Abstract
We describe Caucasian monozygotic twin brothers with rheumatoid arthritis (RA) and discuss influence of predictors to methotrexate (MTX) outcome treatment. Single nucleotide polymorphisms (SNPs) of the MTX metabolic pathways were genotyped. Twins have multiple mutations: a CC mutation of SNP 1298A>C in methylenetetrahydrofolate reductase (MTHFR) gene, CC mutations of three SNPs in the adenosine receptor gene ADORA2A (rs3761422_4217241T>C, rs2267076_4221164T>C, rs2236624_4226593T>C), and a heterozygous genotype in SNPs ATIC_rs2372536_347C>G, MTHFD1_rs2236225_1958G>A. These mutations are known to predict a worse outcome of MTX treatment. The twins had different lifestyles (alcohol drinking and smoking in Twin 1, regular coffee consumption in Twin 2), but a very similar clinical presentation of the outset of RA, radiographic scoring according to the Sharp/van der Heijde method with an almost identical antibodies presentation. The period of the patients before anti-TNFα treatment was characterized by unsuccessful per oral MTX pharmacotherapy in both cases (a low effect of MTX in Twin 1; an early discontinuation of MTX due to an adverse event in Twin 2). In both twins, the outcome of well-controlled anti-TNFα treatment (co-medication with MTX in Twin 1) for 10 years was expressed as low disease activity measured using composite index DAS28. It is interesting that Twin 2 had an unfavorable radiographic scoring after a 10-year follow-up than Twin 1 in spite of the comparable DAS28 in Twin 2 and smoking in Twin 1. In conclusion, co-medication of MTX with biologics may impact on RA radiographic progression despite predicted bad MTX outcome based on pharmacogenetic analysis.
Keywords: methotrexate, pharmacogenetics, rheumatoid arthritis, twins
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease with a complex origin. Previous studies have reported heritability estimated in RA at about 60%. Potential factors influencing disease course and therapeutic outcome can be classified according to clinicopathological variables, which can in turn be divided into patient-related variables (duration, activity, disability, and biomarkers); treatment related variables (compliance, dose, and previous drugs);1,2 and genetic factors such as genetic polymorphisms implicated in key methotrexate (MTX) pathway genes.3–5 Several studies have been performed to evaluate the influence of clinico-pathological variables on clinical responses to MTX.1,6 Nevertheless, there is no consensus on which factors can be used as predictors.2 Clinical predictors of response to treatment also include cigarette smoking status, rheumatoid factor (RF) status, anti-cyclic citrullinated peptide antibody (ACPA) status as well as the use of other/prior disease modifying anti-rheumatic drugs (DMARDs).1,7
Several studies have demonstrated that variations regarding clinical response to MTX could be explained by genetic polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene and in the MTX metabolic adenosine pathway genes.3–8
Epigenetic modifications such as DNA methylation, histone modification, and microRNA signaling regulate gene expression and are sensitive to external stimuli. These factors might bridge the gap between genetic and environmental factors and thus may be ideal targets for new personalized treatments. Recent advances in epigenetics have enhanced knowledge of how environmental factors (ultraviolet [UV] radiation, drugs, infections, etc.) may contribute to epigenetic regulation.9
We describe a pair of Caucasian monozygotic twin brothers who have RA and were participants in a previous MTX pharmacogenetic study of ours.10 In the present study, single nucleotide polymorphisms (SNPs) of the MTX metabolic pathways were genotyped. We discuss genetic predictors and the influence of clinical variables on MTX treatment outcome.
Methods
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Germany). Genotyping was performed using quantitative PCR with allelic discrimination using commercial TaqMan (allele-specific) assays (Life Technologies, USA): C_16189248_10 (ADORA2A, rs2298383), C_2446666_20 (ADORA2A, rs3761422), C_2446667_1 (ADORA2A, rs2267076), C_15954834_20 (ADORA2A, rs2236624), C_33603912_10 (AMPD1, rs17602729), C_16218146_10 (ATIC, rs2372536), C_27465000_10 (ITPA, rs1127354), C_1376137_10 (MTHFD1, rs2236225), C_850486_20 (MTHFR, rs1801131), C_1202883_20 (MTHFR, rs1801133). High quality data were obtained for all samples and SNPs tested. Using a Modular Analyzer from Roche Diagnostics, thurbidimetry was employed for the evaluation of the C-reactive protein (CRP) - normal range 0–5 mg/L. ANA, ACPA, and ELISA analyses were performed using the commercially available kit Immunoscan (Euro-Diagnostica, Sweden). RF level was detected with the ELISA kits RF IgG, IgA, IgM (Orgentec, Germany). Using commercial kits from Grifols, ELISA was performed for the evaluation of anti-infliximab and adalimumab antibodies (anti-infliximab >2 AU/mL; anti adalimumab >3.5 AU/mL, respectively). Serum levels of drugs were detected using ELISA (commercial kits Promonitor – normal level infliximab ⩾1.5 µg/mL; adalimumab ⩾0.8 g/mL, respectively). Both twins gave their written informed consent before genotyping, data presentation, and participation in the pharmacogenetics study. The work was approved by the Ethics Committee of the University Hospital, Hradec Kralove, Czech Republic and was conducted in accordance with the Declaration of Helsinki principles.
Case reports
Twin 1
The first 59-year-old man, with a positive family history (mother suffered from RA) as well as a history of arterial hypertension, developed RA at the age of 46 years (thus meeting the 1987 RA classification criteria of the American College of Rheumatology) with an acute onset of the disease activity (DAS28 = 4.9). This patient is a regular alcohol drinker (three beers/day), a minimal coffee drinker (⩽7 cups/week), and a smoker (15 cigarettes/day).
The onset of RA was characterized by positivity of ACPA 340.5 U/mL, RF positivity 90 U/mL, total ANA negativity, and radiographic scoring according to the Sharp/van der Heijde method was 12.11 Sulphasalazine 2 g/day and prednisone 10 mg/day were started, but RA was not under control. One year later, sulphasalazine was changed to per oral MTX 7.5 mg/week with folate supplementation. Within 3 months the dose was escalated to 15 mg/week. After 23 months’ duration of MTX, the treatment activity still persisted (DAS28 = 6.1), radiographic scoring 18, and functional score of the health assessment questionnaire (HAQ) was 1.13. The patient started anti-TNFα treatment, infliximab (dose 3 mg/kg/8 weeks) was added to MTX 15 mg/week and prednisone 7.5 mg/day. After 6 months of treatment, a low disease activity of RA was reached (DAS28 = 2.5; ∆DAS28 = 3.6) and from the 3rd to 10th years of treatment, remission persisted except for three small peaks of DAS28 to low disease activity (<2.6). After the 10th year, the patient developed anti-infliximab antibodies (4.0 AU/mL) with a decrease of infliximab serum level (0.04 µg/mL); simultaneously RA activity increased to DAS28 = 4.8. Infliximab was escalated to 5 mg/kg but it had no influence on the inflammatory activity. A subsequent step comprising a switch to golimumab 50 mg/month primarily failed (still combined with MTX 15 mg/week). Recently, therapy has been successfully switched to tocilizumab 600 mg/month (DAS28 <2.6). During 14 years of observation, this patient underwent synovectomy of the knee joint. Currently the patient has functional status on the level of HAQ 0.5 (Figure 1), disability pension gradus I; radiographic scoring was 26. The patient remains employed as a watchman.
Figure 1.
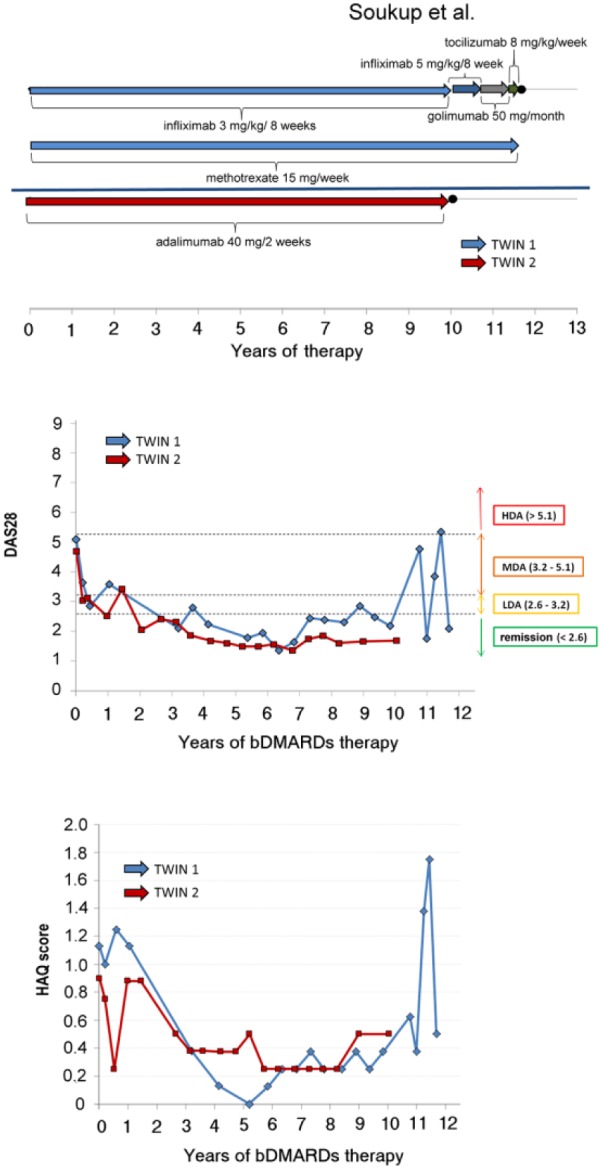
Clinical presentation in twins during period of bDMARDs treatment.
Twin 2
The genetically identical twin of this man developed seropositive erosive RA at the age of 47 years with a similar acute onset (DAS28 = 5.2), radiographic scoring was 28. He also developed ACPA (750.5 U/mL), RF positivity (1:2.560 titer) (total ANA was negative). The patient is a regular coffee drinker (three cups/day - coffee contains 115 g caffeine per cup on average) and a non-smoker. The patient irregularly consumes alcohol, fizzy drinks, and tea.
The first choice treatment was per oral methylprednisolone 16 mg/day and MTX 10 mg/week. After the initial dose, the patient suffered from severe nausea and vomiting. MTX was changed to sulphasalazine 2 g/day and subsequently leflunomide 20 mg/day, but without effect during 3 months. After a 7-month duration of DMARDs treatment, RA activity still persisted (DAS28 = 5.2), radiographic scoring was 46, and functional status measured by assessed HAQ was 0.90. Adalimumab monotherapy in standard dosing was started (40 mg subcutaneously every other week). RA activity has been well-controlled using this monotherapy for 10 years to the present date. After 12 months of treatment, DAS28 was significantly reduced (∆DAS28 = 2.0) to low disease activity (DAS28 = 3.2) and during 10 years of treatment remission has persisted except one small peak (DAS28 = 3.6) (Figure 1). During 13 years of observation, the patient underwent synovectomy of the knee and hip joint replacement. Currently the patient has favorable functional status (HAQ 0.5) (Fig 1.), disability pension gradus III; radiograph scoring according to the Sharp/van der Heijde method was 11911 (Figure 2).
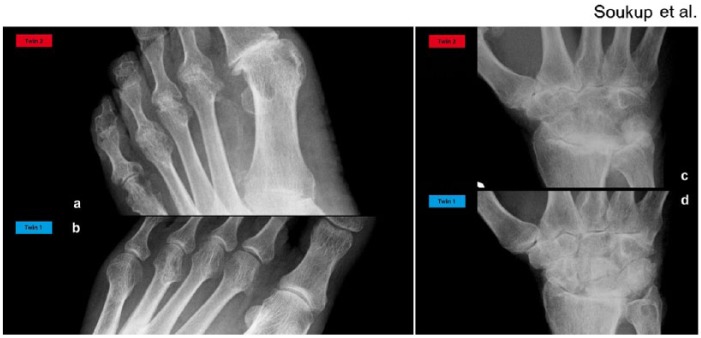
Figure 2.
Radiography of the metatarsophalangeal joints and of the wrist. (a, b) Radiography of the metatarsophalangeal joints. (a) Twin 2: Large marginal erosions of multiple joints, i.e. in the 2nd–5th metatarsophalangeal joints and in the 4th and 5th proximal interphalangeal joints. In the 5th metatarsophalangeal joint associated with mild joint space narrowing, in the 2nd–4th metatarsophalangeal with ankylosis. In the 1st metatarsophalangeal joint combined with osteoarthrosis. Erosion in the base of the 1st metatarsal, calcifications in the dorsal pedis artery. (b) Twin 1: Joint space narrowing of the 4th metatarsophalangeal joint and a large shallow erosion of the medial surface of the metatarsal head. (c, d) Radiography of the wrist. (c) Twin 2: Ankylosis of many joint spaces, i.e. the midcarpal compartment, intercarpal joint spaces, and part of the radiocarpal joint space, marked narrowing of the remaining joint spaces. Erosions of the distal radius, ulnar styloid, and a small erosion of the trapezium. (d) Twin 1: Widespread joint space narrowing of all the compartments of the right wrist, scapholunate dissociation, small erosion of the trapezium, large cystic lesion of the distal ulna, and calcification of the triangular fibrocartilage.
Results
Genotypization showed a high frequency of mutations in SNPs of folate and adenosine pathways in the studied twins. Patients have CC mutation in the MTHFR gene of SNP 1298A>C, CC mutations in three SNPs of adenosine receptor gene ADORA2A (rs3761422 4217241T>C, rs2267076 4221164T>C, rs2236624 4226593T>C), and heterozygous genotype in SNPs ATIC rs2372536 347C>G, MTHFD1 rs2236225 1958G>A. Human leukocyte antigen (HLA) typing revealed HLA-DRB1*0401 genotype in twins.
Discussion
In this case report, the twins have different lifestyles, but the same genetic background and immunopathological profile, very similar clinical manifestations of RA, and almost identical presentations of antibodies. The twins live in the same environment in the same city. The period before biological anti-TNFα treatment was characterized by unsuccessful MTX therapy in both cases. In both twins, anti-TNFα treatment was effective within 10 years and resulted in low disease activity measured using DAS28 and similar good-quality functional status expressed by HAQ. Twin 2 has unfavorable radiographic progression (despite non-smoking in contrast to Twin 1), in spite of the DAS28 remission and comparable DAS28 in Twin 2.
Clinical variables
Earlier studies indicated that clinical variables could play certain roles. The male gender is associated with a better response to MTX therapy.1 On the other hand, smokers respond worst to MTX, presenting a higher disease activity and severity. ACPA and ANAs auto-antibodies found in RA are strongly correlated with erosive disease, worse functional status, and higher disease activity associated with non-response.1 Moreover, coffee drinking is associated with MTX treatment failure, 12 caffeine being an adenosine receptor antagonist.12 In Twin 2, however, MTX treatment was discarded before potential MTX effect and caffeine influence. According to Lima et al., the combination of non-current smoking, ACPA and ANA positivity, higher HAQ, NSAIDs utilization, per oral administration route, and the 677TT MTHFR genotype can be a possible predictive factor of non-response to MTX.13
SNPs of MTHFR gene
A recent meta-analysis included 12 studies comprising a total of 2288 RA patients, the results of which suggest that the C677T and A1298C SNPs are associated with MTX toxicity in RA patients.8 In the case of genotype 1298CC presented in twins, we expect a possible influence on early MTX toxicity development showed in Twin 2.
SNPs of adenosine metabolic pathway
A study of Wessels et al. established a model for predicting the efficacy of MTX in patients with RA.4 The model considered sex, RF and smoking status, the DAS, and four SNPs in the AMPD1, ATIC, ITPA, and MTHFD genes (adenosine pathway). This prediction model was transformed into a scoring system in the range of 0–11.5. Scores of ⩽3.5 had a true positive response rate of 95%. Scores of ⩾6 had a true negative response rate of 86%, which proved to be the case with the scoring of our twins (see Table 1). In Twin 1, the impact of mutations in these SNPs could play role in MTX bad response. In Twin 2, early MTX toxicity (possibly influenced ADORA2A and MTHFR 1298A>C homozygotic mutations) hid the possible impact of these SNPs on MTX inefficacy.
Table 1.
Regression coefficients of the logistic regression model and the assigned scores for predicting efficacy of methotrexate monotherapy at the 6th month by Wessels et al.13
| Variable | Score* | B | Twin 1 | Twin 2 |
|---|---|---|---|---|
| Female sex | ||||
| Premenopausal | 1 | −1.2 | ||
| Postmenopausal | 1 | −0.79 | ||
| Male sex | 0 | – | 0 | 0 |
| DAS at baseline † | ||||
| −3.8 | 0 | – | ||
| >3.8 and ⩽5.1 | ||||
| 2nd quartile | 3 | −2.8 | ||
| 3nd quartile | 3 | −2.7 | 3 | |
| >5.1 | 3.5 | −3.4 | 3.5 | |
| RF status, smoking status | ||||
| RF-negative non-smoker | 0 | − | ||
| RF-negative smoker | 1 | −0.80 | ||
| RF-positive non-smoker | 1 | −0.75 | 1 | |
| RF-positive smoker | 2 | −2.2 | 2 | |
| Studied polymorphisms | ||||
| MTHFD1 1958AA genotype | 1 | −0.98 | ||
| AMPD1 34CC genotype | 1 | −1.2 | 1 | 1 |
| ITPA 94A allele carrier | 2 | −1.7 | ||
| ATIC 347 G allele carrier | 1 | −1.1 | 1 | 1 |
| Total score | 11.5 | 7 | 6.5 | |
Higher scores represent a higher probability of nonresponse to methotrexate.
DAS versus DAS28 transformation formula was used: DAS28 = (1.072 × DAS) + 0.938.
AMPD1, adenosine monophosphatedeaminase 1gene; ATIC, 5-aminoimidazole-4-carboxamide ribonucleotideformyltransferase/inosinemonophosphatecyclohydrolase gene; B, regression coefficient in the definite model; DAS, Disease Activity Score; ITPA, inosinetriphosphatase gene; MTHFD1, methylenetetrahydrofolate dehydrogenase 1 gene; RF, rheumatoid factor.
Adenosine receptor SNPs
According to Hider et al., all five studied SNPs within ADORA2A (which contains a carriage of the ADORA2A mutated allele) were associated with stopping MTX treatment due to the association was specific for gastrointestinal toxicity. No association was observed between ADORA2A and inefficacy outcomes. The twins are mutated in the three SNPs ADORA2A studied by Hider et al.2 Hypothetically, in the case of Twin 2, mutations of ADORA2A may have resulted in the onset of severe nausea and vomiting.
Combination of conventional synthetic and biologic DMARDs
The literature does not show a comparison of infliximab treatment in combination with MTX versus adalimumab monotherapy on radiographic progression. However, apart from radiographic data (according to EULAR recommendations for the management of RA 2013 update), biological DMARDs should be used preferentially in combination with MTX or other conventional synthetic DMARDs. Only tocilizumab has been repeatedly demonstrated to be superior as a monotherapy over MTX or other conventional synthetic DMARDs.14 However, it is unclear if the tocilizumab and MTX combination has a possible impact on radiographic progression.
In conclusion, the early discontinuation of MTX (mainly during the first year of RA duration) may have resulted in the unfavorable radiographic total score in Twin 2.
In summary: (1) in our twin study, the application of the pharmacogenetics model according to Wessels et al.4 and the results of the study by Hider et al.,2 as well as MTHFR 1298A>C homozygotic mutation, predicted negative MTX outcome. The pharmacogenetics of MTX have not been previously applied in twins to our knowledge; (2) MTX is an important anchor drug for RA treatment in monotherapy and useful in combination with conventional synthetic and biologic DMARDs; and (3) our observations suggest that co-medication biologics and MTX influences radiographic progression despite predicted unfavorable MTX outcome in pharmacogenetics analysis.
The combination of clinical and biological variables with SNPs in genes encoding proteins involved in the MTX action mechanism will hopefully help to predict MTX outcome, thus improving the treatment of patients with RA.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by research project MH CZ - DRO (UHHK, 00179906) to TS and supported by research project PRVOUK P37-08 (from Charles University).
References
- 1. Anderson JJ, Wells G, Verhoeven AC, et al. (2000) Factors predicting response to treatment in rheumatoid arthritis: The importance of disease duration. Arthritis and Rheumatism 43: 22–29. [DOI] [PubMed] [Google Scholar]
- 2. Hider SL, Buckley C, Silman AJ, et al. (2005) Factors influencing response to disease modifying antirheumatic drugs in patients with rheumatoid arthritis. Journal of Rheumatology 32: 11–16. [PubMed] [Google Scholar]
- 3. Aggarwal P, Naik S, Mishra KP, et al. (2006) Correlation between methotrexate efficacy & toxicity with C677T polymorphism of the methylenetetrahydrofolate gene in rheumatoid arthritis patients on folate supplementation. Indian Journal of Medical Research 124: 521–526. [PubMed] [Google Scholar]
- 4. Wessels JA, de Vries-Bouwstra JK, Heijmans BT, et al. (2006) Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single-nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis and Rheumatism 54: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 5. Owen SA, Lunt M, Bowes J, et al. (2013) MTHFR gene polymorphisms and outcome of methotrexate treatment in patients with rheumatoid arthritis: Analysis of key polymorphisms and meta-analysis of C677T and A1298C polymorphisms. Pharmacogenomics Journal 13: 137–147. [DOI] [PubMed] [Google Scholar]
- 6. Dervieux T, Furst D, Lein DO, et al. (2004) Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis and Rheumatism 50: 2766–2774. [DOI] [PubMed] [Google Scholar]
- 7. Wessels JA, van der Kooij SM, le Cessie S, et al. (2007) A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis and Rheumatism 56: 1765–1775. [DOI] [PubMed] [Google Scholar]
- 8. Song GG, Bae SC, Lee YH. (2014) Association of the MTHFR C677T and A1298C polymorphisms with methotrexate toxicity in rheumatoid arthritis: A meta-analysis. Clinical Rheumatology 33: 1715–1724. [DOI] [PubMed] [Google Scholar]
- 9. Picascia A, Grimaldi V, Pignalosa O, et al. (2015) Epigenetic control of autoimmune diseases: From bench to bedside. Clinical Immunology 157: 1–15. [DOI] [PubMed] [Google Scholar]
- 10. Soukup T, Dosedel M, Pavek P, et al. (2015) The impact of C677T and A1298C MTHFR polymorphisms on methotrexate therapeutic response in East Bohemian region rheumatoid arthritis patients. Rheumatology International 35: 1149–1161. [DOI] [PubMed] [Google Scholar]
- 11. van der Heijde D. (1999) How to read radiographs according to the Sharp/van der Heijde method. Journal of Rheumatology 26: 743–745. [PubMed] [Google Scholar]
- 12. Montesinos MC, Yap JS, Desai A, et al. (2000) Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: Evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis and Rheumatism 43: 656–663. [DOI] [PubMed] [Google Scholar]
- 13. Lima A, Monteiro J, Bernardes M, et al. (2014) Prediction of methotrexate clinical response in Portuguese rheumatoid arthritis patients: implication of MTHFR rs1801133 and ATIC rs4673993 polymorphisms. BioMed Research International 2014: 368681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smolen JS, Landewe R, Breedveld FC, et al. (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 73: 492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]