Abstract
Chordoid meningioma (CM) is a rare subtype of meningioma, which represents only 0.5% of all meningiomas. It is classified as Grade II according to the World Health Organization classification because of its tendency to relapse. Pathological and clinical characteristics have been studied in order to forecast the future evolution of the lesions. However, information about infiltration of macrophagic elements and mast cells is very scarce. The authors analyzed the immunohistochemical patterns of three cases and a relapse of CM, in order to verify whether infiltrating macrophages are in a polarized state and what would be the proportion between such elements and mastocytes. Results suggest that macrophages in CMs are mainly in a non-polarized or M2 state and their abundance might be associated with a major potential of relapse; additionally, there is an inverse correlation between the number of mast cells and macrophages. Further studies are requested in order to confirm these intriguing data.
Keywords: cancer, chordoid meningioma, innate immunity, macrophage polarization
Introduction
Chordoid meningioma (CM) is a rare subtype of meningioma, accounting to 0.5% of all meningiomas. This lesion normally contains regions that are histologically similar to chordoma, with trabeculae of eosinophilic, vacuolated cells in a myxoid background. The marked tendency to relapse after resection assigned a Grade II by the World Health Organization (WHO).1 Pathological and clinical characteristics of CM have been studied for diagnostic, prognostic, and hypothetical therapeutic purposes,2 but data about innate immunity regulation in such a type of tumors are scarce and mainly described in relation to other meningiomas.2–5 It is well known that mast cells are present in meningiomas, with an increasing number related to the aggressiveness of the neoplasia, but no data are available about the possible relationship between CMs relapse and the abundance of mastocyte population. On the other hand, mononuclear cells that infiltrate meningiomas are mainly composed of T cells and macrophages. Given the intriguing behavior of macrophage populations recruited in the peritumoral space or infiltrating tumor, it seems an important shortcoming for understanding the biology of CM, the lack of studies that analyze the macrophage polarization state. The functional and prognostic significance of the CD68-positive cells detection is currently unclear. Finally, the interaction between innate immunity cells like mast cells and macrophages in meningiomas is actually unknown, in spite of the functional link between these types of cells.5 Here, we present the results of an immunohistochemical analysis of three cases and one relapse of CM, in order to verify the polarization state engaged among macrophages population and what is the proportion between such elements and mastocytes.
Materials and methods
Three patients underwent neurosurgical resection for endocranial tumor. Histopathological diagnosis of CM was confirmed. All subjects expressed their consent for their inclusion in this study, and the expression of markers for macrophages and mast cells characterization was examined by immunohistochemistry. One of the patients had a relapsing lesion, afterward (Table 1).
Table 1.
Characteristics of patients suffering from CMs.
| Case | Sex | Age | Tumor location | DFAR (years) |
|---|---|---|---|---|
| CM1 | M | 73 | Orbito-sphenoidal | 13 |
| CM2 | F | 42 | Sphenoidal | 7 |
| CM3 | M | 57 | Middle cranial fossa | 3 |
| CM3-Ra | M | 60 | Middle cranial fossa | 8 |
DFAR: disease free after resection.
CM3-R is a relapse of case CM3.
Five-micrometer-thick sections, prepared from the tumoral tissue of all cases, were analyzed using an Autostainer Link 48 Dako (Dako Italia, Milano), testing the immunoreactivity with a 1:500 dilution of mouse anti-human CD68 antibody and 1:50 dilution of mouse anti-human CD31 (Dako Italia, Milano), 1:100 dilution of mouse anti-human inducible nitric oxide synthase (iNOS) (Santa Cruz, Milano), 1:200 dilution of mouse anti-CD163 (Leica Biosystems), and 1:150 dilution of mouse monoclonal (clone 10D11) anti-human tryptase antibodies (Leica, Mannheim, Germany). CD68+ cells were considered as M0, M1, and M2 mixed macrophages population; CD163+ cells were recognized as M2 polarized macrophages, while iNOS+ cells were considered typical M1 macrophages, when a consistent morphology was also appreciable. Mast cells in all cases were recognized for anti-tryptase immunoreactivity.6 The abundance of infiltrating tryptase+, CD68+, iNOS+, and CD163+ cells was evaluated analyzing 10 different areas, randomly selected over the entire section. A direct cell count has been carried out using the cell count function in Image J 1.427 software analyzing over 10 fields for all cases involved in this study. Each field consisted of a photo obtained at 400× magnification.
A correlation index between mastocytes and macrophage counting data was obtained applying the least squares fitting method.
Results
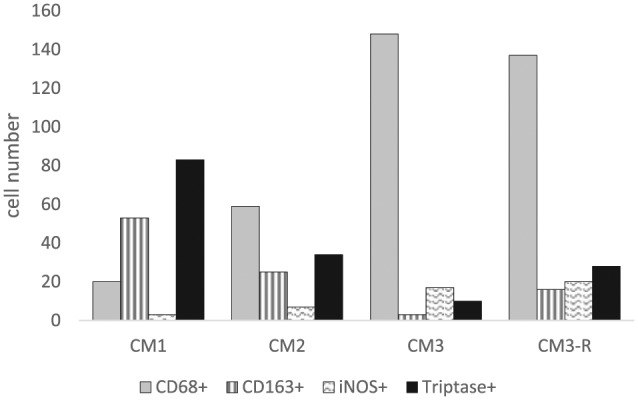
The results relative to the CD68, CD163, iNOS, and tryptase-positive cells counting are reported in Table 2. The numerosity of CD68-positive macrophages is higher in case CM3 and in its relapse (CM3-R) and, in all cases, CD68-positive macrophages outnumber the count of CD163-positive cells. We found a negative correlation coefficient (r = −0.886; P = 0.114) between macrophages CD68+ and tryptase-positive mast cells number. Inducible-NOS immunoreactivity pattern allowed us to stain only rare cells with a compatible morphology that would resemble the macrophage phenotype. An iNOS immunostaining was also observed in tumoral cells, in relapsing meningioma (case CM3-R) (Figures 1 and 2).
Table 2.
The absolute cell count obtained from 10 fields.
| Case | CD68+ | CD163+ | iNOS+ | Tryptase+ |
|---|---|---|---|---|
| CM1 | 20 | 53 | 3 | 83 |
| CM2 | 59 | 25 | 7 | 34 |
| CM3 | 148 | 3 | 17 | 10 |
| CM3-R | 137 | 16 | 20 | 28 |
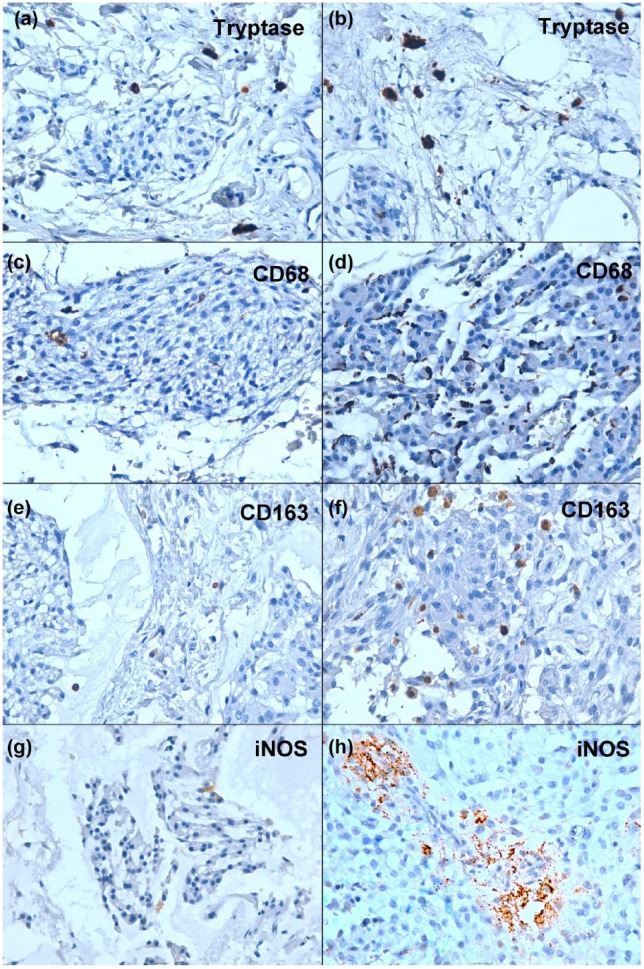
Figure 1.
(a) and (b) immunostaining for anti-tryptase in case CM1 and case CM3 respectively. (c) and (d) immunostaining for anti-CD68 in case CM2 and CM3 respectively. Please note that in panel (c) only few scattered elements are positive and in panel d positive cells are more numerous. (e) and (f) Immunostaining for anti-CD163 in case CM2 and CM3, respectively; the positive elements are more abundant in CM3 case than in CM2. (g) Very rare cells positively stained with anti-iNOS in case CM3. (h) The same immunoreactivity in the CM3 relapse (case CM3-R) with iNOS positivity detectable in tumoral cells.
Figure 2.
Immune cells infiltrating chordoid meningiomas.
Discussion
Innate immunity acts as a key factor in growth and diffusion of tumors. Macrophages and mast cells have a pivotal role on the regulation of various events in cancer tissues.8,9 All interactions between mast cells and other subset of immune cells are always bidirectional: the most important role of mastocytes in innate responses appears to be the capacity to recruit the innate immunity cells such as neutrophils, eosinophils, and macrophages.5 In turn, macrophages polarization and imbalance can affect mast cell activities.10 In the tumoral pathology, the functional interactions between mast cells and polarized macrophages have not been studied previously. In our study, we found, in all cases, an inverse correlation between macrophages and mast cells numerosity; this is probably due to the direct influence of the neoplastic cells or the particular polarization state of macrophages showing phenotype between M0 and M2. Indeed, the main macrophage population we found is CD68+/CD163+ (or CD68+/CD163±) and classically M2 cells are CD68+/CD163+.
M2 polarized macrophages are the typical tumor-associated macrophages (TAMs) promoting malignancy of the primary tumors; TAMs can stimulate angiogenesis and enhance tumor cell invasiveness, motility, and intravasation.11 Also the CMs’ tendency to relapse may be due to the particular polarized, pro-tumoral state of macrophages. However, a small mastocyte delegation has been found in some types of malignant tumors and we cannot exclude that a particular balance between macrophages and mast cells in CMs, could reflect a favorable pattern of innate immunity reaction, for growth and relapse of neoplasia. Besides, the iNOS expression is important in tumors and other diseases.12–14 In cancer, during the tumor growth phases, the cancerous epithelial cells exert tumorigenic properties via iNOS activation while the environmental stroma population, among which TAMs, may bring into play tumoricidal activity also via iNOS activity.12 In relapsing CM, iNOS expression was detected in tumoral cells. This characteristic may be related to progression of such a kind of lesions.15 In conclusion, we are aware that the small sample size of this study limits the generalization of results. However, we consider that the findings of this work, mainly the presence of macrophages and mast cells in that neoplasia with an inverse profile, may open new interesting perspective for the knowledge about CMs biology and may provide insight for novel possibilities in therapy and clinical management. In fact, in cases of arduous surgical access and subtotal removal or multiple recurrences, it could be possible to pharmacologically modulate these tumor recruited populations, in such a way to influence the growth of that neoplasm.9,11
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Perry A, Louis DN, Budka H, et al. (2016) Meningioma variants. In: Louis DN, Ohgaki H, Wiestler OD, et al. (eds) WHO Classification of Tumours of the Central Nervous System, 4th edn. Lyon: IARC, pp. 237–245. [Google Scholar]
- 2. Yang Y, Li D, Cao XY, et al. (2016) Clinical features, treatment, and prognostic factors of chordoid meningioma: Radiological and pathological features in 60 cases of chordoid meningioma. World Neurosurgery 93: 198–207. [DOI] [PubMed] [Google Scholar]
- 3. Polyzoidis S, Koletsa T, Panagiotidou S, et al. (2015) Mast cells in meningiomas and brain inflammation. Journal of Neuroinflammation 12: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bø L, Mørk SJ, Nyland H. (1992) An immunohistochemical study of mononuclear cells in meningiomas. Neuropathology and Applied Neurobiology 18(6): 548–558. [DOI] [PubMed] [Google Scholar]
- 5. Cardamone C, Parente R, De Feo G, et al. (2016) Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunology Letters 178: 10–14. [DOI] [PubMed] [Google Scholar]
- 6. Donato G, Conforti F, Camastra C, et al. (2014) The role of mast cell tryptases in cardiac myxoma: Histogenesis and development of a challenging tumor. Oncology Letters 8(1): 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collins TJ. (2007) ImageJ for microscopy. Biotechniques 43(1): 25–30. [DOI] [PubMed] [Google Scholar]
- 8. Chanmee T, Ontong P, Konno K, et al. (2014) Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 6: 1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dyduch G, Kaczmarczyk K, Okoń K. (2012) Mast cells and cancer: Enemies or allies? Polish Journal of Pathology 63(1): 1–7. [PubMed] [Google Scholar]
- 10. Hasan D, Chalouhi N, Jabbour P, et al. (2012) Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. Journal of Neuroinflammation 9: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noy R, Pollard JW. (2014) Tumor-associated macrophages: From mechanisms to therapy. Immunity 41(1): 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vannini F, Kashfi K, Nath N. (2015) The dual role of iNOS in cancer. Redox Biology 6: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J, Bae EH, Ma SK, et al. (2016) Altered nitric oxide system in cardiovascular and renal diseases. Chonnam Medical Journal 52: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perrotta I, Brunelli E, Sciangula A, et al. (2011) iNOS induction and PARP-1 activation in human atherosclerotic lesions: An immunohistochemical and ultrastructural approach. Cardiovascular Pathology 20: 195–203. [DOI] [PubMed] [Google Scholar]
- 15. Donato G, Ferraro G, Signorelli F, et al. (2006) Chordoid meningioma: Case report and literature review. Ultrastructural Pathology 30(4): 309–314. [DOI] [PubMed] [Google Scholar]