3
In the past, we showed in vivo that the levels of HSP60 decrease during the carcinogenic steps of bronchial mucosa in patients affected by COPD and, noteworthy, the normal epithelium close to the tumor shows a reduced immunopositivity for HSP60 compared to normal epithelium of healthy subjects as determined in mucosal biopsies.4 In contrast, the intracellular HSP60 levels are increased in the bronchial epithelium of patients without cancer but with severe/very severe COPD compared to smoker and non-smoking subjects.5 Interestingly, oxidative stress induced increased levels of HSP60 in human bronchial epithelial cells and is actively released by them.5 Extracellular HSP60 (eHSP60) can bind various surface receptors, typically present on immune cells, including Toll-like receptor (TLR)-4 and TLR-2, which are involved in the inflammatory response.6 TLRs activate MyD88 and, in turn, this leads to the release of pro-inflammatory cytokines.7 It has been shown that TLR signaling activated by eHSP60 can induce either pro-inflammatory or anti-inflammatory responses, probably depending on the immune cell type involved.7,8 However, the signal pathways responding to eHSP60 stimulation have not been elucidated in airway epithelial cells, potentially involved in the inflammatory mechanisms occurring in COPD.9
Our present results show for the first time that HSP60 can have pro-inflammatory effects which are mediated by TLR-4 in bronchial epithelial cells.
Materials and methods
Cell cultures and treatments
We used the 16-HBE (human bronchial epithelial) cell line, which retains the differentiated morphology and functions of normal human airway epithelia.10 16-HBE cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) and supplemented with 50 μg/mL penicillin, 50 μg/mL streptomycin, 1× non-essential amino acids, 1 mM sodium pyruvate, and 2 mM glutamine. Cell cultures were maintained in a humidified atmosphere of 5% CO2 in air at 37°C. When cells were at 70%–80% confluence, prior to each treatment, cells were starved with DMEM without FBS for 24 h to make them quiescent. This ensured that all the cells were synchronized in the same phase of the cell cycle and that when stimulated their responses would be comparable. The experiments were performed on 24-well culture plates for mRNA measurements. The experiments started when the cells were at 60%–70% confluence.
The 16-HBE cells were cultured for 1, 2, 4, 8, and 24 h in the presence and in the absence of lipopolysaccharide (LPS, 10 μg/mL; Sigma–Aldrich, Milan, Italy), or H2O2 (100 μM; Sigma–Aldrich), or cytomix (IL-1β 1 ng/mL, + TNFα 10 ng/mL, + INF-γ 10 ng/mL, all from R&D System, Minneapolis, MN) and human low endotoxin HSP60 (1 μg/mL; Enzo Life Sciences, Lausanne, Switzerland), the latter to mimicking eHSP60. In some experiments, the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (Santa Cruz Biotechnology, Dallas, TX; 10 μM, 30 min) was added to the 16-HBE cell cultures before stimulation with LPS, or with eHSP60. At the end of the stimulation period, cell pellets and cell culture supernatants were collected for study. Cells were harvested immediately after incubation. Before mRNA isolation, cell cultures were initially washed with phosphate-buffered saline (PBS). All experiments were performed in quadruplicate.
Extraction and quantification of RNA from 16-HBE cells and quantitative reverse transcription polymerase chain reaction from 16-HBE cells
Total cellular RNA from treated and non-treated cultures was purified and isolated, using RNAspin Mini RNA Isolation kit (GE Healthcare, Buckinghamshire, UK) and following the manufacturer’s instructions. Total RNA was re-suspended in 100 μL nuclease-free water. RNA concentration was determined using a UV/visible spectrophotometer (λ260/280 nm, Eppendorf BioPhotometer plus) and stored at −80°C until use.
Gene expression was measured using the Syber green for quantitative reverse transcription polymerase chain reaction (qRT-PCR) in a Rotor-Gene Q (Qiagen) system. One-step real-time PCR was carried out by amplifying mRNA using the QuantiFastTM SYBER Green RT-PCR Kit (Qiagen) according to the manufacturer’s instructions, and gene specific primers (Qiagen), as follows: CXCL8 (IL-8) (Cat. No. QT00000322); IL-10 (Cat. No. QT00041685); p38α (Cat. No. QT00079345); JNK1 (Cat. No. QT00091056); ERK1 (Cat. No. QT00065933); NF-κB p65 subunit (Cat. No. QT01007370); and CREB1 (QT00092435). A single quantitative PCR measurement was performed for each type of treatment (eHSP60, H2O2, cytomix, or LPS) and each 16-HBE time exposure (1, 2, and 4 h). Briefly, the PCR reaction mix, prepared in a total volume of 25 μL, was run on the Rotor-Gene Q (Qiagen) and the following PCR run protocol was used: 55°C for 10 min (reverse transcription), 95°C for 5 min (PCR initial activation step), 40 amplification cycles of 95°C for 5 s (denaturation), and 60°C for 10 s (combined annealing/extension), followed by melting curve analysis to ensure the specificity of PCR amplification. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cat. No. QT01192646) was used as the reference gene for every target gene per sample, and the data were normalized against the respective GAPDH signaling. Cycle threshold (CT) values were determined using the Rotor-Gene Q software (Rotor-Gene Q Series Software 2.0.2). The expression levels of all genes studied were normalized to GAPDH levels in each sample to determine the expression between treated and non-treated cells using the 2−ΔΔCt method.11
ELISA tests in the 16-HBE cell culture supernatants
IL-8 and IL-10 proteins were quantified in the cell culture supernatants. These assays were performed according to the manufacturer’s instructions, using the Invitrogen ELISA Kit (Cat. No. KHC0081) for human IL-8, standard curve sensibility range: 0–1000 pg/mL, sensitivity <5 pg/mL; Invitrogen ELISA Kit (Cat. No. KHC0101) for human IL-10, standard curve sensibility range: 0–500 pg/mL, sensitivity <1 pg/mL.
Statistical analysis
All data were expressed as mean and standard deviation (SD). Individual experiments were performed in quadruplicate (n = 4). Data analysis was performed using the Stat View SE Graphics program (Abacus Concepts Inc., Berkeley, CA). Significant differences between groups were determined using the independent t-test. A P-value ⩽0.05 was considered the threshold for significance.
Results
MTT tests, applied to 16-HBE cells in our preliminary experiments, showed 100% survival for all single and combined treatments at 4 h. H2O2 treatment at 4 h showed 98% and 95% survival at a dosages of 100 and 200 μM, respectively. A dosage of 100 μM of H2O2 was then chosen for our experiments.
IL-8 and IL-10 mRNA expression after single stimulation with eHSP60, H2O2, cytomix, or LPS
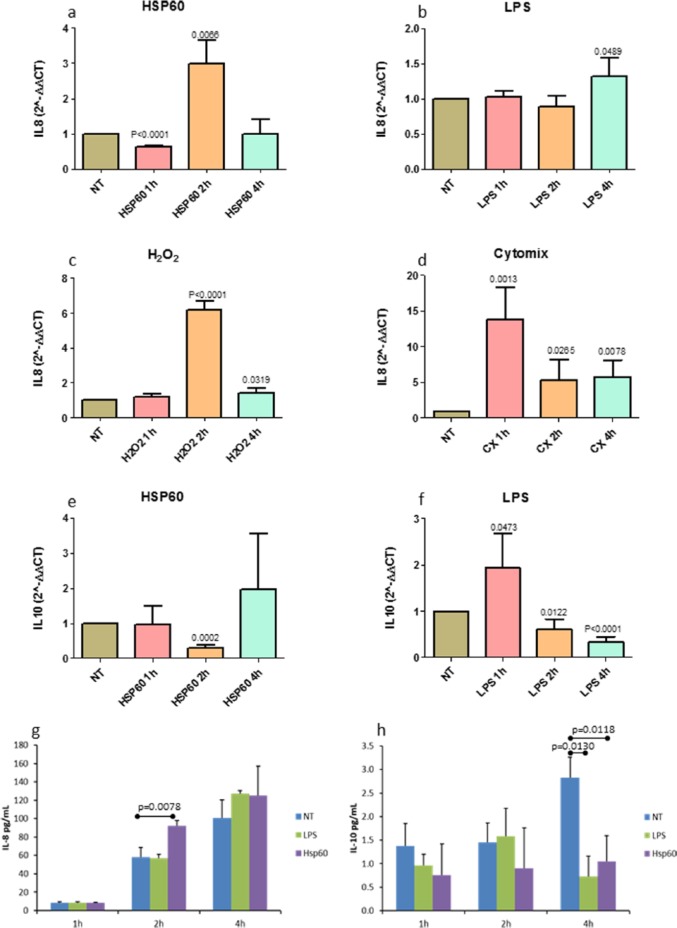
16-HBE cells were stimulated with eHSP60, H2O2, cytomix, or LPS, for 1, 2, and 4 h. IL-8 and IL-10 expression was examined by qRT-PCR. eHSP60 up-regulated IL-8 mRNA after 2 h of exposure (non-treated vs treated, P = 0.0066) (Figure 1(a)). LPS up-regulated IL-8 mRNA after 4 h of exposure (P = 0.0489) (Figure 1(b)). H2O2 up-regulated IL-8 mRNA at 2 and 4 h of exposure (P < 0.0001 and P < 0.0319, respectively) (Figure 1(c)). Cytomix up-regulated IL-8 at 1, 2, and 4 h of exposure (P = 0.0013, P = 0.0265, and P = 0.0078, respectively) (Figure 1(d)). eHSP60 down-regulated IL-10 at 2 h of exposure (P = 0.0002) (Figure 1(e)). LPS transitorily up-regulated IL-10 at 1 h (P = 0.047) followed by down-regulation at 2 h (P = 0.0122) and 4 h (P = 0.0001) of exposure (Figure 1(f)).
Figure 1.
IL-8 and IL-10 mRNA and protein expression after single challenges. IL-8 mRNA in 16-HBE cells treated with (a) eHSP60, (b) lipopolysaccharide from P. aeruginosa (LPS), (c) H2O2, or (d) cytomix (IL-1β+TNFα+IFNγ) (CX). (e) Treatment with eHSP60 down-regulated IL-10 mRNA at 2 h. (f) The treatment with LPS, up-regulated transitorily IL-10 expression at 1 h after exposure, which was followed by down-regulation at 2 and 4 h after exposure. (g) eHSP60 stimulation of 16-HBE cells up-regulated IL-8 protein secretion after 2 h of exposure. (h) IL-10 protein secretion was down-regulated by both LPS and eHSP60 stimulations after 4 h of exposure. Data are presented as mean ± SD of quadruplicate experiments both for mRNA and secreted proteins. The expression of all genes studied were normalized to GAPDH levels in each sample to determine the difference in expression between treated and non-treated cells using the 2−ΔΔCt method (see the “Materials and methods” section); NT indicates not treated cells.
Quantification of secreted IL-8 and IL-10 in the supernatants of epithelial cells
Secreted IL-8 in the supernatant of bronchial epithelial cells was increased (P = 0.0078) after 2 h of exposure to eHSP60 (Figure 1(g)). Secreted IL-10 was decreased after 4 h of exposure to LPS (P = 0.0130) and after 4 h of exposure to eHSP60 (P = 0.0118) (Figure 1(h)), confirming at the protein level, the data obtained for mRNA.
TLR-4 pathway mediators after eHSP60 stimulation
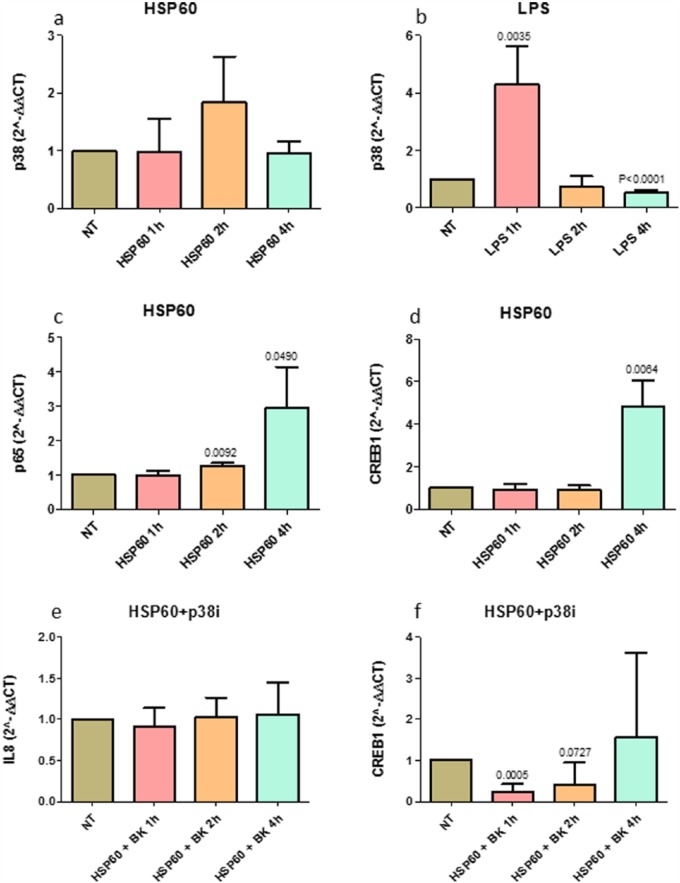
We quantified the expression of the TLR-4 pathway mediators: p38α, JNK1, ERK1, NF-κB p65 subunit, MyD88, and CREB1 after eHSP60 or LPS stimulation. We did not quantify their expression after H2O2 and cytomix since they do not activate directly TLR-4. JNK1 and ERK1 expression did not significantly change after the treatments (data not shown).
P38α mRNA tended to increase at 2 h of treatment with eHSP60 (non-treated vs treated, P = 0.0733) (Figure 2(a)). LPS up-regulated p38 mRNA at 1 h of exposure (P = 0.0035) (Figure 2(b)). NF-κB p65 subunit mRNA was significantly increased after treatment with eHSP60 for 2 h (P = 0.0092) and 4 h (P = 0.0490) (Figure 2(c)). MyD88 mRNA expression showed a tendency to increase after 4 h of exposure to eHSP60 (P = 0.1232) (not shown). CREB1 mRNA significantly increased at 4 h of exposure to eHSP60 (P = 0.0064) (Figure 2(d)).
Figure 2.
TLR-4 pathway mediators’ expression after eHSP60 stimulation, and IL-8 and CREB1 mRNA expression after p38 inhibition. p38α mRNA after (a) eHSP60 and (b) LPS stimulations; (c) p65 mRNA and (d) CREB1 mRNA after eHSP60 stimulation. p38α tended to increase at 2 h of treatment with eHSP60 (P = 0.0733); LPS up-regulated p38α at 1 h of exposure; eHSP60 exposure up-regulated p65 at 2 and 4 h of exposure as well as CREB1 at 4 h of exposure. (e) IL-8 and (f) CREB1 mRNA after a 30-min pre-treatment with the p38 inhibitor SB203580 (p38i) followed by treatment with eHSP60. The p38 inhibitor inhibited IL-8 mRNA up-regulation after 2 h (e) of exposure previously observed after the eHSP60 stimulation and abolished the CREB1 up-regulation after 4 h of exposure to eHSP60. Data are presented as mean ± SD of quadruplicate experiments.
This set of data suggested that the pro-inflammatory effects of eHSP60 are accompanied by TLR-4 pathway activation in bronchial epithelial cells.
Effect of p38 inhibition on IL-8 and IL-10 mRNA production after eHSP60 stimulation
Since p38α is a key regulator for activation of transcription of nuclear genes involved in inflammation, included IL-8, we performed a 30-min pre-treatment of cultured 16-HBE cells with a p38 inhibitor followed by 1, 2, and 4 h of eHSP60 stimulation.
The blocking of the p38α activity abolished the up-regulation of IL-8 at 1, 2, and 4 h after eHSP60 challenge (P = 0.4820, P = 0.7735, and P = 0.7460, respectively) (Figure 2(e)). Blocking p38α activity also abolished the CREB1 mRNA production at 1, 2, and 4 h after eHSP60 stimulation (P = 0.0005, P = 0.0727, and P = 0.5605, respectively) (Figure 2(f)).
This set of experiments supported the hypothesis that p38α activation is at least in part, involved in the pro-inflammatory effects and IL-8 up-regulation caused by eHSP60.
Discussion
The purpose of this study was to investigate whether HSP60 has pro- or anti-inflammatory effects on bronchial epithelial cells. Stimulation of 16-HBE with eHSP60 caused an up-regulation of IL-8 mRNA and protein, while IL-10 mRNA and protein were down-regulated.
We looked at relevant intracellular mediators, and we observed a significant involvement of p38α in the regulation of IL-8 production.
These data, taken together, indicate that eHSP60 can have pro-inflammatory activity in bronchial epithelial cells.
Along this line, we already reported an increase in intracellular HSP60 in the epithelium and lamina propria of bronchial biopsies of severe COPD patients compared to control smokers and non-smokers, suggesting a possible cytoprotective function for this chaperonin,5 in agreement with published reports showing that HSPs can protect cells from death caused by oxidative stress.12 However, the data reported here suggest that this intracellular cytoprotective effect can be altered when HSP60 is secreted in the external side of the cell environment, producing opposite effects.
To obtain clues on how eHSP60 induces cell-activation after binding TLRs, we also measured levels of MyD88 (the principal TLRs adapter) and of other TLR pathway mediators. MyD88 mRNA tended to be up-regulated 2 h after stimulation with eHSP60. This may confirm the activation of TLRs via the MyD88-dependent signaling pathway. Treated 16-HBE cells with eHSP60 also showed an up-regulation of p38α kinase and CREB1 nuclear transcription factor (phosphorylated by p38α activation), compared to untreated cells but, in contrast, JNK1 mRNA and ERK1 mRNA levels were not changed. In agreement, an involvement of activated CREB1 in human fibroblast-like synoviocytes inducing IL-8 increased secretion has been recently reported.13 These results suggest that p38α and CREB1 play a more relevant role in inducing IL-8 up-regulation and IL-10 decrease than ERK1 and JNK1 do in bronchial epithelial cells after eHSP60 stimulation.
Pre-treatment of eHSP60-treated 16-HBE cells with a p38 inhibitor abolished the IL-8 mRNA and CREB1 mRNA increase, confirming the involvement of this kinase in IL-8 up-regulation. These data are in part, in contrast, with those from others who propose that the main MAPKs involved in the up-regulation of IL-8 after stimulation of 16-HBE with LPS are ERK1 and ERK2.14 This discrepancy may be related to the different experimental models used and to the different epithelial cell stimulus applied.
We speculate that eHSP60 induces the activation of CREB1, which is known to be crucial for the transcriptional regulation of IL-8 and IL-10 expression.15 Notably, activation of CREB1 has also been demonstrated to suppress the transcriptional activity of NF-κB by competing with the NF-κB p65 subunit for the transcription co-factor CREB-binding protein (CBP).16
Our present data show that stimulation of 16-HBE cells with human eHSP60 causes up-regulation of IL-8 through the nuclear transcription factor CREB1. However, CREB1 is not able to suppress the activity of NF-κB p65 subunit that we found increased in eHSP60-treated cells. We hypothesize that activation of NF-κB p65 subunit is only partially dependent on eHSP60 stimulation. Distinct but parallel mechanisms, which use separate molecular pathways, play a role in this inflammatory process mediated by NF-κB p65 subunit.
In conclusion, our data show that HSP60 has pro-inflammatory activity in bronchial epithelial cells, possibly mediated by p38α activation and CREB1 up-regulation.
Acknowledgments
This work was done under the umbrella of the agreement between IEMEST and IMET signed March 2012 (this is IMET contribution number IMET 17–213).
Footnotes
Author contribution: C.S. and D.V. participated in the design of the study, carried out the experiments and data analysis, and drafted part of the manuscript; I.G. and A.G. carried out the laboratory work and participated in data analysis; F.C., A.L., F.B., B.B., P.B., A.J.L.M., E.C.de M, and A.D.S. conceived and designed the study and helped draft the manuscript. All authors gave final approval for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the Istituti Clinici Scientifici Maugeri, IRCCS, Ricerca Corrente. F.C. and A.J.L.M. were partially supported by IEMEST. E.C.M. and A.J.L.M. were partially supported by IMET. F.C. was also partially supported by the University of Palermo, FFR-BIONEC.
References
- 1. Martel G, Bérubé J, Rousseau S. (2013) The protein kinase TPL2 is essential for ERK1/ERK2 activation and cytokine gene expression in airway epithelial cells exposed to pathogen-associated molecular patterns (PAMPs). PLoS ONE 8: e59116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macario AJL, Cappello F, Zummo G, et al. (2010) Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Annals of the New York Academy of Sciences 1197: 85–93. [DOI] [PubMed] [Google Scholar]
- 3. Cappello F, Macario AJL, Di Stefano A. (2015) Hsp27 and Hsp70 in chronic obstructive pulmonary disease: Certainties vs doubts. Cell Stress and Chaperones 20(5): 721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappello F, Di Stefano A, D’Anna SE, et al. (2005) Immunopositivity of heat shock protein 60 as a biomarker of bronchial carcinogenesis. Lancet Oncology 6: 816. [DOI] [PubMed] [Google Scholar]
- 5. Cappello F, Caramori G, Campanella C, et al. (2011) Convergent sets of data from in vivo and in vitro methods point to an active role of Hsp60 in chronic obstructive pulmonary disease pathogenesis. PLoS ONE 6: e28200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tian J, Guo X, Liu XM, et al. (2013) Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovascular Research 98: 391–401. [DOI] [PubMed] [Google Scholar]
- 7. Cohen-Sfady M, Pevsner-Fischer M, Margalit R, et al. (2009) Heat shock protein 60, via MyD88 innate signaling, protects B cells from apoptosis, spontaneous and induced. Journal of Immunology 183: 890–896. [DOI] [PubMed] [Google Scholar]
- 8. Yoo CG, Lee S, Lee CT, et al. (2000) Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. Journal of Immunology 164: 5416–5423. [DOI] [PubMed] [Google Scholar]
- 9. Di Stefano A, Ricciardolo FLM, Caramori G, et al. (2017) Bronchial inflammation and bacterial load in stable COPD is associated with TLR4 overexpression. European Respiratory Journal 49(5): 1602006. [DOI] [PubMed] [Google Scholar]
- 10. Cozens AL, Yezzi MJ, Kunzelmann K, et al. (1994) CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. American Journal of Respiratory Cell and Molecular Biology 10: 38–47. [DOI] [PubMed] [Google Scholar]
- 11. Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 12. Takuma K, Mori K, Lee E, et al. (2002) Heat shock inhibits hydrogen peroxide-induced apoptosis in cultured astrocytes. Brain Research 946: 232–238. [DOI] [PubMed] [Google Scholar]
- 13. Zhao C, Hui W, Fernandes MJ, et al. (2014) Lysophosphatidic acid-induced IL-8 secretion involves MSK1 and MSK2 mediated activation of CREB1 in human fibroblast-like synoviocytes. Biochemical Pharmacology 90(1): 62–72. [DOI] [PubMed] [Google Scholar]
- 14. Pace E, Ferraro M, Siena L, et al. (2008) Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 124: 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu X, Paik PK, Chen J, et al. (2006) IFN- suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 24: 563–574. [DOI] [PubMed] [Google Scholar]
- 16. Parry GC, Mackman N. (1997) Role of cyclic AMP response element binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. Journal of Immunology 159: 5450–5456. [PubMed] [Google Scholar]