Abstract
The importance of replacing synthetic pigments with natural types is increasing day by day in the food industry due to the harmful effects of some synthetic pigments. Microorganisms are a major source of natural pigments, which nowadays have attracted the attention of researchers. In this study, carotenoid pigments were produced by Micrococcus roseus and Rhodotorula glutinis, and some of their biological properties such as antimicrobial, antioxidant, anticancer, and anti-inflammatory activities were evaluated. Given the results, bacteria, especially gram-positive bacteria, had higher sensitivity to the pigments extracted from M. roseus (PEM) and R. glutinis (PER) compared to molds so that Bacillus cereus and Alternaria citri had the highest and the lowest sensitivity, respectively. PER showed a higher antioxidant activity compared with PEM in the various methods of measuring antioxidant activity. In vitro and in vivo anti-tumor-promoting activities of PER were measured significantly more than PEM (P <0.05). Both pigment extracts remarkably inhibited the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation, so that ID50 (50% inhibitory dose) of PEM and PER were 0.22 and 0.09 mg/ear, respectively.
Keywords: biological activities, Micrococcus roseus, pigment, Rhodotorula glutinis
Introduction
Nowadays, food consumers and patients are seeking natural additives and/or traditional medicines in foods instead of synthetic types. For instance, one in three people in the US has used at least one form of the alternative drug. Plants and microorganisms still represent a large source of natural bioactive compound that might serve as leads for the development of novel drugs.1
In recent years, numerous studies have been done for the development of isolation methods of known metabolites and reducing the price of novel compounds isolated from microorganisms. It is revealed that natural product chemists and microbiologist are interested in less investigated drug sources, such as some microorganisms, which turned out to be a large unutilized supply of metabolic diversity. Hence, the study of biological activity of natural products derived from microorganisms has increased remarkably in recent years due to the demand for the substances having potential pharmaceutical applications or economic value as food additives, cosmetics, drugs, and functional personal-care products.2,3
Carotenoids are one of the important pigments, which are achieved from plant and microbial sources. These pigments that belong to the chemical group known as isoprenoid polyenes are lipid-soluble and yellow-orange-red in color.3,4 Carotenoids derived from plants have various issues, such as high sensitivity to heat, light, pH, and chemicals, and also have some practical difficulties for industrial use, such as low solubility and inconvenient access during the year. Nowadays, researchers seek pigment production from microorganisms in various industries. The rapid growth of pigment-producing microorganisms, inexpensive mediums, relatively easy procedure to extract pigment, independent to the weather conditions, and the wide variety of colors are the benefits of pigment production from microorganisms, in addition to benefits in human and animal health.5,6 Pigments producing microorganisms are completely ordinary in nature. Several microorganisms such as Micrococcus, Bacillus, Monascus, Rhordosporidium, Rhodotorula, Sporobolomyces, Sporobolomyces, and Phaffia are considered as potential pigment sources.4
The risk of the food-borne diseases caused by microbial contamination, the incidence of some chronic diseases such as cancer, sensitivity and inflammation, and common side effects of traditional chemotherapy, indicating a crucial need for new approaches to the control microbial contamination.7,8
Carotenoids have been studied comprehensively and verified to show different beneficial effects on human health through serving as precursors of vitamin A, anti-inflammatory effect, antimicrobial and antioxidant activity (AA), etc.8–12 Also, it has been proven that carotenoids derived from plants (e.g. α-carotene,13 β-carotene,14 β-carotene, canthaxanthin, and astaxanthin15) and carotenoids extracted from fruits of red paprika (Capsicum annuum L.),16 lutein,7 and saffron17 could have a potential chemopreventive effect against cancer.
In recent years, several studies focused on the production and characterization of pigments from M. roseus and R. glutinis such as Schwartzel and Cooney,18 Jagannadham et al.,19 Jagannadham et al.,20 Chattopadhyay et al.,21 Zhang et al.,22 and Kot et al.23 Nevertheless, there is no study available in the literature which focused on the comprehensive biological activities of carotenoids extracted from M. roseus and R. glutinis. Thus, we decided to evaluate the antimicrobial, antioxidant, anti-inflammatory, and cancer chemopreventive activity of pigments extracted from M. roseus (PEM) and R. glutinis (PER).
Materials and methods
Microorganism and materials
M. roseus (PTCC 1411) and R. glutinis (PTCC 5257) were obtained from Persian Type Culture Collection (PTCC), the biotechnological Department of Iranian Research Organization for Science and Technology (IROST), Tehran, Iran. All chemical materials and culture media were of analytical grade and purchased from Merck (Germany) and Sigma-Aldrich (UK).
Condition of pigment production
M. roseus
For inoculum preparation, a loopful of M. roseus from agar plate was transferred into an Erlenmeyer flask containing 100 mL GPY medium (1 g glucose, 10 g peptone, and 6 g yeast extract). This Erlenmeyer flask was incubated at 180 rpm and 30°C for 48 h. A total of 10 mL of prepared inoculums was added into an Erlenmeyer containing 100 mL trypticase soy broth (TSB; Difco) that was supplemented with yeast extract, biological grade dextrose (Oxoid), and phosphate. This Erlenmeyer was then incubated at 180 rpm and 30°C for 72 h in order to produce pigment. The pH-value culture was controlled at 7 using 2 M NaOH and 2 M HCl solutions.24
R. glutinis
A single colony was transferred from the stock culture on YPG (10 g yeast extract, 20 g peptone, and 20 g galactose in 1 L water) agar to 50 mL YPG broth followed by incubation at 27°C for an overnight period. A total of 3 mL of YPG broth was used for inoculation of 100 mL semi-synthetic medium in a 500 mL flask, then incubated in a shaker incubator at 150 rpm and 27°C for 72 h.25
Pigment extraction and spectrophotometric analysis
Microorganism cells were harvested by centrifugation at 10,000 rpm for 20 min, followed by washing with distilled water and centrifuged again. To extract carotenoid pigments, the method described by Bhosale and Gadre26 was used with some modification. Briefly, cells were ruptured twice with 10 mL of acetone and broken by homogenizer (Scilogex D500, USA). The suspension was then centrifuged and the supernatant was gathered. The extracts were collected and carotenoid pigments were extracted with the same volume of petroleum ether.
Total carotenoid (TC) was measured spectrophotometrically using the method described by Hornero-Méndez et al.27 In practice, after dissolving the pigment concentrates in 10 mL acetone, the absorbance was measured by spectrophotometer at 472 and 508 nm and TC was calculated using the following formulas:
where CY and CR represent the yellow and red isochromic families, respectively.
Microorganisms and inoculum preparation
Antimicrobial activities of PEM and PER were assayed against Staphylococcus aureus (PTCC 1431), Bacillus cereus (PTCC 1539), Streptococcus pyogenes (PTCC 1447), Escherichia coli (PTCC 1269), Salmonella enteritidis (PTCC 1709), Enterococcus faecalis (PTCC 1393), and Listeria monocytogenes (PTCC 1163). Microorganisms were grown overnight at 35°C in TSB. Overnight cultures were adjusted to match a 0.5 McFarland standard followed by diluted 1:100 with Mueller–Hinton broth. The dilution was used as the inoculum for antibacterial activity assay.
Alternaria citri and Penicillium digitatum were cultured on sabouraud dextrose agar (SDA). Spores and hyphae were scraped off with a sterile wire loop. The sterile tubes containing A. citri and P. digitatum were diluted by ringer solution until the solution turbidity equalizes with 0.5 McFarland standard solutions through spectrophotometrically measuring turbidity at 530 nm to obtain a final concentration of 1.5 × 10 CFU/mL.
Antimicrobial activity
Kirby–Bauer method
The disk diffusion method was carried out to evaluate the antimicrobial activity of PEM and PER. Briefly, an amount of inoculums (0.1 mL) was spread on Mueller–Hinton agar (MHA) (for bacteria) and SDA (for fungi). Sterile paper discs (6 mm in diameter, Sigma-Aldrich Company, USA) were soaked in PEM and PER at concentrations of 0.5, 1.5, 2.5, 3.5, and 5 mg/mL. After incubation of plates at 37°C for 24 h (for bacteria) and 27°C for 72 h (for fungi), the halo of inhibition was measured. Penicillin or gentamicin discs were placed in the plates as a comparative standard. The result was obtained by measuring the microbial free zone area diameter. The experiment was performed three times to minimize the error and the mean values are presented.11,29
Determination of minimum inhibitory concentration (MIC)
Agar dilution method was used to determine MIC values of PEM and PER against some microorganisms. In practice, the pigment at concentrations of 2, 4, 8, 16, 32, 64, 128, and 256 mg/mL was mixed with sterile MHA (for bacteria) and SDA (for fungi), and then 0.1 mL of microbial suspension (0.5 McFarland) was plated using the pour plate method. These plates were incubated at 37°C for 24 h (for bacteria) and 27°C for 72 h (for fungi), and then the MIC of the pigments was evaluated. The MIC was defined as the lowest concentration of PEM and/or PER that prevents visible growth of a bacterium in comparison with positive (medium + microorganism) and negative (medium) control groups.30
Determination of minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC)
The agar dilution method was also used to determine MBC and MFC of PEM and PER. With this difference, bacteria and molds were sub-cultured on nutrient agar (NA) and yeast extract glucose chloramphenicol (YGC) agar, respectively, from the plate containing MIC of the pigment. If the sub-cultured microorganisms cannot grow, the MIC and MBC and/or MFC concentrations will be equal. However, in contrast to the situation, the grown bacteria were sub-cultured in MHA (for bacteria) and SDA (for fungi) containing further pigment concentrations (8, 16, 32, 64, 128, and 256 mg/mL) and the MBC was considered as a concentration of the pigment that bacteria did not grow.11,29
Antioxidant and free radical scavenging activity
Radical scavenging activity
PEM and PER were evaluated for their radical scavenging capacity. In practice, 1 mL of DPPH+ solution (0.3 mM) in ethanol: hexane (equal proportions) was mixed with 1 mL pigment extract (100 µg/mL) in ethanol: hexane. This blend was agitated and kept at 21°C in a dark place for 15 min and then the absorbance was measured spectrophotometrically at 540 nm using an ultraviolet visible (UV-Vis) spectrophotometer (Shimadzu, Japan). The inhibition activity was calculated by the following formula:30
Abssample: absorbance value of pigment extract plus DPPH
Abscontrol: absorbance value of DPPH solution
Abscontrol: absorbance value of pigment solution
β-carotene bleaching test
Evaluation of AA by β-carotene bleaching test was carried out using the method described by Conforti et al.31 In practice, emulsion of β-carotene was prepared using the above method and 5 mL of this emulsion was transferred to test tubes containing a 0.2 mL sample of ethanol (70%) at various concentrations. The emulsion containing 0.2 mL ethanol was used as the control. A solution of propyl gallate was used for comparison. After keeping the tube at 45°C for 60 min, the absorbance of the samples was measured spectrophotometrically at 470 nm using a UV-Vis spectrophotometer against a blank (emulsion without β-carotene). The practice was done at the first time and then at 30 and 60 min. The AA was expressed as successful bleaching β-carotene using the following formula:
where A0 and A0 represent the absorbance values at the first time for sample and control, respectively. “t” is time of incubation.
Bovine brain peroxidation
The lipid peroxidation activity was evaluated using the thiobarbituric acid (TBA) test. PEM and PER were evaluated for their antioxidant activity versus liposomes that were supplied from bovine brain extract in phosphate-buffered saline (PBS) (5 mg/mL). The absorbance was measured at 532 nm and the inhibition of lipid peroxidation was calculated using the following formula:
Anticancer activity
In vitro Epstein–Barr virus (EBV) early antigen
The experiment was performed using the method described by Maoka et al.16 with some modifications. In brief, Raji cells (indicator cells) were grown to a concentration of 106 cell/mL, gently centrifuged, and the pellet was resuspended in medium containing n-butyrate (4 mM), as inducer, 12-O-tetradecanoylphorbol-13-acetate (TPA) (10 ng/mL) and different concentrations of PEM or PER. The cells were then incubated at 37°C for 72 h and followed by the cells were harvested and dissolved in PBS (pH 7.2). Smears were prepared from the cell suspension. The active cells were stained with high titer EBV-early positive sera from nasopharyngeal carcinoma (NPC) patients and detected using an indirect immunofluorescence method. For this purpose, more than 500 cells were counted and the experiments were done in three replicates.
In vivo carcinogenesis
This test was carried out on mice skin papillomas promoted by TPA. The mice used in this study were divided into three experimental classes (each including 10 mice aged 6 weeks). Mice were locally treated with 100 µg 7,12-dimethylbenz[a]anthracene (DMBA) as a promoter in 0.1 mL acetone through a shave in their backs. One week after beginning, papilloma forming was promoted twice a week by using 1 µg TPA in acetone to the skin of mouse. Classes I and II were locally treated with PEM (85 nmol) and PER (85 nmol) in acetone (0.1 mL), respectively, 1 h before the TPA treatment. Class III was locally treated alone with TPA. Papillomas and the incidence were evaluated weekly for 15 weeks.16
Anti-inflammatory activity
The topical anti-inflammatory activity was studied as inhibition of the TPA-induced ear edema in mice. Mice (25–30 g aged 6 weeks) were held for 1 week before the experiment at 21±°C. In practice, a solution of TPA (1 µg) in acetone (20 µL) was applied to the inner surface of the right ear (1 cm2) by a micropipette. Control mice received only TPA, whereas other animals received the irritant together with PEM or PER. Ear thickness was gauged before and 6 h after the treatment. The inhibitory ratio (%) was calculated by the following formula:32
Edema A is induced by TPA alone (b–a)
Edema B is induced by TPA plus sample (b′–a)
Each of the above amounts was the average of individual measuring from five mice. ID50 values were determined by the method of probit-graphic interpolation.
Statistical analysis
All experiments and measurements were carried out in triplicate. All statistical analyses were performed using Minitab® version 16.1.1 (Minitab Inc., USA. 2010). Data from the experiments were subjected to Student’s t-test. P values <0.05 were considered to be significant.
Results
Pigment production
Table 1 shows TC, yellow, and red isochromic fractions contents of PEM and PER. A significant difference (P <0.05) was observed between the extracted pigment, so that PER had a significantly higher TC than PEM.
Table 1.
TC, yellow, and red isochromic fractions contents of PEM and PER (μg/g).
| Sample | TC content (CT) | Yellow isochromic fraction (CY) | Red isochromic fraction (CR) |
|---|---|---|---|
| PEM | 2395 ± 14* | 773 ± 15* | 1622 ± 8* |
| PER | 2965 ± 9† | 1172 ± 5† | 1793 ± 11† |
Means (± SD) within a column with the different sign letters are significantly different (p<0.05).
Means (± SD) within a column with the different sign letters are significantly different (p<0.05).
Antimicrobial activity
Kirby–Bauer method
Given the results, PEM and PER had an antimicrobial effect on all examined bacteria, so that the effect was increased by increasing pigment concentration, as is shown in Table 2. The antimicrobial activity of PER against all pathogenic bacteria was higher than PEM.
Table 2.
Average diameter (mm) of microbial free zone area of PEM and PER.
| Microorganism | 0.5 mg/mL | 1.5 mg/mL | 2.5 mg/mL | 3.5 mg/mL | 5 mg/mL | Antibiotic disk | |
|---|---|---|---|---|---|---|---|
| PEM | B. cereus | 9.5 | 12.9 | 15.3 | 16.9 | 17.9 | 13.5 P |
| E. coli | 7.9 | 9.5 | 11.4 | 12.3 | 13.1 | 12 G | |
| S. aureus | 8.8 | 11.6 | 13.1 | 13.9 | 15.3 | 32 P | |
| S. enteritidis | 7.3 | 8.1 | 9 | 10.9 | 12.2 | 10 G | |
| L. monocytogenes | 7.5 | 9.2 | 10.2 | 11.6 | 13.5 | 22 P | |
| S. pyogenes | 9.1 | 12.3 | 14.1 | 15.3 | 16.5 | 14 P | |
| E. faecalis | 8.4 | 10.3 | 12.0 | 12.9 | 14.4 | 13.5 P | |
| A. citri | 5.6 | 7.9 | 9.2 | 10.0 | 10.7 | ||
| P. digitatum | 6.8 | 8.6 | 10.1 | 11.2 | 11.9 | ||
| PER | B. cereus | 10.6 | 13.0 | 15.9 | 18.0 | 19.6 | 13.5 P |
| E. coli | 9.1 | 11.0 | 12.4 | 13.5 | 14.1 | 12 G | |
| S. aureus | 9.9 | 12.5 | 15.2 | 17.0 | 18.5 | 32 P | |
| S. Enteritidis | 8.9 | 11.4 | 12.4 | 13.2 | 13.8 | 10 G | |
| L. monocytogenes | 9.1 | 11.6 | 13.8 | 14.5 | 15.8 | 22 P | |
| S. pyogenes | 9.8 | 12.9 | 15.0 | 16.9 | 19.1 | 14 P | |
| E. faecalis | 9.6 | 12.4 | 14.6 | 16.0 | 17.3 | 13.5 P | |
| A. citri | 6.9 | 8.9 | 10.0 | 10.9 | 11.8 | ||
| P. digitatum | 8.1 | 10.4 | 11.4 | 12.5 | 13.1 |
G, antibiotic use of gentamicin; P, antibiotic disc of penicillin.
Determination of MIC
MIC of both pigments for molds was remarkably more than bacteria, so that the highest MIC was observed for A. citri (Table 3). The MIC of PER for all examined microorganisms, excluding A. citri, was less than MIC of PEM. MIC of PER and PEM for A. citri was the same (128 mg/mL).
Table 3.
MIC of the PEM and PER.
| Microorganism | 2 mg/mL | 4 mg/mL | 8 mg/mL | 16 mg/mL | 32 mg/mL | 64 mg/mL | 128 mg/mL | 256 mg/mL | Negative control | Positive control | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEM | B. cereus | + | + | + | − | − | − | − | − | − | + |
| E. coli | + | + | + | + | + | − | − | − | − | + | |
| S. aureus | + | + | + | − | − | − | − | − | − | + | |
| S. enteritidis | + | + | + | + | + | − | − | − | − | + | |
| L. monocytogenes | + | + | + | + | + | − | − | − | − | + | |
| S. pyogenes | + | + | + | − | − | − | − | − | − | + | |
| E. faecalis | + | + | + | + | − | − | − | − | − | + | |
| A. citri | + | + | + | + | + | + | − | − | − | + | |
| P. digitatum | + | + | + | + | + | + | − | − | − | + | |
| PER | B. cereus | + | + | − | − | − | − | − | − | − | + |
| E. coli | + | + | + | + | − | − | − | − | − | + | |
| S. aureus | + | + | + | − | − | − | − | − | − | + | |
| S. enteritidis | + | + | + | + | − | − | − | − | − | + | |
| L. monocytogenes | + | + | + | − | − | − | − | − | − | + | |
| S. pyogenes | + | + | − | − | − | − | − | − | − | + | |
| E. faecalis | + | + | + | − | − | − | − | − | − | + | |
| A. citri | + | + | + | + | + | + | − | − | − | + | |
| P. digitatum | + | + | + | + | + | − | − | − | − | + |
+, grew; −, did not grow.
Determination of MBC and MFC
MBC and MFC of PEM and PER are shown in Table 4. At the used concentrations of PEM and PER, a bactericidal effect was observed for both pigments against all bacteria. At the applied concentrations of PEM and PER, a fungicidal effect was observed for PER against A. citri and P. digitatum; whereas, no fungicidal effect was observed for PEM against A. citri.
Table 4.
MBC and MFC of PEM and PER.
| Microorganism | 8 mg/mL | 16 mg/mL | 32 mg/mL | 64 mg/mL | 128 mg/mL | 256 mg/mL | |
|---|---|---|---|---|---|---|---|
| PEM | B. cereus | + | + | − | − | − | − |
| E. coli | + | + | + | + | − | − | |
| S. aureus | + | + | − | − | − | − | |
| S. enteritidis | + | + | + | + | − | − | |
| L. monocytogenes | + | + | + | − | − | − | |
| S. pyogenes | + | + | − | − | − | − | |
| E. faecalis | + | + | − | − | − | − | |
| A. citri | + | + | + | + | + | + | |
| P. digitatum | + | + | + | + | + | − | |
| PER | B. cereus | + | + | − | − | − | − |
| E. coli | + | + | + | − | − | − | |
| S. aureus | + | + | − | − | − | − | |
| S. enteritidis | + | + | + | − | − | − | |
| L. monocytogenes | + | + | − | − | − | − | |
| S. pyogenes | + | + | − | − | − | − | |
| E. faecalis | + | + | − | − | − | − | |
| A. citri | + | + | + | + | + | − | |
| P. digitatum | + | + | + | + | − | − |
G, antibiotic dose of gentamicin; P, antibiotic disc of penicillin.
Antioxidant and free radical scavenging activity
Table 5 shows IC50 values of antioxidant activities of PEM and PER. The antioxidant and free radical scavenging activities of PER were higher compared to PEM.
Table 5.
IC50 values of antioxidant activities of PEM and PER.
| Sample | DPPH (ppm) | β-carotene |
Lipid peroxidation (µg/mL) | |
|---|---|---|---|---|
| 30 min | 60 min | |||
| PEM | 572.5 ± 0.15* | 6.5 | 11.5 | 14.4 |
| PER | 555.5 ± 0.05† | 2.5 ± 0.02 | 3.9 ± 0.05 | 7.9 |
| Propyl galat | − | 1.5 ± 0.05 | 1.5 ± 0.05 | 7.4 ± 0.05 |
| β-carotene | 553.5 ± 0.02† | − | − | − |
Means (± SD) within column with the different sign letters are significantly different (p<0.05).
Means (± SD) within column with the different sign letters are significantly different (p<0.05).
In vitro and in vivo anti-tumor-promoting
Table 6 shows the inhibitory effects of PEM and PER on the activation and viability of the Raji cells so that the inhibitory effect was increased by increasing concentration (mol ratio/TPA). Figure 1 shows the inhibition of TPA-induced tumor promoting by PEM and PER.
Table 6.
Relative ratio of EBV-EA activation with respect to positive control (100%) in the presence of PEM and PER.
| Sample | Inhibition of inflammation |
Relative ratio of EBV-EA activation* |
|||
|---|---|---|---|---|---|
| ID50 (mg/ear) | Concentration (mol ratio/TPA)† |
||||
| 1000 | 500 | 100 | 10 | ||
| PEM | 0.22 | 3.1 (70)‡ | 31.7 (>80) | 87.5 (>80) | 98.7 (>80) |
| PER | 0.09 | 0.0 (70) | 22.8 (>80) | 65.5 (>80) | 89.9 (>80) |
| Reference compound | |||||
| β-carotene | − | 2.5 (70) | 27.5 (>80) | 90.5 (>80) | 100.0 (>80) |
| Indomethacin | 0.33 | − | − | − | − |
Values represent relative percentage of the positive control value (100%).
TPA concentration was 20 ng (32 pmol)/mL.
Values in parentheses are viability percentages of Raji cells.
Figure 1.
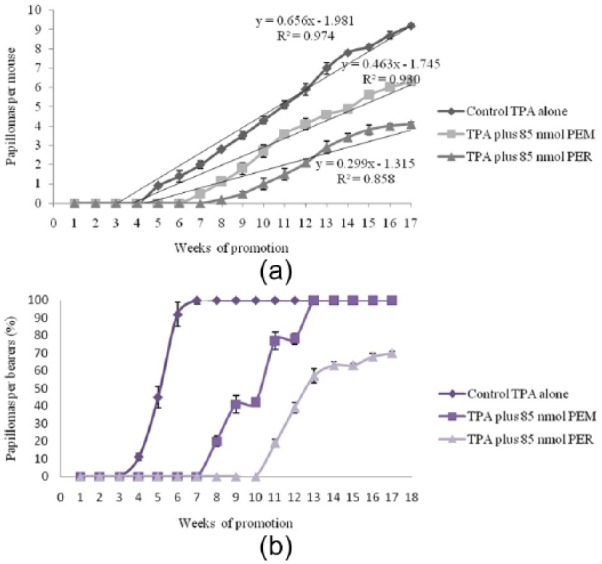
Inhibition of TPA-induced tumor promoting by PEM and PER. (a) Percentage of mice bearing papillomas; (b) average number of papillomas/mouse.
Anti-inflammatory activity
The TPA-induced anti-inflammatory activities of PEM and PER on mice are shown in Table 6. According to the result, the anti-inflammatory activity of PER was higher than PEM.
Discussion
Before the study of biochemical properties, measuring the amount of carotenoids is important. Therefore, according to Table 1, PER had significantly (P <0.05) higher TC (2965 ± 9) compared to PEM (2395 ± 14). Also, yellow and red isochromic fractions were measured as significantly higher (P <0.05) for PER compared to PEM, as shown in Table 1. Several researchers have reported that the yellow fraction of carotenoids exhibits significant AA, as the results showed in the antioxidant section.33–35 Jagannadham et al.19 purified and identified the major carotenoid pigment of a psychrotrophic M. roseus strain as bisdehydro-β-carotene-2-carboxylic acid. They also reported that the purified pigment was different from canthaxanthin, the major carotenoid pigment from a mesophilic M. roseus strain. Ungers and Cooney36 studied the isolation and characterization of carotenoid pigments of M. roseus and reported that canthaxanthin, phoenicoxanthin, α- and β-carotene were the main pigments. Perrier et al.37 used chromatographic analyses to identify and characterize the carotenoid pigments extracted from Rhodotorula spp. They reported that β-carotene, torulene, and torularhodin carotenoid were isolated from these microorganisms as main pigments.
B. cereus and A. citri had the highest and the lowest sensitivity to both pigments, respectively (Table 1). The antimicrobial activity of pigment extracted from both microorganisms on the bacteria was more than molds. The results are in agreement with studies about essential oils of Turkish plant,38 rosemary extract,39 Finnish plant extract,40 and extracts of various onions,41 which reported that the resistance of fungi to natural antimicrobial substances is higher compared to bacteria.
PER had more antimicrobial activity compared to PEM. This fact could be related to the type of carotenoids that were produced by each microorganism. Siva et al.42 studied the antimicrobial properties of eight food dyes against ten bacteria and five fungal organisms and reported that red dyes showed best antibacterial activity, while yellow dyes showed better antifungal activity.
The results of MIC assay revealed that PEM and PER had more antibacterial effects against gram-positive bacteria than gram-negative bacteria, so that B. cereus and S. enteritidis showed the lowest and the highest MIC, respectively. The similar results were observed by several researchers such as Smith-Palmer et al.43 and Galindo-Cuspinera et al.44 for plant essential oils and essences, and annatto extract (2.8% norbixin), respectively. This fact is likely due to the presence of lipopolysaccharide in the cell wall of gram-negative bacteria. This lipopolysaccharide can impede the influx of active compounds to cytoplasmic membrane of these bacteria.45 L. monocytogenes had the lowest sensitivity to PEM and PER among gram-positive bacteria so that MIC of PER for L. monocytogenes was observed equally with S. enteritidis and E. coli (Table 3). This phenomenon could be due to the presence of lipoteichoic acids in the cell wall of L. monocytogenes that resemble the lipopolysaccharides of gram-negative bacteria in both structure and function, being the only amphipathic polymers at the cell surface.46
PER had less MIC for all the microorganisms except A. citri, compared to PEM (Table 3). Umadevi and Krishnaveni47 studied antibacterial activity of pigment produced from M. luteus KF532949 and reported that the isolated strain M. luteus is able to act against both gram-positive and gram-negative bacteria.
PER had bactericidal and fungicidal effects on all tested bacteria and fungi; however, the opposite is true for PEM, so that no fungicidal activity against A. citri was observed for PEM at the applied concentrations (Table 4). MFC values of pigments were observed higher than MBC values, which indicate a more bactericidal activity of these pigments. The lowest MBC of PEM and PER was observed on B. cereus.
Carotenoids are pigments that play a major role in the protection of pigment-producing microorganisms against some environmental shocks such as decreasing temperature. This role could be due to some features of carotenoids such as AA and membrane fluidity increase.48 According to the results of this study, PEM and PER had antioxidant and free radical scavenging activities (Table 5). The effect of antioxidants on DPPH radical scavenging was believed to be because of their hydrogen-donating ability. PEM and PER were able to reduce the stable free radical DPPH to the yellow 1,1-diphenyl-2-picrylhydrazyl. DPPH radical scavenging of PER (IC50 = 555.5 ± 0.05 µg/mL) was measured significantly less compared to PEM (P <0.05). As an interesting result, no significant difference was observed between the radical scavenging activities of PER and β-carotene as reference (IC50 = 553.5 ± 0.02 µg/mL).
In the β-carotene bleaching test after 30 min of incubation, PER showed the highest inhibition of linoleic acid oxidation (2.5 ± 0.02), even more than the standard sample (IC50 = 1.5 ± 0.05 µg/mL). The AA of the pigment extracts decreased during the reaction time. However, the IC50 of propyl gallate was 1.5 µg/mL after 30 and 60 min incubation. This is probably due to the thermal sensitivity of carotenoids so that the AA of carotenoid was reduced at high temperatures.48
Using liposomes prepared from bovine brain, the highest AA was observed for PER (IC = 14.4 µg/mL), which was significantly more compared to PEM (P <0.05).
The differences in the AA of PEM and PER could be attributed to the higher β-carotene content of PER, since this carotenoid is known to exert significant AA.49–51 Hernández-Ortega et al.8 studied antioxidant, antinociceptive, and anti-inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.) and reported that carotenoid extracts of guajillo pepper showed good AA and had the best scavenging capacity for the DPPH+ (24.2%).
In vitro anti-tumor promoting activities of PEM, PER, and β-carotene as well as a strong anti-tumor-promoter,52 were examined using the EBV activation assay in Raji cells. PEM and PER showed an inhibitory effect on EBV-EA induction without significant cytotoxicity on Raji cells in this test. PER showed notably inhibitory effect at 1000 mol/TPA ratio. Furthermore, at 10 and 100 mol/TPA ratio, PEM was more active than β-carotene, whereas the opposite is true at the high mol/TPA ratios (Table 6). Wang et al.7 studied some biological activity of lutein and their results provided scientific evidence for the safe use and health beneficial effects of lutein.
The in vivo anti-tumor-promoting activities of PEM and PER were evaluated by a two-stage carcinogenesis method for mouse skin papillomas promoted by TPA. The incidence (%) of papilloma-bearing mice and the average number of papillomas per mouse are shown and compared with a positive control (initiated with 390 nmol of DMBA and promoted with TPA) in Figure 1. When PEM (85 nmol) and PER (85 nmol) were applied before each TPA treatment, they notably hinder the formation of papillomas and reduced the number of papillomas per mouse, as shown in Figure 1. In the positive control mice, the first papillomas emerged in the fifth week of promotion, and in mice treated with PEM and PER in the seventh and eighth weeks, respectively. After 7 weeks of promotion, the positive control mice presented a complete incidence of papillomas, while in mice treated with PEM the complete incidence was observed at the 13th week. As an interesting result, the complete incidence papilloma was not observed for the group treated with PER, so that even at week 17 only 70% of mice bore papillomas. After 17 weeks of promotion, 9.2, 6.3, and 4.1 papillomas were found per mouse in the positive control mice and the groups treated with PEM and PER, respectively. Previous researchers reported a direct relationship between the anticancer and antioxidant activities for carotenoids.7,53,54 Also in this study, a close correlation was found between these two properties for the extracted pigments. Both antioxidant and anticancer activities were higher for PER compared to PEM. Nesaretnam et al.55 reported that rats that were fed palm-oil diets have a lower incidence of mammary cancers than those that were fed other dietary fats; these results were partly related to the presence of carotenoids or tocopherols and tocotrienols.
PEM and PER extracts were assessed with respect to their anti-inflammatory activity against TPA-induced inflammation in mice and compared with a commercially available anti-inflammatory drug (indomethacin). As shown in Table 6, both pigment extracts remarkably hinder the TPA-induced inflammation, so that ID50 of PEM and PER were measured as 0.22 and 0.09 mg/ear, respectively, which was more inhibitory than indomethacin (ID50 = 0.33 mg/ear). Nam et al.12 studied the anti-inflammatory effects of crocin and crocetin (natural carotenoids mainly found in the crocus flower) in rat brain microglial cells and reported that crocin and crocetin provide neuroprotection by reducing the production of various neurotoxic molecules from activated microglia. Heo et al.56 suggested that using fucoxanthin (a xanthophyll, which is one of two major divisions of the carotenoid group) could be a useful therapeutic approach for the various inflammatory diseases.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors thanks Research’s Deputy of Baqiyatallah Medical Sciences University for providing the cost of this project and help to the implementation of this project, which adopted of the Research Council of Health and Nutrition Research Center.
References
- 1. Yolmeh M, Najafi MBH, Farhoosh R, et al. (2014) Modeling of antibacterial activity of annatto dye on Escherichia coli in mayonnaise. Food Bioscience 8: 8–13. [Google Scholar]
- 2. Debbab A, Aly AH, Lin WH, et al. (2010) Bioactive compounds from marine bacteria and fungi. Microbial Biotechnology 3L 544–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dufossé L, Galaup P, Yaron A, et al. (2005) Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends in Food & Science Technology 16: 389–406. [Google Scholar]
- 4. Frengova GI, Beshkova DM. (2009) Carotenoids from Rhodotorula and Phaffia: Yeasts of biotechnological importance. Journal of Industrial Microbiology & Biotechnology 36: 163–180. [DOI] [PubMed] [Google Scholar]
- 5. Kim CH, Kim SW, Hong SI. (1999) An integrated fermentation separation process for the production of red pigment by Serratia sp. KH-95. Process Biochemistry 35: 485–490. [Google Scholar]
- 6. Venil CK, Zakaria ZA, Ahmad WA. (2013) Bacterial pigments and their applications. Process Biochemistry 48: 1065–1079. [Google Scholar]
- 7. Wang M, Tsao R, Zhang S, et al. (2006) Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anti-clastogenicity of lutein from marigold flowers. Food and Chemical Toxicology 44: 1522–1529. [DOI] [PubMed] [Google Scholar]
- 8. Hernández-Ortega M, Ortiz-Moreno A, Hernández-Navarro MD, et al. (2012) Antioxidant, antinociceptive, and anti-inflammatory effects of carotenoids extracted from dried pepper (Capsicum annuum L.). Journal of Biomedicine & Biotechnology 2012: 524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han S, Yang Y. (2005) Antimicrobial activity of wool fabric treated with curcumin. Dyes and Pigments 64: 157–161. [Google Scholar]
- 10. Yolmeh M, Khomeiri M. (2016) Using physical and chemical mutagens for enhanced carotenoid pro-duction from Rhodotorula glutinis (PTCC 5256). Biocatalysis and Agricultural Biotechnology 8: 158–166. [Google Scholar]
- 11. Yolmeh M, Habibi-Najafi MB, Shakouri S, et al. (2015) Comparing antibacterial and antioxidant activity of annatto dye extracted by conventional and ultrasound-assisted methods. Zahedan Journal of Research in Medical Science 7: 1–6. [Google Scholar]
- 12. Nam KN, Park YM, Jung HJ, et al. (2010) Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. European Journal of Pharmacology 648: 110–116. [DOI] [PubMed] [Google Scholar]
- 13. Murakoshi M, Nishino H, Satomi Y, et al. (1992) Potent preventive action of α-carotene against carcinogenesis: Spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by α-carotene than by β-carotene. Cancer Research 52: 6583–6587. [PubMed] [Google Scholar]
- 14. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. New England Journal of Medicine 330: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 15. Chew BP, Park JS, Wong MW, et al. (1998) A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Research 19: 1849–1853. [PubMed] [Google Scholar]
- 16. Maoka T, Mochida K, Kozuka M, et al. (2001) Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Letters 172: 103–109. [DOI] [PubMed] [Google Scholar]
- 17. Chermahini SH, Majid FAA, Sarmidi MR, et al. (2010) Impact of saffron as an anti-cancer and anti-tumor herb. African Journal of Pharmacy and Pharmacology 4: 834–840. [Google Scholar]
- 18. Schwartzel EM, Cooney JJ. (1972) Isolation of 4’-hydroxyechinenone from Micrococcus roseus. Journal of Bacteriology 112: 1422–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jagannadham MV, Narayanan K, Rao CM, et al. (1996) In vivo characteristics and localisation of carotenoid pigments in psychrotrophic and mesophilic Micrococcus roseus using photoacoustic spectroscopy. Biochemical and Biophysical Research Communications 227: 221–226. [DOI] [PubMed] [Google Scholar]
- 20. Jagannadham MV, Chattopadhyay MK, Shivaji S. (1996) The major carotenoid pigment of a psychrotrophic Micrococcus roseus strain: fluorescence properties of the pigment and its binding to membranes. Biochemical and Biophysical Research Communications 220: 724–728. [DOI] [PubMed] [Google Scholar]
- 21. Chattopadhyay MK, Jagannadham M V, Vairamani M, et al. (1997) Carotenoid pigments of an antarctic psychrotrophic bacterium Micrococcus roseus: Temperature dependent biosynthesis, structure, and interaction with synthetic membranes. Biochemical and Biophysical Research Communications 239: 85–90. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z, Zhang X, Tan T. (2014) Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresource Technology 157: 149–153. [DOI] [PubMed] [Google Scholar]
- 23. Kot AM, Błażejak S, Kurcz A, et al. (2016) Rhodotorula glutinis-potential source of lipids, carotenoids, and enzymes for use in industries. Applied Microbiology and Biotechnology 100: 6103–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Wandawi H. (2014) Carotenoid biosynthesis in Micrcoccus luteus grown in the presence of different concentrations of nicotine. International Journal of Pure and Applied Sciences and Technology 24: 31–41. [Google Scholar]
- 25. Sadat Naghavi F, Hanachi P, Soudi MR, et al. (2015) The capability of Rhodotorula slooffiae to produce carotenoid. Zahedan Journal of Research in Medical Science 17: 52–56. [Google Scholar]
- 26. Bhosale P, Gadren R. (2001) Production of β-carotene by a mutant of Rhodotorula glutinis. Applied Microbiology and Biotechnology 55: 423–427. [DOI] [PubMed] [Google Scholar]
- 27. Hornero-Méndez D, Mínguez-Mosquera MI. (2001) Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. Journal of Agricultural and Food Chemistry 49: 3584–3588. [DOI] [PubMed] [Google Scholar]
- 28. Ahmad I, Beg AZ. (2001) Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology 74: 113–123. [DOI] [PubMed] [Google Scholar]
- 29. Espinel-Ingroff A, Fothergill A, Peter J, et al. (2002) Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS Col-laborative Study. Journal of Clinical Microbiology 40: 3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu D, Shi J, Ibarra AC, et al. (2008) The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT - Food Science and Technology 41: 1344–1349. [Google Scholar]
- 31. Conforti F, Sosa S, Marrelli M, et al. (2008) In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. Journal of Ethnopharmacology 116: 144–151. [DOI] [PubMed] [Google Scholar]
- 32. Akihisa T, Nakamura Y, Tagata M, et al. (2007) Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the Fungus Ganoderma lucidum. Chemistry & Biodiversity 4: 224–231. [DOI] [PubMed] [Google Scholar]
- 33. Mortensen A, Skibsted LH, Sampson J, et al. (1997) Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Letters 418: 91–97. [DOI] [PubMed] [Google Scholar]
- 34. Polyakov NE, Kruppa AI, Leshina TV, et al. (2001) Carotenoids as antioxidants: Spin trapping EPR andoptical study. Free Radical Biology & Medicine 31: 43–52. [DOI] [PubMed] [Google Scholar]
- 35. Young AJ, Lowe GM. (2001) Antioxidant and prooxidant properties of carotenoids. Archives of Biochemistry and Biophysics 385, 20–27. [DOI] [PubMed] [Google Scholar]
- 36. Ungers GE, Cooney JJ. (1968) Isolation and characterization of carotenoid pigments of Micrococcus roseus. Journal of Bacteriology 96: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perrier V, Dubreucq E, Galzy P. (1995) Fatty acid and carotenoid composition of Rhodotorula strains. Archives of Microbiology 164: 173–179. [DOI] [PubMed] [Google Scholar]
- 38. Özcan M, Erkmen O. (2001) Antimicrobial activity of the essential oils of Turkish plant spices. European Food Research and Technology 212: 658–660. [Google Scholar]
- 39. Campo JD, Amiot MJ. (2000) Antimicrobial effect of rosemary extracts. Journal of Food Protection 63: 1359–1368. [DOI] [PubMed] [Google Scholar]
- 40. Rauha JP, Remes S, Heinonen M, et al. (2000) Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology 56: 3–12. [DOI] [PubMed] [Google Scholar]
- 41. Benkeblia N. (2004) Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT - Food Science and Technology 37: 263–268. [Google Scholar]
- 42. Siva R, Palackan MG, Maimoon L, et al. (2011) Evaluation of antibacterial, antifungal, and antioxidant properties of some food dyes. Food Science and Biotechnology 20: 7–13. [Google Scholar]
- 43. Smith-Palmer A, Stewart J, Fyfe L. (1998) Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Letters in Applied Microbiology 26: 118–122. [DOI] [PubMed] [Google Scholar]
- 44. Galindo-Cuspinera V, Westhoff DC, Rankin SA. (2003) Antimicrobial properties of commercial annatto extracts against selected pathogenic lactic acid and spoilage microorganisms. Journal of Food Protection 66: 1074–1078. [DOI] [PubMed] [Google Scholar]
- 45. McKeegan KS, Borges-Walmsley MI, Walmsley AR. (2002) Microbial and viral drug resistance mechanisms. Trends in Microbiology 10: S8–S14. [DOI] [PubMed] [Google Scholar]
- 46. Low JC, Donachie W. (1997) A review of Listeria monocytogenes and listeriosis. Veterinary Journal 153: 9–29. [DOI] [PubMed] [Google Scholar]
- 47. Umadevi K, Krishnaveni M. (2013) Antibacterial activity of pigment produced from Micrococcus luteus KF532949. International Journal of Chemical and Analytical Science 4: 149–152. [Google Scholar]
- 48. Joshi VK, Attri D, Bala A, et al. (2003) Microbial pigments. Indian Journal of Biotechnology 2: 362–369. [Google Scholar]
- 49. Miller NJ, Sampson J, Candeias LP, et al. (1996) Antioxidant activities of carotenes and xanthophylls. FEBS Letters 384: 240–242. [DOI] [PubMed] [Google Scholar]
- 50. Müller L, Fröhlich K, Böhm V. (2011) Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chemistry 129: 139–148. [Google Scholar]
- 51. Böhm V, Puspitasari-Nienaber NL, Ferruzzi MG, et al. (2002) Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. Journal of Agricultural and Food Chemistry 50: 221–226. [DOI] [PubMed] [Google Scholar]
- 52. Temple NJ, Basu TK. (1987) Protective effect of β-carotene against colon tumors in mice. Journal of the National Cancer Institute 78: 1211–1214. [PubMed] [Google Scholar]
- 53. Guerin M, Huntley ME, Olaizola M. (2003) Haematococcus astaxanthin: Applications for human health and nutrition. Trends in Biotechnology 21: 210–216. [DOI] [PubMed] [Google Scholar]
- 54. Tanaka T, Shnimizu M, Moriwaki H. (2012) Cancer chemoprevention by carotenoids. Molecules 17: 3202–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nesaretnam K, Khor HT, Ganeson J, et al. (1992) The effect of vitamin E tocotrienols from palm oil on chemically-induced mammary carcinogenesis in female rats. Nutrition Research 12: 63–75. [Google Scholar]
- 56. Heo SJ, Yoon WJ, Kim KN, et al. (2010) Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food and Chemical Toxicology 48: 2045–2051. [DOI] [PubMed] [Google Scholar]


