Abstract
Decreasing levels of cytokines are associated with better responses to therapies, while increasing levels are related to progression or recurrence and decreased survival. NF-κB’s role in the cell cycle and its ubiquity are only stressed out by the evidence for the importance of activation (aberrant activation in the majority of cancers) of both canonical and non-canonical pathways in advanced basal cell carcinomas (aBCCs), a subset of basal cell carcinoma (BCC). NF-κB acts through its canonical, or classical, form activated by interleukin-1 (IL-1), regulates cytoprotective, innate, and adaptive immune responses. However, NF-κB2 often acts through its non-canonical or alternate pathway. During the two-year study period, we selected 21 patients presenting with aBCCs due to delay in accessing medical attention with an advanced form of BCCs (n = 19) and infiltrative BCCs (n = 2). Initial diagnosis of BCCs of head and neck was made clinically and verified by skin biopsy. Venous blood was drawn and serum was obtained. Samples were collected at baseline and every three days thereafter (days 3, 6, 9, etc. until surgery). Antigenes’ quantities (cytokines) were determined by ELISA kits. Initially, the mean value of all cytokine subjects was significantly different related to the control group (P <0.05). Changes in serum levels of circulating soluble receptor activator of NF-κB and interleukins-1 (α and β) were observed following the surgery. Changes in serum levels of circulating soluble receptor activator of NF-κB and interleukins-1 (α and β) are evident throughout our study period and a certain regularity in its dynamics is evident as the follow-up period moves away. It was therefore concluded that measurement of these factors might be useful in predicting the overall outcome of patients with aBCCs. This study highlights the systemic effects of aBCCs, but further studies are required on this topic.
Keywords: advanced basal cell carcinoma (aBCC), facial surgery, locally advanced basal cell carcinoma, pro-inflammatory serum cytokines
Introduction
Advanced basal cell carcinomas (aBCCs) are a subset of basal cell carcinomas (BCCs), whose incidence is increasing, and can be difficult to treat either due to their local invasiveness, proximity to vital structures, or metastasis.1 Over 2.8 million new cases of BCC are diagnosed and are estimated to result in over 3000 deaths annually in the United States alone.2 Luckily, thanks to early diagnosis, BCCs are usually treated, the survival rate is relatively high, and five-year recurrence rates are <3 %.3,4 Many of these may become locally advanced, or tough in rare cases, metastasize.
There are, inasmuch, BCCs that are extensive and infiltrate structures below the skin or abut vital structures such as the brain or eyes. Such carcinomas may be difficult to clear surgically without significant morbidity. When combined, there is a small but significant recurrence rate and relatively high incidence; there is a large number of BCCs that are not cured surgically.
Generally speaking, evidence for aberrant constitutive activation of NF-κB was found for human carcinomas of the head and neck that are not melanomas, using cell lines derived from patient tumors.5 In addition to the NF-κB’s role to promote cancer cell survival directly, chemokines and cytokines that result from NF-κB target gene sequencer provide selective advantages for tumor growth in the microenvironment through stimulation of inflammatory cell infiltration and angiogenesis.6,7
Not only does NF-κB play a crucial role in the malignancy of human carcinomas of the head and neck, but it is also activated in squamous dysplasias and carcinomas in general.8 Also, in tobacco-associated and viral-related head and neck carcinomas, detected NF-κB activation had been found aberrant. Moreover, progression of dysplasia and decreased survival can be assessed by the intensity of nuclear immunostaining.3,9 In accordance to this, IL-1, the most pleiotropic cytokine from the classical NF-κB target gene program, regulates NF-κB activation and thus initiates pro-inflammatory responses and triggers a cascade of chemokines/cytokines expression in non-melanomas.10
Concisely, we aimed to establish the predictive significance of circulating soluble receptor activator of NF-κB and interleukins-1 (α and β) regarding the overall outcome of patients with aBCCs. Furthermore, we will demonstrate the dynamics of all cytokines of our interest for the post-surgery follow-up period.
We hypothesize a significant decrease of serum levels of circulating soluble receptor activator of NF-κB and interleukins-1 (α and β) followed by a constant increase during follow-up.
Material and methods
Patients
With informed consent, and under a protocol approved by the institutional review board, sera were obtained from 21 patients (14 men, 7 women; mean age, 68 [±SD = 2.18] years) in the period from 1 January 2013 to 31 December 2015.
Our experimental group was compiled of patients presenting with aBCCs due to delay in accessing medical attention. Initial diagnosis of BCCs of the head and neck was made clinically and verified by skin biopsy. During the two-year study period, we have selected 21 patients with an advanced form of BCCs (n = 19) and infiltrative BCCs (n = 2).
Patients were treated at The First Hospital of Jilin University, China and received standard treatment with surgery and radiation. Standard surgical excision was performed in 85.7% (n = 18) patients, while three patients (14.3%) were treated by Mochs micrographic surgery. External-beam radiotherapy was administered in all patients after the surgery according to the standard treating protocol.
Only patients with clinically confirmed T2 or more advanced disease or nodal involvement were included (Table 1).
Table 1.
Clinical features of participants.
| Clinical features | n |
|---|---|
| T2 | 1 |
| T3 | 28 |
| >T3 | 12 |
| N LOCAL | 28 |
| N REMOTE | 13 |
Exclusion criteria for participating in study:
recurrent BCCs that have recurred following previous radiation treatment;
chemotherapeutic treatment modalities – including smoothened chemotherapeutics and traditional chemotherapy.
The control group consisted of 20 healthy patients (12 men, 8 women; mean age, 67.3 [±SD = 2.43] years).
Laboratory data
Fasting blood samples (3 mL of venous blood) were drawn and dispensed in BD Vacutainers for serum (BD Franklin Lakes, NJ, USA). Serum was obtained by centrifuging blood at 1600 × g for 30 min; sera were collected and stored at −80°C until further use. Samples were collected at baseline, and every three days thereafter (days 3, 6, 9, etc.) until hospitalization. Soluble receptor activator of NF-κB (sRANKL) was determined by sandwich ELISA kits (Koma Biotech, Republic of Korea) according to the manufacturer’s protocol. On available serum samples, IL-1β was measured with The AssayMax Human IL-1beta ELISA kit (Assaypro, MO, USA). Boster Picokine™ ELISA Kit (Boster Immunoleader, CA, USA) was used for quantitative detection of human IL-1α. The optical density readings in the linear range of the dose-response curve were used for calculating the concentrations. The final concentrations (pg/mL) were obtained after multiplying the values by a dilution factor. The sensitivity and range of cytokine detection ranged differently for different cytokines (pg/mL) as reported by the manufacturer and was taken into consideration for the analysis.
Statistical analysis
Statistical analyses were performed using Statistica 12 (Dell Software, TX, USA). For association analyses, participants were divided into three groups based on the length of survival period Table 2. Data were expressed as the means ± SE from at least three independent experiments. Differences between experimental groups were evaluated for statistical significance using Student’s t-test where P <0.05 were considered to be statistically significant.
Table 2.
Survival time after the surgical treatment.
| Survival time (days) | n |
|---|---|
| <3 | 2 |
| 3–30 | 19 |
| >30 | 18 |
Ethical conduct
All authors warrant that this article is their work, which does not infringe the intellectual property rights of any other person or entity, and cannot be construed as plagiarizing any other published work, including their own previously published work.
We declare that we followed national and international procedures that govern the ethics of experimentation on humans. Research reported in the paper was conducted in an ethical and responsible manner, in full compliance with all relevant codes of experimentation.
Results
Baseline serum assessment
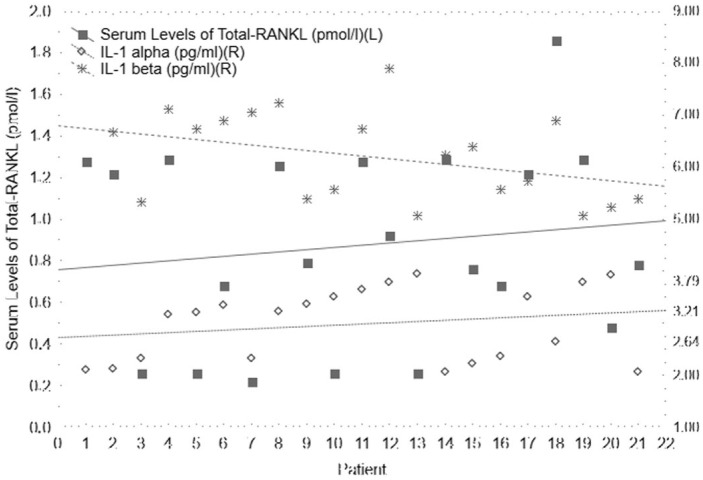
Serum levels of total-RANKL are 0.87 (±SD = 0.46) pmol/L at the baseline (Figure 1). Mean levels of total-RANKL in the sera of patients from the control group are 0.16 (±SD = 0.02) pmol/L. The two-sided t-test revealed that total-RANKL values significantly differ (P = 0.0000) comparing these two groups. At the same time, mean serum values of IL-1α is 2.98 (±SD = 0.69) pg/mL (Figure 1). In the panel of controls, IL-1α is 1.43 (±SD = 0.23) pg/mL. Statistical difference between these two groups is P = 0.0000. Even serum levels of IL-1β differ significantly (P = 0.0000) between experimental and control groups (6.21 [±SD = 0.83] pg/mL and 3.57 [±SD = 0.36] pg/mL, respectively) (Figure 1).
Figure 1.
Scattered plot of serum levels of total-RANKL, IL-1α, and IL-1β at the baseline and in the group of counterpart controls.
Serum assessment after three days
In the first three days after surgery, two patients died (26 h and 34 h after the surgery; aged 68 and 69 years, respectively; cause of death in both cases was congestive heart failure), and the sera of remaining 19 patients contained significantly different serum levels of total-RANKL, IL-1α, and IL-1β (Figure 2).
Figure 2.
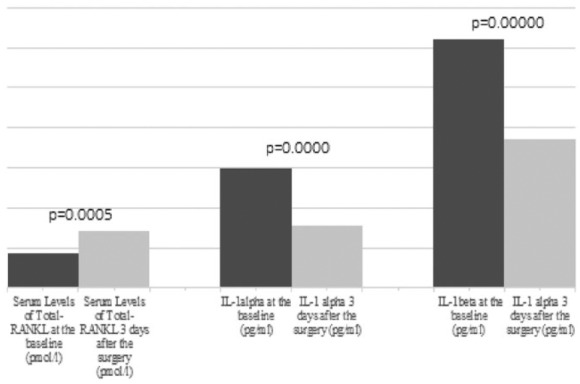
Column diagrams with compared serum levels of total-RANKL, IL-1α, and IL-1β at baseline and the early post-surgery phase (three days). P values <0.05 are denoted between two columns in the set.
Cytokines after one month
We demonstrate serum levels of total-RANKL, IL-1α, and IL-1β (Figure 3), and after comparison with baseline values, these levels are significant (P = 0.0000).
Figure 3.
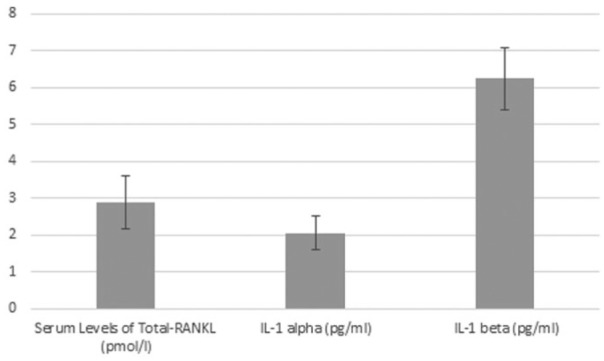
Column diagram with serum levels of total-RANKL, IL-1α, and IL-1β 30 days after surgery.
Discussion
BCC does not commonly occur in African and Asian races, and that should explain the scarce histopathological findings available in this study. Namely, there have been no comprehensive analyses, including the histopathological features of BCC, except for the Caucasian race. Since there have been few reviews that have described the clinical overview of BCC among Asians, our study design and stratification system are different from the standard. Kikuchi et al. describe 59 BCC counterparts of a similar age to our study, over 20 years.11 We successfully confirmed fluctuation in levels of circulating soluble receptor activator of NF-κB and IL-1 (α and β) depending on the survival length, which was our hypothesis.
When non-surgical modalities, such as radiation or chemotherapy, are utilized the cost of treating aBCCs rises significantly, although there are no studies to date that have officially quantitated this. Even though therapeutic innovations like those influencing NF-κB system present a significant financial cost, they may influence beneficially on quality of life and the length of productive lifespan.1 Though medicamentous treatment of aBBCs sometimes exceeds $7500 per month and there are few data as to when to discontinue drugs, costs for health systems should be attentively analyzed in the future, to assure that prolonged survival time correlates with an acceptable quality of life.
For that exact reason, we have high hopes for the NF-κB system; that is canonically activated by various components of pathogens or cytokines such as or IL-1. It regulates genes that function to mediate cytoprotective responses as well as innate and adaptive immune responses. Both cytokines (IL-1α and IL-1β) have thus far been reported to play a pivotal role in enhancing the transcription and expression of other cytokines in the instigation of cytokine cascade (such as IL-6, IL-8, and GM-CSF). More than a few early transcription factors involved in the transcription of pro-inflammatory cytokine genes can be induced by IL-1.12 This enhancement of activation of pro-inflammatory cascade suggests that IL-1 may serve as an important autocrine and/or exocrine factor in coordinating expression of this range of cytokines.
NF-κB2 often acts through its non-canonical or alternate pathway, after being activated by other stimuli. The non-canonical pathway has been indicated to have a more limited role in B lymphocyte development and immunity, at least in transgenic mice. However, we must not neglect the canonical pathway, where IL-1, as an autocrine factor, can stimulate BCCs to produce IL-6 and IL-8, granulocyte-macrophage-colony-stimulating factor (GM-CSF), and vascular endothelial growth factor.13 On the other hand, a paracrine agent stimulates the production of hepatocyte growth factor by stromal fibroblasts.14 Also, at a systemic level, it mediates acute-phase reactions, increases catabolic state and cachexia, and so is characteristic for patients with aBCCs.15 Cytokines produced by BCCs are abundant and of sufficient stability to enable their elevated levels to be detected in the serum of patients.15,16 Decreasing levels of cytokines signify better responses to therapies while increasing levels were related to progression or recurrence and decreased survival.17 This is evident in our results, as well; in the early postoperative phase levels of cytokines drop significantly, while in the later follow-up these values increase again.
NF-κB’s role in the cell cycle and its ubiquity is only stressed out by the evidence on the importance of activation (aberrant activation in the majority of cancers) of both canonical and non-canonical pathways in aBCCs.6,18
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Mohan SV, Chang AL. (2014) Advanced basal cell carcinoma: Epidemiology and therapeutic innovations. Current Dermatology Reports 3: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gould A, Missailidis S. (2011) Targeting the hedgehog pathway: The development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Reviews in Medicinal Chemistry 11: 200–213. [DOI] [PubMed] [Google Scholar]
- 3. Alam M, Goldberg LH, Silapunt S, et al. (2011) Delayed treatment and continued growth of nonmelanoma skin cancer. Journal of the American Academy of Dermatology 64: 839–848. [DOI] [PubMed] [Google Scholar]
- 4. Chren MM, Linos E, Torres JS, et al. (2013) Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Journal of Investigative Dermatology 133: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen CT, Ricker JL, Chen Z, et al. (2007) Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head & Neck 29: 959–971. [DOI] [PubMed] [Google Scholar]
- 6. Van Waes C. (2007) Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clinical Cancer Research 13: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 7. Chen Z, Yan B, Van Waes C. (2008) The role of the NF-kappaB transcriptome and proteome as biomarkers in human head and neck squamous cell carcinomas. Biomarkers in Medicine 2: 409–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duffey DC, Chen Z, Dong G, et al. (1999) Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Research 59: 3468–3474. [PubMed] [Google Scholar]
- 9. Zhang PL, Pellitteri PK, Law A, et al. (2005) Overexpression of phosphorylated nuclear factor-kappa B in tonsillar squamous cell carcinoma and high-grade dysplasia is associated with poor prognosis. Modern Pathology 18: 924–932. [DOI] [PubMed] [Google Scholar]
- 10. Wolf JS, Chen Z, Dong G, et al. (2001) IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clinical Cancer Research 7: 1812–1820. [PubMed] [Google Scholar]
- 11. Kikuchi A, Shimizu H, Nishikawa T. (1996) Clinical and histopathological characteristics of basal cell carcinoma in Japanese patients. Archives of Dermatology 132: 320–324. [PubMed] [Google Scholar]
- 12. Kishimoto T, Taga T, Akira S. (1994) Cytokine signal transduction. Cell 76: 253–262. [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Colon I, Ortiz N, et al. (1998) Effects of interleukin-1alpha, interleukin-1 receptor antagonist, and neutralizing antibody on proinflammatory cytokine expression by human squamous cell carcinoma lines. Cancer Research 58: 3668–3676. [PubMed] [Google Scholar]
- 14. Dong G, Chen Z, Li ZY, et al. (2001) Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Research 61: 5911–5918. [PubMed] [Google Scholar]
- 15. Chen Z, Malhotra PS, Thomas GR, et al. (1999) Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clinical Cancer Research 5: 1369–1379. [PubMed] [Google Scholar]
- 16. Tamura M, Arakaki N, Tsubouchi H, et al. (1993) Enhancement of human hepatocyte growth factor production by interleukin-1 alpha and -1 beta and tumor necrosis factor-alpha by fibroblasts in culture. Journal of Biological Chemistry 268: 8140–8145. [PubMed] [Google Scholar]
- 17. Allen C, Duffy S, Teknos T, et al. (2007) Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clinical Cancer Research 13: 3182–3190. [DOI] [PubMed] [Google Scholar]
- 18. Karin M. (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436. [DOI] [PubMed] [Google Scholar]