Abstract
Introduction:
Studies on biomaterials involve assays aimed to assess the interactions between the biomaterial and the cells seeded on its surface. However, the morphology of biomaterials is heterogeneous and it could be tricky to standardize the results among different biomaterials and the classic plastic plates. In this light, we decided to create, by means of computer-aided design (CAD) technology, a standardized sample model, with equal shape and sizes, able to fit into a classic shape of a 96-wells tissue culture plate (TCP).
Methods:
The design of this sample consists of a hole in the top in order to allow the injected cells to settle without them being able to slip from the sides of the sample to the bottom of the TCP wells. This CAD project is made using the software Pro-Engineer. The sample will totally fill the wells of the 96-well TCP. Dental pulp stem cells have been used to assess the ability of the different sample to support and promote the cell proliferation.
Results:
Twelve titanium, 12 gold–palladium, and 12 zirconium oxide customized samples were designed by means of the software cam powermill, by importing the .stl file created in Pro-Engineer software. The proliferation rate of the tested scaffolds showed to be similar to the control in the group with the customized shape.
Conclusion:
We think that our method can be useful to test different types of scaffolds when a greater accuracy of the measurements is desirable in order to verify the cell behavior of these scaffolds. Our innovative method can improve the standardization process in the evaluation of cell behavior on different biomaterials to open the way to more reliable tests on biomatrices functionalized with drugs or growth factors applied to the future regenerative medicine.
Keywords: biomaterials, regenerative medicine, stem cells
Introduction
Studies on biomaterials need several investigations about the interactions between the biomaterial and the cells commonly seeded on its upper surface. The interaction between the upper surface of a biomaterial and the cells seeded on it is the first and main check to understand how the biomaterials will interact with the biological components of a biological tissue.1
It is important to test every new biomaterial by using several in vitro experiments to check some important parameters such as the cell adhesion, the ability of the scaffold to support the cell growth or to promote the cell differentiation; these experiments should be performed on many samples and every test should be controlled by many replicates. In this light, it is important that the biomaterials are tested in comparison with a “control.” Usually, the control is represented by the plastic tissue culture plate (TCP). Another concern is about the upper surface of the biomaterial to be tested: this surface, in fact, should be properly juxtaposed in the circular shape of the plastic culture wells, in order not to disperse an important number of cells and, therefore, to have an amount of cell comparable to what is found in the “control” wells.2
We decided to create a standardized sample by means of a computer-aided design (CAD) project. The resulting shape of the sample will be able to perfectly fit the morphology of a 96-wells plastic culture plate. This study represents an innovation in the understanding of the interaction between the bioscaffolds and cells, giving a more reliable and standardized point of view in the studies on the cell biology related to different biomaterials. This study will allow also the performing of reliable studies on the behavior of cells seeded onto scaffolds functionalized with drugs or growth factors (GFs), giving a strong support to the research on the interaction between the drugs and the biological tissues.
Materials and methods
The purpose of this study was to design and create scaffolds made of different biomaterials but with the same shape: the main requirement of this shape was to make the scaffolds fit into the holes of a 96-wells plastic culture plate. Moreover, it was necessary that this shape was able to retain the cells on the upper surface of the scaffold after they were seeded on its top.
Sample design
We designed a structure characterized by a large hole on its top: this hole is delimited by a wall to allow the injected cells to be retained on the surface of the scaffold, without slipping from the sides of the sample to the bottom of the wells as commonly happens in similar experiments. This structure was designed by means of a CAD project, by using the software Pro-Engineer (Pro-E) (Figure 1).
Figure 1.

Pro-E CAD project of the first sample.
The dimensions of this customized sample (Figure 2) are:
Figure 2.

Drawing of the customized sample.
External diameter: 6 mm
Hole diameter: 5 mm
External height: 4 mm
Hole height: 2 mm
The gap between the external surface of the sample and the internal surface of the plastic wells was measured to be 0.175 mm. Moreover, the amount of culture medium (CM) that can be placed into the hole inside the sample was estimated to be about 40 μL.
We tested the customized samples made of different materials to verify the cell behavior after the cells were seeded on each sample.
We chose to build a first series of customized samples with the following test materials: titanium, gold–palladium alloy, and zirconium oxide. These materials are commonly used for realizing several type of prostheses, such as dental prostheses.
Cell culture
We decided to use dental pulp stem cells (DPSCs) for our experiments, given their high clonogenic potential and in the light of their ease of harvest.6–8
DPSCs were isolated from dental pulp tissue by using the enzymatic digestion: dental pulp was collected after a minimally traumatic tooth extraction, and the selected teeth were free of caries of other infections. Human dental pulp tissue was obtained from two healthy female volunteers (age, 33 and 31 years) who provided written informed consent at the Calabrodental dental clinic (Crotone, Italy). The study was carried out under approved guidelines of the internal Ethical Committee. Culture protocol was carried out following previously described procedures.6–8
Cell counting
We chose to calculate the population doubling (PD) rate to better understand the cell viability on our three different biomaterials with respect to TCP control. DPSCs were seeded (baseline density: 200 × 103 cells in 6 wells) in the classic CM. Cells were re-plated and cultured until passage 6. Cumulative population doublings (cPD) index was calculated at each passage so to create a time-dependent curve.
Cell proliferation
The selected biomaterials underwent cell proliferation assay. We directly put 50,000 DPSCs on our customized samples made from the following materials: titanium, gold–palladium alloy, and zirconium oxide. A similar amount of cells was plated on the same biomaterials but did not undergo any milling process. DPSCs were cultured into 100 μl of CM and properly replaced when necessary, in agreement with our previously published protocol.6
We performed the cell count at days 3 and 5 after the first cell placement. We only considered vital cells in our results. Cell proliferation assays were performed in triplicate.
Results were calculated as the mean ± SD of three autonomous experiments and the statistical significance was evaluated by one-way analysis of variance (ANOVA) and by Student’s t-test. Different results were considered significant with P <0.05 (*).
Results
The main aim of this research was to evaluated the feasibility of customized samples made of different biomaterials with a standardized shape to ensure better suitability of each material during the in vitro analyses.
As a primary result, we made the scaffolds following the description in the methods section.
Titanium customized samples
We created the model using the software Cam Powermill by importing the .stl file saved by Pro-E software. We used a titanium plate with a diameter of 100 mm and a height of 16 mm. We loaded 12 samples on the plate in the computer-aided manufacturing (CAM) software and we worked on the project (Figure 3). Before the milling, we linked each structure with sustaining pins to the plate to immobilize them during the milling process.
Figure 3.

The customized scaffolds. Four titanium samples, four gold–palladium samples, and four zirconia samples.
After 11 working hours we obtained the customized samples made in titanium.
Gold–palladium customized samples
We realized 12 customized samples in a gold–palladium alloy. Samples were previously realized in c-cast materials using the software Cam Powermill, to be ready for the milling process. Subsequently, we melted these c-cast samples to obtain the customized samples in a gold–palladium alloy.
We used a c-cast plate with a diameter of 100 mm and a height of 20 mm. We then loaded 12 samples on the plate in the CAM software and we started the milling process, after having linked each structure to the main plate with sustaining pins to have stability during the milling process.
After melting, the customized samples were successfully obtained.
Zirconium oxide customized samples
We used a zirconia plate with a diameter of 100 mm and a height of 16 mm with a retraction factor of 1245. We loaded 12 samples on the plate in the CAM software and then we processed the project.
After melting, the customized samples were successfully obtained.
We verified by cell counter the amount of cells retained on the top of the customized scaffolds with respect to the amount of cells plated and retained on the free surface of casual shaped scaffolds as controls.
Cell proliferation
The evaluation of proliferation rates by measuring cell viability at various time points indicated that DPSCs had a higher proliferation rate on a titanium surface. The zirconium oxide shows a very good proliferation score, while the gold–palladium alloy reports the worst performance, even if these last two materials are otherwise better than the control (TCPs) (Figure 4).
Figure 4.
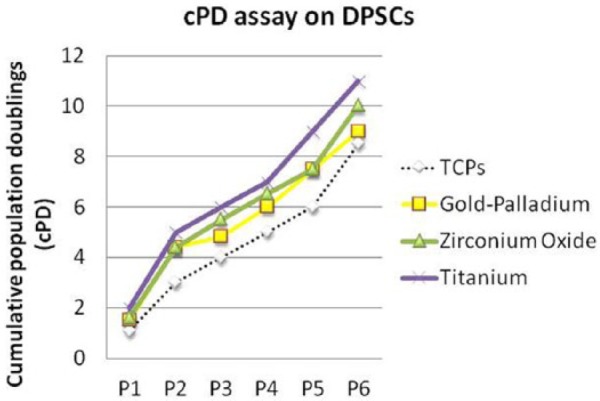
Cumulative population doublings (cPD) of dental pulp stem cells (DPSCs). The cPD was obtained with the following method: PD = [ log10(N) − log10(Ns)] / log10(2), (N = harvested cell number; Ns = the initial plated cell number).
Figure 5.

Cell-seeded on different scaffolds, either the customized shape and the free shape. TCPs were used as control.
We also evaluate the DPSCs proliferation on titanium, gold–palladium alloy and zirconium oxide; the same evaluations have been performed on the same biomaterials but did not undergo any milling process. The cell count was performed at day 1, day 3, and day 5. TCPs were used as control.
At day 1, we observed the same amount of cells on the different samples, customized and not, as those found on TCPs.
At day 3, the scaffolds with customized shape showed a cell count similar to TCPs; on the other hand, the same scaffold with the free shape had the worst performances, even if only the variation shaped/not-shaped related to the titanium scaffolds were statistically significant.
At day 5, the scaffolds with customized shape continued to register a cell count similar to TCPs; moreover, the variation shaped/not-shaped related to the titanium scaffolds confirmed to be statistically significant.
The overall consideration is that the customized scaffolds showed a cell proliferation rate similar to the TCPs; often we found a better proliferation rate, as registered with the titanium scaffolds, and in only one case we found values lower than the control (gold–palladium alloy).
Discussion
The biocompatibility of a material, as well as the capacity of a material to allow cell growth, are topics commonly studied in pre-clinical stages in order to verify if the investigated material could be used in the construction of prostheses or other biological devices. The innovative biomaterials are used in different clinical applications, but their performances are widely investigated to ensure their best performance. Furthermore, the working phases to which the biomaterials are subjected during the milling processes related to the CAD–CAM technology are studied for many years and no strong evidence has been reported against this production process. In conclusion, the reported biomechanical tests seem to confirm that the biomaterials subjected to CAM milling processes retain their physic properties and the only alterations are due to errors in the design stage.3 Therefore, the comparability between two or more materials is an important prerequisite to clearly define the characteristics of the investigated materials. In recent years, regenerative medicine issues have stimulated research to investigate an elevated number of new biomatrices4 aimed at improving tissue healing; in this light, the old concept of bioscaffolds has given way to new concepts, such as acellular scaffolds,5 cell niche, and cell commitment.6–10
Regarding the scaffolds, the exposed surface area and porosity play major roles in the proliferative response of cultured cells.11 Moreover, the architecture and the design of scaffolds have a clear effect on cells seeding and culturing.12,13
Finally, the superficial modifications of the used biomaterials as well as the modifications of the culturing conditions may change the response of the cells cultured and grown on the scaffold.14,15
In view of this, a standardization of the shape and the surface of the scaffolds, even when they are composed of different materials, is an important factor for normalization of the results obtained from the analysis of the cellular response on the tested scaffolds.
Conclusions
We conclude that the use of this standardized design to test different types of scaffolds may be a way to ensure a greater accuracy of the measurements in the assessment of the cellular response on a biomaterial. This method could reduce bias during measurements and, furthermore, could allow a greater standardization of tests and results. Finally, we propose that the same approach can also be applied, in the near future, to study the effects of different three-dimensional biomaterials on cell behavior, especially in the field of stem cell research.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Ballini A, Cantore S, Capodiferro S, et al. (2009) Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. International Journal of Medical Sciences 6: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tatullo M, Marrelli M, Falisi G, et al. (2016) Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. International Journal of Immunopathology and Pharmacology 29: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marrelli M, Maletta C, Inchingolo F, et al. (2013) Three-point bending tests of zirconia core/veneer ceramics for dental restorations. International Journal of Dentistry 2013: 831976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marrelli M, Tatullo M. (2013) Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. European Review for Medical and Pharmacological Sciences 17: 1958–1962. [PubMed] [Google Scholar]
- 5. Perniconi B, Coletti D, Aulino P, et al. (2014) Muscle acellular scaffold as a biomaterial: Effects on C2C12 cell differentiation and interaction with the murine host environment. Frontiers in Physiology 5: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marrelli M, Paduano F, Tatullo M. (2013) Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. International Journal of Biological Sciences 9: 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tatullo M, Marrelli M, Shakesheff KM, et al. (2015) Dental pulp stem cells: Function, isolation and applications in regenerative medicine. Journal of Tissue Engineering and Regenerative Medicine 9: 1205–1216. [DOI] [PubMed] [Google Scholar]
- 8. Tatullo M, Marrelli M, Paduano F. (2015) The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. International Journal of Medical Sciences 12: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marrelli M, Paduano F, Tatullo M. (2015) Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. Journal of Dental Research 94: 843–852. [DOI] [PubMed] [Google Scholar]
- 10. Aulino P, Costa A, Chiaravalloti E, et al. (2015) Muscle extracellular matrix scaffold is a multipotent environment. International Journal of Medical Sciences 12: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carletti E, Motta A, Migliaresi C. (2011) Scaffolds for tissue engineering and 3D cell culture. Methods in Molecular Biology 695: 17–39. [DOI] [PubMed] [Google Scholar]
- 12. Melchels FP, Tonnarelli B, Olivares AL, et al. (2011) The influence of the scaffold design on the distribution of adhering cells after perfusion cell seeding. Biomaterials 32: 2878–2884. [DOI] [PubMed] [Google Scholar]
- 13. Melchels FP, Barradas AM, van Blitterswijk CA, et al. (2010) Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomaterialia 6: 4208–4217. [DOI] [PubMed] [Google Scholar]
- 14. Ballini A, Mastrangelo F, Gastaldi G, et al. Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: A good promise for tissue engineering. Journal of Biological Regulators and Homeostatic Agents 29: 813–822. [PubMed] [Google Scholar]
- 15. Marrelli M, Falisi G, Apicella A, et al. (2015) Behaviour of dental pulp stem cells on different types of innovative mesoporous and nanoporous silicon scaffolds with different functionalizations of the surfaces. Journal of Biological Regulators and Homeostatic Agents 29: 991–997. [PubMed] [Google Scholar]


