Abstract
Kruppel-like factor 6 (KLF6) as a novel tumor suppressive gene participates in multiple biological behaviors and plays an important role in regulating tumor cell growth and invasion. However, the functions of KLF6 in hepatocellular carcinoma (HCC) remain poorly understood. The expression level of KLF6 was examined by immunohistochemical assay in human HCC tissues, and KLF6-overexpressed HCC cells (SMCC-7721 and HepG2) were used for evaluating cell proliferation and invasion by MTT and Transwell assays. A subcutaneous HCC tumor model was established for assessing tumor growth in vivo. Our results showed that the expression of KLF6 was significantly downregulated in HCC tissues compared with the adjacent non-cancerous tissues (50.0% vs. 72.0%, P = 0.034) and negatively associated with the lymph-vascular space invasion (LVSI) in HCC patients (P = 0.003). Furthermore, overexpression of KLF6 reduced cell proliferation and weakened the cell invasive potential followed with the decreased expression of PCNA and MMP-9 in HCC cells. The in vivo experiment indicated that KLF6 overexpression suppressed the xenograft tumor growth. Therefore, our findings show that KLF6 suppresses growth and invasion of HCC cells in vitro and in vivo, suggesting a tumor suppressive function in HCC and provides the potential therapeutic target for the treatment of HCC.
Keywords: growth, hepatocellular carcinoma, invasion, Kruppel-like factor 6
Introduction
Hepatocellular carcinoma (HCC), one of the most common tumors and deadliest cancers worldwide, is a multifactorial disease linked with many causal risk factors such as infection with hepatitis viruses, alcohol, and obesity.1 Patients with chronic liver disease are at high risk for the tumorigenicity of HCC. Previously, liver transplantation and resection were considered the only available treatments for HCC, but until now high mortality of HCC patients has not been improved.2 Due to the increasing incidence of HCC, molecular biology and medical genetics have been made to understand cellular and molecular mechanisms of oncogene and anti-oncogene in this disease. The novel therapeutic strategies can be taken to combat liver cancer.
Krueppel-like factor (KLF) is a group of master regulators of gene expression conserved from flies to human, of which KLF6 is a member of the KLF family of transcription factors and regulates cell proliferation, differentiation, apoptosis, and angiogenesis.3 The expression of KLF6 mRNA and protein in tumor tissues such as gastric carcinoma,4 osteosarcoma,5 malignant melanoma,6 and breast cancer7 is significantly lower than that in normal tissues. Moreover, the expression of KLF6 is found in gastric cancer with early or advanced stage, suggesting the significant correlation of KLF6 expression with the progression of cancer.8 In addition, decreased expression of KLF6 is markedly related with histological differentiation, TNM stage, lymph node metastasis, and distant metastasis in gastric cancer4 and with a 3-year survival rate in malignant melanoma.6
But some reports indicate no association between KLF6 expression and increased risk of cervical cancer.9 To elucidate the function of KLF6 in cancer, we hypothesized that KLF6 might function as a tumor suppressor in HCC and serve as a potential therapeutic target for the treatment of cancer.
Materials and methods
Materials
Human HCC cell lines (SMCC-7721 and HepG2) were from the Institute of Biochemistry and Cell Biology (Shanghai, PR China). Lentivirus-mediated KLF6 overexpression was purchased from GENECHEM Biotech Co., Ltd. (Shanghai, PR China). All antibodies including KLF6 (specifically recognizing wild-type KLF6), PCNA, and MMP-9 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The tissue microarray was from Outdo Biotech Co., Ltd. (Shanghai, PR China). Six-week-old female immune-deficient nude mice (BALB/c-nu) were purchased from the Shanghai Laboratory Animal Center of Chinese Academy Sciences.
Drugs and reagents
Dulbecco’s Modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Gaithersburg, MD, USA); 3-(4,5)-dimethylthiahiazo (-z-yl)-3,5- di-phenytetrazoliumromide (MTT) was from Dingguo Biology (Shanghai, PR China); Apoptosis Kits were from KeyGEN biology (Nanjing, PR China); TRIzol Reagent and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA, USA); M-MLV Reverse Transcriptase was from Promega (Madison, WI, USA); SYBR Green Master Mixture was from Takara (Otsu, Japan); ECL-PLUS/Kit was obtained from Beyotime (Hainan, PR China).
Clinical samples and data
HCC tissues and the adjacent non-tumor tissues were acquired from the First Affiliated Hospital of Zhengzhou University from May 2010 to Dec 2015 (Supplementary Table 1). Our present study was approved by Medical Ethics Committee of Zhengzhou University and written informed consent was received from the HCC patients or their parents before sample collection. Two pathologists decided and checked the HCC cases.
Immunohistochemical staining
KLF6 antibody was used for immunohistochemical (IHC) examination of the protein expression of KLF6 in HCC tissue microarray. KLF6 antibody was applied at 1:200 dilutions. Endogenous peroxidase was blocked by incubation with freshly prepared 3% hydrogen peroxide with 0.1% sodium azide. Non-specific staining was blocked with 0.5% casein and 5% normal serum. Staining was developed with diaminobenzidine substrate and sections were counterstained with hematoxylin.
Quantification of protein expression
The expression of KLF6 was quantitatively evaluated according to the total immunostaining scores calculated as the product of a proportion score and an intensity score. The proportion and intensity of the staining was determined by two observers. The proportion score reflected the fraction of positive staining cells (score 0, <5%; score 1, 5–10%; score 2, 10–50%; score 3, 50–75%; score 4, >75%), and the intensity score represented the staining intensity (score 0, no staining; score 1, weak positive; score 2, moderate positive; score 3, strong positive). KLF6 was considered as negative expression if the score was less than 2, and positive expression if the score was more than 2.
Cell culture and infection
HCC cells, placed in a humidified atmosphere containing 5% CO2 at 37°C, were cultured in DMEM medium supplemented with 10% heat-inactivated FBS, 100 U/mL of penicillin and 100 μg/mL of streptomycin. When the cells reached more than 50% confluence, they were infected with lentivirus vector wild-type KLF6 or negative control virus and cultured at 37°C and 5% CO2. The clone infected with KLF6 overexpression was set as KLF6 group, that infected with negative control vectors was considered as NC group and non-infected HCC cells were as the Ctrl group.
Quantitative real-time PCR
To quantitatively examine the mRNA expression of KLF6 in HCC cells, Real-time PCR was implemented. Total RNA of each clone was extracted with TRIzol according to the manufacturer’s protocol. Reverse-transcription was performed using M-MLV and cDNA amplification was done using SYBR Green Master Mix kit. KLF6 gene was amplified using specific oligonucleotide primer sense 5’-AAGGTACCATGGACGTGCTCCCTATGTGC-3’ and anti-sense 5’-CGTCTAGA TCAGAGGTGCCTCTTCATGTG-3’ and GAPDH gene was used as an endogenous control.
Western blot assay
HCC cells were harvested and total protein extracts were prepared using lysis buffer (Tris-HCl, SDS, Mercaptoethanol, Glycerol). Cell extracts were boiled for 5 min in loading buffer and then equal amount of cell extracts were separated on 15% SDS-PAGE gels. Separated protein bands were transferred into polyvinylidene fluoride (PVDF) membranes and the membranes were blocked in 5% skim milk powder. The primary antibodies against KLF6, PCNA, and MMP-9 were diluted according to the instructions of antibodies and incubated overnight at 4°C. Horseradish peroxidase-linked secondary antibodies were added at a dilution ratio of 1:1000 and incubated at room temperature for 2 h. The membranes were washed with PBS for three times and the immunoreactive bands were visualized using ECL-PLUS/Kit according to the kit’s instruction.
Cell proliferation assay
Cell proliferation was analyzed with the MTT assay. HCC cells infected with KLF6 overexpression were incubated in 96-well plates with DMEM supplemented with 10% FBS. HCC cells were treated with 20 μL MTT dye at 0, 24 h, and 48 h and incubated with 150 μL of DMSO for 5 min. The color reaction was measured at 570 nm with enzyme immunoassay analyzer (Bio-Rad, American).
Transwell invasion assay
Transwell assay was performed by using a Transwell chamber (Qiagen, German) with pore size of 8.0 μm. The Transwell chamber was coated with Matrigel. A total of 1 × 106 cells were suspended in 200 μL serum-free medium and seeded in the upper compartment of the chamber. The lower compartment was loaded with 750 μL full culture medium containing 10% FBS. After being incubated at 37°C for 12 h, the membrane was fixed with formaldehyde and stained with hematoxylin. Then the trans-membrane cells were counted.
In vivo tumor xenograft studies
Six-week-old female immune-deficient nude mice (BALB/c-nu) were bred at the laboratory animal facility (Institute of Chinese Academy of Sciences, Shanghai, PR China), and were housed individually in micro-isolator ventilated cages with free access to water and food. All experimental procedures were performed according to the regulations and internal biosafety and bioethics guidelines of Shanghai Jiaotong University and the Shanghai Municipal Science and Technology Commission. Two mice were injected subcutaneously with 1 × 106 HCC cells in 50 µL of PBS pre-mixed with an equal volume of matrigel matrix (Becton Dickinson). Mice were monitored daily and developed a subcutaneous tumor. When the tumor size reached approximately 5 mm in length, they were surgically removed, cut into 1–2 mm3 pieces, and reseeded individually into other mice. When tumor size reached approximately 5 mm in length, the mice were randomly assigned as Ctrl (n = 5), NC (n = 5), and KLF6 groups (n = 5). In NC and KLF6 groups, 15 µL of lentivirus (MOI = 100), mediated NC, or KLF6 was injected into subcutaneous tumors using a multi-site injection format. Injections were repeated every other day after initial treatment. The tumor volume was measured with a caliper, using the formula: volume = (length × width)2 / 2. After 21 days, mice were sacrificed and their tumors were dissected for weight measurement.
Statistical analysis
The result of each experiment was shown as mean ± SD while applicable. All experiments were carried out three times. A statistically significant difference in each assay was determined by SPSS version 11.5. The difference in each group was tested for significance using the Kruskal–Wallis H test and ANOVA analysis of variance. P <0.05 was considered significant.
Results
The expression of KLF6 in HCC tissues
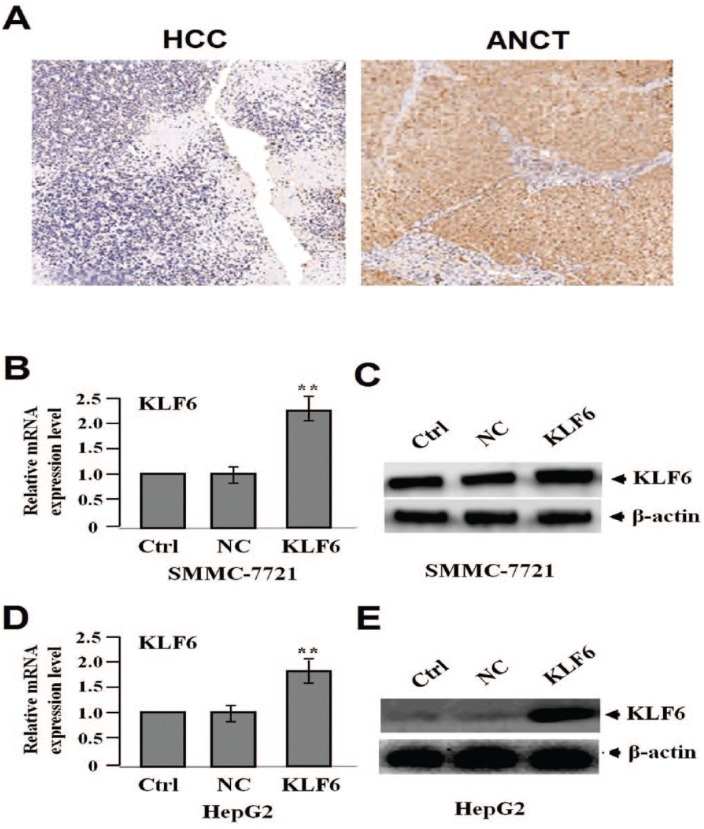
The protein expression level of KLF6 was detected by IHC staining. The positive expression of KLF6 was found in HCC tissues and para-carcinoma tissue cells and differential expression level of KLF6 (low expression in HCC vs. high expression in para-carcinoma) was demonstrated in Figure 1a. As shown in Table 1, the positive expression rate of KLF6 accounted for 50.0% in HCC tissues, but 72.0% in para-carcinoma tissues (P = 0.034).
Figure 1.
The protein expression of KLF6 in HCC tissues (200). (a) HCC tissues were immunohistochemically stained with the anti-KLF6 antibody. The positive expression of KLF6 was decreased in HCC tissues compared with the adjacent non-cancer tissues (ANCT). After HCC cell lines (SMMC-7721 and HepG2) were infected with lentivirus-mediated KLF6 overexpression for 24 h, the expression of KLF6 was examined by real-time PCR (b, d) and western blot assays (c, e) (**P <0.01).
Table 1.
Expression of KLF6 in HCC tissues.
| Target | Group | n | Grade |
Positive rate (%) | P | |||
|---|---|---|---|---|---|---|---|---|
| None | Low | Intermediate | High | |||||
| KLF6 | HCC | 50 | 25 | 15 | 7 | 3 | 50.0 | |
| ANCT | 50 | 14 | 21 | 9 | 6 | 72.0 | 0.034 | |
ANCT, adjacent non-cancerous tissues; HCC, hepatocellular carcinoma.
The correlation between KLF6 expression and clinicopathologic parameters in HCC patients
The association between KLF6 expression and clinicopathologic factors was analyzed in HCC. As demonstrated in Table 2, decreased expression of KLF6 was not correlated with age, sex, tumor size, pathologic stage, and TNM stage in HCC patients (each P >0.05). However, the downregulation of KLF6 was related with liver cirrhosis (P = 0.016) and LVSI (P = 0.003).
Table 2.
Correlation of KLF6 expression with clinicopathologic factors in HCC patients.
| Variables | Cases (n) | KLF6 expression |
χ2 | P | |
|---|---|---|---|---|---|
| − 25 |
+ 25 |
||||
| Age (years) | |||||
| <60 | 35 | 19 | 16 | ||
| ⩾60 | 15 | 6 | 9 | 0.840 | 0.359 |
| Sex | |||||
| Male | 41 | 21 | 20 | ||
| Female | 9 | 4 | 5 | 0.133 | 0.716 |
| Liver cirrhosis | |||||
| No | 34 | 13 | 21 | ||
| Yes | 16 | 12 | 4 | 5.765 | 0.016 |
| Pathological staging | |||||
| I–II | 37 | 21 | 16 | ||
| III–IV | 13 | 4 | 9 | 2.547 | 0.111 |
| Tumor size (cm) | |||||
| <5 | 39 | 22 | 17 | ||
| ⩾5 | 11 | 3 | 8 | 2.855 | 0.091 |
| TNM staging | |||||
| T1 + T2 | 35 | 17 | 18 | ||
| T3 + T4 | 15 | 8 | 7 | 0.093 | 0.760 |
| Lymph-vascular space invasion (LVSI) | |||||
| No | 34 | 12 | 22 | ||
| Yes | 16 | 13 | 3 | 9.007 | 0.003 |
Establishing KLF6-overexpressed HCC cells
After HCC cell lines (SMCC-7721 and HepG2) were infected with lentivirus-mediated KLF6 overexpression for 24 h, the RNA (Figure 1b, d) and protein (Figure 1c, e) expression levels of KLF6 were tested by real-time PCR and western blot assays, indicating the increased expression of KLF6 in KLF6 group compared with the NC and Ctrl groups (**P <0.01) and KLF6-overexpressed HCC cell lines (SMMCC-7721 and HepG2) were successfully constructed.
The effect of KLF6 overexpression on cell proliferation
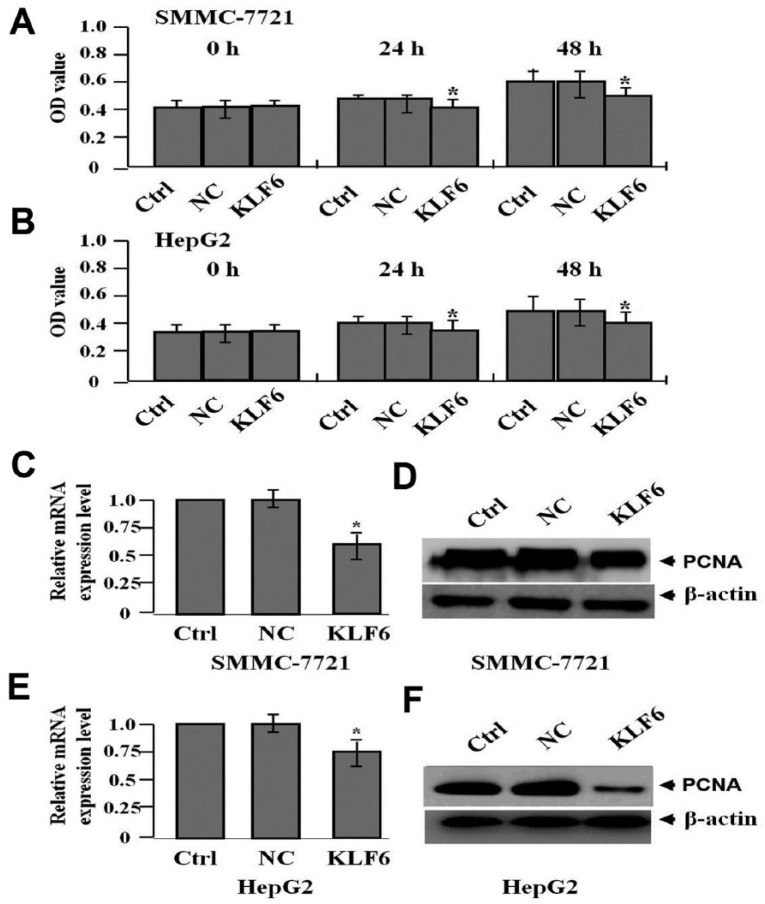
To assess the effect of KLF6 overexpression on HCC cell proliferation, MTT assay was conducted. Results showed that cell viability was suppressed in KLF6 group compared to those in NC and Ctrl groups (*P <0.05, Figure 2a, b). In addition, the RNA (Figure 2c, e) and protein (Figure 2d, f) expression levels of PCNA, tested by real-time PCR and western blot assays, were found decreased in KLF6 group compared to those in NC and Ctrl groups (*P <0.05).
Figure 2.
The effect of KLF6 overexpression on cell proliferation. (a, b) MTT assay was carried out to determine cell proliferative activity, indicating that cell proliferation was markedly suppressed in a time-dependent manner in KLF6 group compared with the NC and Ctrl groups (*P <0.05). The RNA (c, e) and protein (d, f) expression levels of PCNA were detected by real-time PCR and western blot assays (*P <0.05).
The effect of KLF6 overexpression on cell invasion
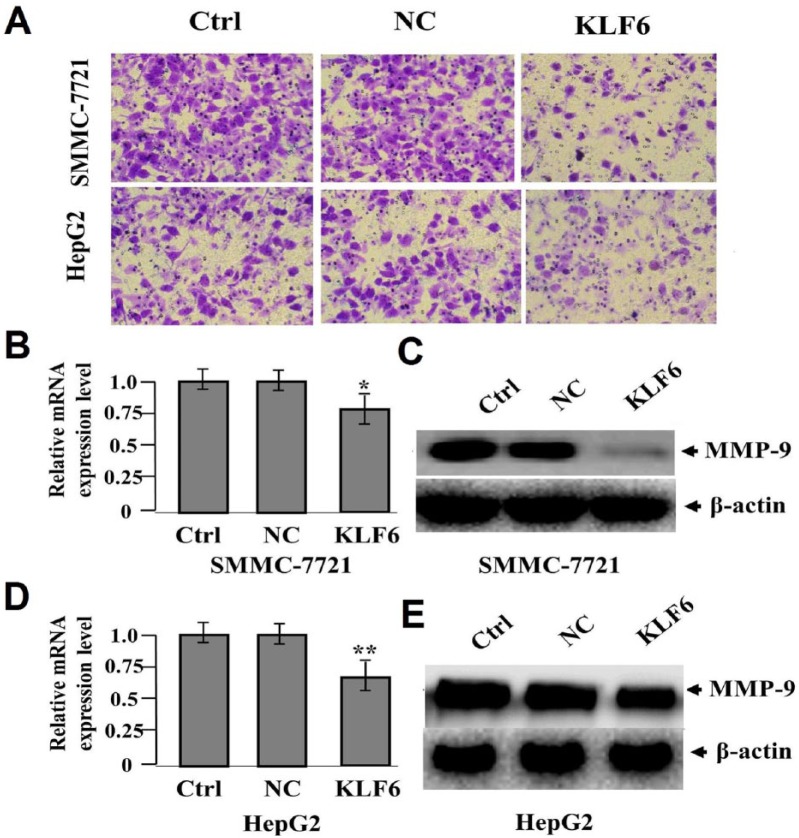
To appraise the effect of KLF6 overexpression on cell invasive potential in HCC cells, Transwell assay was performed, indicating that the invasive potential of SMMC-7721 and HepG2 cells were attenuated in KLF6 group (42 ± 9 cells/field and 63 ± 11 cells/field) compared to those in NC (84 ± 15 cells/field and 73 ± 12 cells/field) and Ctrl groups (82 ± 13 cells/field and 75 ± 14 cells/field) (**P <0.01) (Figure 3a). The RNA (Figure 3b, d) and protein (Figure 3c, e) expression levels of MMP-9 examined by real-time PCR and western blot assays were found downregulated in KLF6 group compared to those in NC and Ctrl groups (*P <0.05; **P <0.01).
Figure 3.
The effect of KLF6 overexpression on cell invasion. (a) Transwell assay was applied to assess cell invasive potential, which was retrained in KLF6 group compared with the NC and Ctrl groups in HCC cells (**P <0.01). The RNA (b, d) and protein (c, e) expression levels of MMP-9 were evaluated by real-time PCR and western blot assays (*P <0.05; **P <0.01).
The effect of KLF6 on xenograft tumor growth
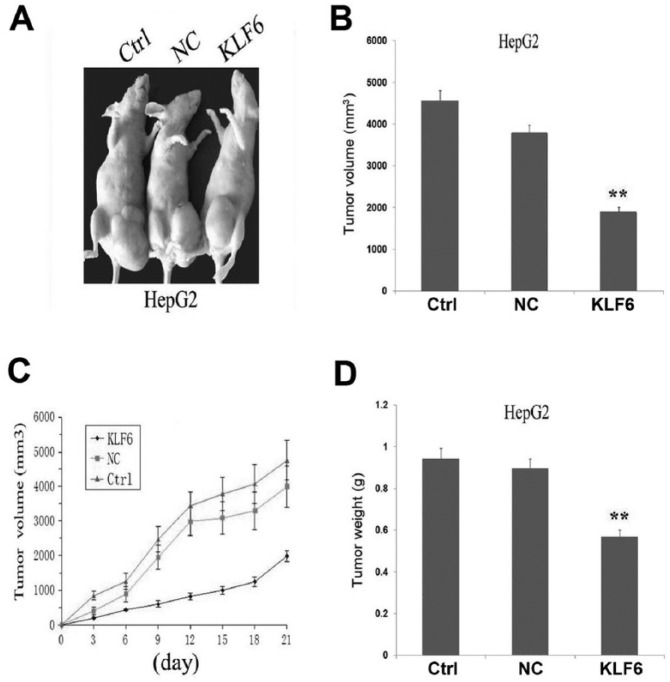
Our in vitro experiments demonstrated the inhibitory effect of KLF6 overexpression on tumor growth in HCC cells. Thus, it is valuable to further explore the effect of KLF6 overexpression on xenograft tumor growth in vivo. The mean volume of tumors in the experimental mice before treatment was 57.23 ± 22.75 mm3. During the whole tumor growth period, the tumor growth activity was measured. The tumors treated with KLF6 grew substantially slower compared to the Ctrl and NC groups (Figure 4a, c). When the tumors were harvested, the average volume and weight of the tumors in KLF6 group were significantly smaller than those of the Ctrl and NC groups (*P <0.05; **P <0.01) (Figure 4b, d).
Figure 4.
The effects of KLF6 overexpression on HCC xenograft tumor growth. (a, c) During the whole tumor growth period, the tumor growth activity was measured. HCC xenograft tumors treated with KLF6 grew significantly slow compared to those in NC group and Ctrl groups. (b, d) The average volume and weight of the tumors in KLF6 group were significantly smaller than those of the NC and Ctrl group (**P <0.01).
Discussion
The expression of KLF6, decreased in primary hepatocarcinoma (PHC) tissues,10 HCC,11 and ovarian cancer,12 is positively associated with cellular differentiation degree in PHC, but negatively associates with liver cirrhosis, tumor size, extra hepatic metastases, portal vein thrombus, and metastatic lymph nodes.10 Functionally, stable expression of KLF6 inhibits cell proliferation, migration, and angiogenesis of HCC,13 suggesting that KLF6 may play a dual role in the tumorigenesis of some malignancies. In the present study, we found that KLF6 was lowly expressed in HCC tissues and was negatively correlated with the liver cirrhosis and LVSI in HCC patients, indicating that loss of KLF6 might be related with the progression of HCC.
A dominant negative oncogenic splice variant of KLF6 (KLF6-SV1) is detected in a number of human cancers, such as breast cancer,14 glioblastoma,15 and ovarian cancer.12 The transcriptional and translational levels of KLF6-SV1 are overexpressed in glioblastoma,15 and results in an increased tumorigenicity of gastric cancer.16 KLF6-SV1 antagonizes tumor suppressor KLF6 via directly binding KLF6 to accelerate its degradation.17 In addition, KLF6-SV1 is a key driver of metastasis in breast cancer14 and inhibition of KLF6-SV1 decreases cellular proliferation, invasion, angiogenesis, and tumorigenicity.12 Targeted reduction of KLF6-SV1 restores chemotherapy sensitivity in resistant lung adenocarcinoma, which may provide a potential therapeutic target.18 In our study, to confirm the role of KLF6 in HCC, we found that overexpression of KLF6 inhibited the proliferation and invasive potential and induced apoptosis of HCC cells in vitro and in vivo, suggesting that KLF6 might function as a tumor suppressor in HCC.
Moreover, KLF6 suppresses the expression levels of B-cell lymphoma-2 (bcl-2), matrix metalloprotein-9 (MMP-9), proliferating cell nuclear antigen (PCNA), and murine double minute2 (MDM2), and promotes the expression of p21 and p53 leading to the suppression of tumor proliferation in gastric cancer.16,19,20 In addition, KLF6 inhibits the proliferation of HCC via downregulation of pituitary tumor transforming gene 1 (PTTG1)21 and suppresses cell growth of breast cancer via a c-Src mediated pathway and its downstream kinases Erk and AKT.22 However, the molecular mechanism of KLF6 involving HCC is still unclear. In the present study, we found that overexpression of KLF6 decreased the expression of PCNA and MMP-9, indicating that KLF6 might block the development of HCC through inhibition of PCNA and MMP-9 expression.
In conclusion, loss of KLF6 expression is closely correlated with the liver cirrhosis and tumor invasion, and overexpression of KLF6 inhibits the cell proliferation and invasive potential in HCC cells in vitro and in vivo, indicating that KLF6 may function as a tumor suppressor in HCC.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the Joint Funds of the National Natural Science Foundation of China (Grant no. U1504804) and Key scientific research projects in Colleges of Henan Province (Grant no. 15A320032).
References
- 1. Aravalli RN. (2013) Role of innate immunity in the development of hepatocellular carcinoma. World Journal of Gastroenterology 19: 7500–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molla N, AlMenieir N, Simoneau E, et al. (2014) The role of interventional radiology in the management of hepatocellular carcinoma. Current Oncology 21: e480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gehrau RC, D’Astolfo DS, Andreoli V, et al. (2011) Differential expression of the KLF6 tumor suppressor gene upon cell damaging treatments in cancer cells. Mutation Research 707: 15–23. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Q, Tan XP, Yuan YS, et al. (2010) Decreased expression of KLF6 and its significance in gastric carcinoma. Medical Oncology 27: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 5. Chen K, Chen Y, Zhu XD, et al. (2012) Expression and significance of Kruppel-like factor 6 gene in osteosarcoma. International Orthopaedics 36: 2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai D, Zhao J, Sun Q. (2014) Kruppel-like factor 6 in the progression and prognosis of malignant melanoma. Journal of International Medical Research 42: 184–190. [DOI] [PubMed] [Google Scholar]
- 7. Ozdemir F, Koksal M, Ozmen V, et al. (2014) Mutations and Kruppel-like factor 6 (KLF6) expression levels in breast cancer. Tumour Biology 35: 5219–5225. [DOI] [PubMed] [Google Scholar]
- 8. Chen H, Chen L, Zhang QF. (2010) The Kruppel-like factor 6 genotype is associated with gastric cancer in a Chinese population. Journal of International Medical Research 38: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 9. Marrero-Rodriguez D, Taniguchi-Ponciano K, Jimenez-Vega F, et al. (2014) Kruppel-like factor 5 as potential molecular marker in cervical cancer and the KLF family profile expression. Tumour Biology 35: 11399–11407. [DOI] [PubMed] [Google Scholar]
- 10. Zhenzhen Z, De’an T, Limin X, et al. (2012) New candidate tumor suppressor gene KLF6 and its splice variant KLF6 SV2 counterbalancing expression in primary hepatocarcinoma. Hepatogastroenterology 59: 473–476. [DOI] [PubMed] [Google Scholar]
- 11. Wang S, Kang L, Chen X, et al. (2010) Frequent down-regulation and deletion of KLF6 in primary hepatocellular carcinoma. Journal of Huazhong University of Science and Technology Medical Sciences 30: 470–476. [DOI] [PubMed] [Google Scholar]
- 12. DiFeo A, Narla G, Martignetti JA. (2009) Emerging roles of Kruppel-like factor 6 and Kruppel-like factor 6 splice variant 1 in ovarian cancer progression and treatment. Mount Sinai Journal of Medicine 76: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang WC, Wang Y, Xiao LJ, et al. (2014) Identification of miRNAs that specifically target tumor suppressive KLF6-FL rather than oncogenic KLF6-SV1 isoform. RNA Biology 11: 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hatami R, Sieuwerts AM, Izadmehr S, et al. (2013) KLF6-SV1 drives breast cancer metastasis and is associated with poor survival. Science Translation Medicine 5: 169ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tchirkov A, Sapin V, Marceau G, et al. (2010) Increased expression of the oncogenic KLF6-SV1 transcript in human glioblastoma. Clinical Chemistry and Laboratory Medicine 48: 1167–1170. [DOI] [PubMed] [Google Scholar]
- 16. Sangodkar J, Shi J, DiFeo A, et al. (2009) Functional role of the KLF6 tumour suppressor gene in gastric cancer. European Journal of Cancer 45: 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vetter D, Cohen-Naftaly M, Villanueva A, et al. (2012) Enhanced hepatocarcinogenesis in mouse models and human hepatocellular carcinoma by coordinate KLF6 depletion and increased messenger RNA splicing. Hepatology 56: 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sangodkar J, DiFeo A, Feld L, et al. (2009) Targeted reduction of KLF6-SV1 restores chemotherapy sensitivity in resistant lung adenocarcinoma. Lung Cancer 66: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jianwei Z, Enzhong B, Fan L, et al. (2013) Effects of Kruppel-like factor 6 on osteosarcoma cell biological behavior. Tumour Biology 34: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 20. Tarocchi M, Hannivoort R, Hoshida Y, et al. (2011) Carcinogen-induced hepatic tumors in KLF6+/- mice recapitulate aggressive human hepatocellular carcinoma associated with p53 pathway deregulation. Hepatology 54: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee UE, Ghiassi-Nejad Z, Paris AJ, et al. (2010) Tumor suppressor activity of KLF6 mediated by downregulation of the PTTG1 oncogene. FEBS Letters 584: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Du T, Yuan Y, et al. (2010) KLF6 inhibits estrogen receptor-mediated cell growth in breast cancer via a c-Src-mediated pathway. Molecular and Cellular Biochemistry 335: 29–35. [DOI] [PubMed] [Google Scholar]