Abstract
Inhaled corticosteroids (ICS)/long-acting beta-agonists (LABA) association offers a better asthma control than a higher steroid dose with short-acting beta-agonists as needed. In this study, we evaluated the effect of the association on bronchial hyperreactivity (BHR) and peak expiratory flow (PEF) variability, as such parameters are positively correlated with increased asthma morbidity and exacerbations. Thirty-six adult patients with mild persistent asthma were enrolled. After a 7-day run-in, they were randomly assigned to three therapy regimens for 6 weeks: Group 1, fluticasone 125 μg + formoterol 5 μg in the same device; Group 2, fluticasone 125 μg + formoterol 12 μg as needed; Group 3, fluticasone 250 μg + formoterol 12 μg as needed. We evaluated changes induced in weekly PEF variability (measured during the entire study and 4 weeks of follow-up) and pre- and post-study PD20 methacholine (MCH). Weekly PEF variability decreased in all groups during treatment with the greatest reduction in Group 1, followed by Group 3, and finally Group 2. During the follow-up, no significant changes were detected in Group 1, whereas a trend towards an increased variability was found in Groups 2 and 3. Post-treatment PD20 MCH was significantly higher versus the pre-treatment. The increase observed in Group 1 was significantly higher compared to Groups 2 and 3 and that observed in Group 3 in respect to Group 2. The study proves that both BHR and PEF variability are influenced by ICS. This effect was greater with fluticasone/formoterol association compared to fluticasone alone with formoterol as needed even at higher steroid dose.
Keywords: bronchial hyperreactivity, fluticasone/formoterol association, methacholine, peak expiratory flow (PEF) variability
Introduction
Inhaled corticosteroids (ICS), the cornerstone of the treatment of bronchial asthma, can be used alone or in association with long- and short-acting bronchodilators, anti-leukotrienes, mast cell stabilizers, and theophylline. Dose-response studies have shown that ICS significantly improves lung function and reduces exacerbations in a dose-dependent manner.1 It has also been shown that treatment of asthma exacerbations with a temporary increase of ICS, at the first sign of asthma worsening, offers similar effects as a regular higher dose of the drug.2 The association with ß2-agonists and anti-leukotrienes is useful when symptoms persist in spite of daily assumption of ICS.3 The addition of long-acting beta-agonists (LABA) to ICS treatment results in a better asthma control compared to the increasing the dose of ICS, with reduction of the incidence of severe asthma exacerbations.4,5 The effectiveness of ICS–LABA association is due to their complementary interactions at a molecular level as ICSs enhance the expression of β2-adrenoceptor and LABA amplifies the anti-inflammatory effects of ICS.6 In addition to the maintenance doses taken for day-to-day asthma control, ICS–LABA association (Formoterol/Budesonide) is also used as needed if symptoms occur.
Reduction of bronchial hyperreactivity (BHR) and of fluctuations in lung function is essential in asthma control. Several studies suggest a positive correlation of BHR with increased asthma morbidity, exacerbations, and airway inflammation.7,8 Therefore, increased BHR may indicate the presence of under-treated airway inflammation and thus be a useful therapeutic target in asthma.9,10 Lung function variability, in particular of peak expiratory flow (PEF), is variously related to clinical parameters so PEF fluctuations can predict the response to long-term asthma treatment.11
The present study aims at evaluating the effect of a combination therapy of fluticasone propionate with formoterol on BHR and PEF variability. In particular, it compares the effects of this combination with those obtained with fluticasone alone at the same and at a high dose (plus ß2-agonists as needed) in the treatment of mild persistent asthma, to evaluate the best treatment choice.
Methods
Patients
Thirty-six consecutive adult patients, referring to the allergy clinic of G. d’Annunzio University, Chieti, Italy, suffering from mild persistent asthma, with an allergy to Dermatophagoides pteronyssinus defined by positive skin test and specific IgE were recruited. The diagnosis of asthma was made at the first visit according to the GINA criteria for asthma severity. The study was approved by EC and all patients gave written informed consent.
Inclusion criteria: FEV1 >80% of predicted and positivity of the methacholine (MCH) challenge test (PD20 <1600 µg) on the first visit.
Exclusion criteria: unstable asthma; respiratory tract infection or exacerbation of asthma during the 4 weeks before entry into the study; current smoking or cessation of smoking within the year preceding the study; history of any pulmonary disease other than asthma; use of oral steroids, inhaled chromones, or leukotriene antagonists during the 2 months before the study; use of antihistamines within 2 weeks before entry into the study; pregnancy or breast feeding; any severe chronic disease; and alcohol or drug abuse.
Protocol
During a 7-day run-in period, all patients underwent PEF measurement twice daily (morning and evening) and were treated by formoterol as needed. The MCH challenge was executed at the end of the run-in week in which no LABA or ICS were administered. In the following period, for 6 weeks they were randomly (1:1:1) assigned to different doses of fluticasone associated with formoterol in the same device or as needed. In Group 1, 12 patients were treated with fluticasone 125 μg + formoterol 5 μg in association twice daily + the combination as needed; in Group 2, 12 patients were treated with fluticasone 125 μg twice daily + formoterol 12 μg as needed; and in Group 3, 12 patients were treated with fluticasone 250 μg twice daily + formoterol 12 μg as needed. The choice of the treatment timing was justified by the fact that some studies have shown that maximal or near maximal ICS effects are achieved around 6 weeks.12 During the treatment all patients performed and recorded a series of three pre-bronchodilator PEF measurements twice daily, in the morning and in the evening. Weekly PEF variability was calculated on the best value of each series with the following formula: (max–min)/[(max+min)/2]×100.
After the treatment period, all patients repeated PEF measurement for 4 weeks and then a further MCH challenge was executed. During the follow-up period, only formoterol as needed was allowed. The MCH challenge was executed with a standard procedure indicated by the American Thoracic Society,13 with the PD20 MHC capped at 1600 μg/L. The doctor who followed the patients during the study was a different person from those who visited the patients in the outpatient clinic and assigned the patients to the three different treatments.
The main characteristics of the study population and treatment schedule are reported in Table 1.
Table 1.
Characteristics of the study population and treatment protocol.
| Group 1 | Group 2 | Group 3 | P | |
|---|---|---|---|---|
| Age (years) | 36 ± 10 | 34 ± 15 | 38 ± 9 | n.s. |
| Sex | 7/5 | 8/4 | 7/5 | |
| BMI | 25.4 ± 3.2 | 26 ± 4 | 25.6 ± 2.8 | n.s. |
| Rhinitis | 8 patients | 6 patients | 8 patients | |
| Basal FEV1 (% pred) | 80.1 ± 4 | 82.2 ± 3.4 | 80.4 ± 3.8 | n.s. |
| FEV1/VC | 79.3 ± 5 | 77.6 ± 4.8 | 76.6 ± 5.3 | n.s. |
| Pre-study PD20 MCH µg/l | 92.1 ± 26.8 | 105 ± 41.6 | 101.5 ± 20 | n.s. |
| Pre-study weekly PEF variability (%) | 35.65 | 34.63 | 36.86 | n.s. |
| Fluticasone (µg) | 125 | 125 | 250 | |
| Formoterol (µg) | 5 in the same device | 12 as needed | 12 as needed |
Statistics
The variables are reported as median, first, and third quartiles. Since these data do not involve any distributional assumptions, we applied the non-parametric methods and, in particular, being these methods based on analysis of ranks, we employed the Mann–Whitney U test to compare percentage of variations of efficacy parameters between the groups; non-parametric repeated measures comparisons were carried out according to the Friedman’s test. The statistical significance of the differences between the groups was evaluated at an alpha level of 0.05.
Statistical analysis was performed using SPSS software 11.0 (SPPS Inc., Chicago, IL, USA).
Results
At enrollment, there were no significant differences both in basal FEV1 and FEV1/VC%, in PD20 MCH values, in weekly PEF variability, presence of rhinitis, and BMI (Table 1) of the three groups.
Seven patients did not report a sufficient number of PEF measurements (<60% of measurements) and therefore results are described for 10 patients in Group 1, nine patients in Group 2, and 10 patients in Group 3.
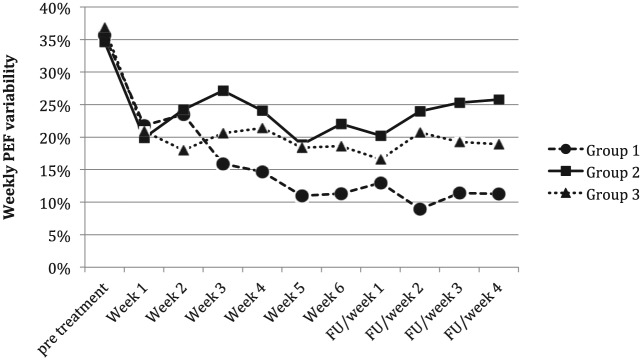
Weekly PEF variability decreased during the treatment in all three groups during the 6 weeks of treatment (Figure 1). The greatest reduction was detected in Group 1 (formoterol/fluticasone association), from 35% (range, 31.3–40.5%) to 13.1% (range, 7.4–14.2%) (P <0.001), then in Group 3 (high dose fluticasone), from 35.6% (range, 32.2–42.3%) in the pretreatment period to 17.3% (range, 13.4–22.6%) at week 6 (P = 0.02), and in Group 2 (low dose fluticasone) from 32.5% (range, 28.5–41.7%) to 21.2% (range, 19.3–24.9%) (P <0.05). In particular, Group 1 showed a greater significant decrease in weekly PEF variability from week 3 compared to Group 2 and from week 4 in respect to Group 3, whereas no significant differences were found between Groups 2 and 3 (Table 2).
Figure 1.
Trend of mean values of weekly PEF variability during the treatment period and follow-up in the three treated groups. FU, follow-up.
Table 2.
Comparison of the weekly PEF variability among the three groups during the treatment and post-treatment periods.
| Variable | Group 1 vs. Group 2 | Group 1 vs. Group 3 | Group 2 vs. Group 3 |
|---|---|---|---|
| Pretreatment | n.s. | n.s. | n.s. |
| Week 1 | n.s. | n.s. | n.s. |
| Week 2 | n.s. | n.s. | n.s. |
| Week 3 | P = 0.002 | n.s. | n.s. |
| Week 4 | P = 0.002 | P = 0.009 | n.s. |
| Week 5 | P = 0.006 | P = 0.004 | n.s. |
| Week 6 | P <0.001 | P = 0.017 | n.s. |
| Week 1 (PT) | P = 0.007 | n.s. | n.s. |
| Week 2 (PT) | P <0.001 | P <0.001 | n.s. |
| Week 3 (PT) | P <0.001 | P = 0.020 | n.s. |
| Week 4 (PT) | P <0.001 | P = 0.009 | n.s. |
Mann–Whitney U test.
n.s., not significant; PT, post-treatment.
After treatment, patients treated with the association (Group 1) always had a weekly PEF variability <20%, whereas in Group 2 (low dose fluticasone treated) the majority of patients (7/10) had a variability >20%. Group 3, treated with high fluticasone dose, had an intermediate trend with only two patients with a variability >20%. Group 1 showed the lowest variability both compared with Group 2 (P = 0.01) and Group 3 (P <0.45).
During the follow-up period of 1 month, there was a decreasing trend in the weekly PEF variability of Group 1 (from a mean value of 13% ± 2.42 in week 1 to a mean value of 11.26% ± 3.54 at week 4), whereas a trend towards an increased variability in Groups 2 and 3 was found (Group 2: from 20.25% ± 5.90 to 25.80% ± 9.70; Group 3: 16.56% ± 5.60 to 18.90% ± 7.30). The weekly PEF variability of Group 1 was significantly lower than Group 2 during all 4 weeks, and lower than Group 3 in weeks 2, 3, and 4 (Table 2). No significant differences were found between Groups 2 and 3.
PD20 MCH detected at the end of the follow-up period was >1600 µg/L in 5/10 evaluated patients of Group 1 and in 3/10 evaluated patients of Group 3, whereas all nine evaluated patients of Group 2 had a PD20 <1600 µg/L. The average PD20 of patients in Group 1 was 952 ± 709 µg (compared to 92.2 ± 26.8 µg/L before the study, P <0.001), that of Group 2 was 355 ± 263 µg/L (compared to 105 ± 41.6 µg/L, P <0.002), and that of Group 3 was 652 ± 657 µg/L (compared to 101.5 ± 20 µg/L, P <0.01). The increase observed in Group 1 was significantly higher compared with Group 2 (P <0.005) and Group 3 (P <0.05) and that observed in Group 3 compared with Group 2 (P <0.05). PD20 MHC >1600 µg/L was always considered 1600 for statistical evaluations.
Discussion
The present study shows that both BHR and PEF variability are influenced by ICS/LABA. This effect was not influenced by the dose, in fact, no significant differences were found between Group 3 treated by 250 µg twice a day compared to Group 2 treated by 125 µg twice a day during the treatment period. The importance to determine the effect of an asthma treatment on PEF variability is underlined by the fact that fluctuation analysis of lung function is useful since it can assess the risk for future loss of asthma control.14 It is of interest that Group 1 treated with the fixed association of fluticasone/formoterol induced the best reduction of PEF variability even at a lower ICS dose in respect to Group 3: 125 versus 250 µg twice/day. These results agree with several studies demonstrating the clinical benefit of ICS/LABA association in the treatment in asthma,15 with similar levels of asthma control at relatively low doses of ICS, compared with a higher dose of ICS alone, with as-needed short-acting beta-agonists.16 The usefulness of the ICS/LABA association is also underlined by their interaction at a molecular level.17,18 A further interesting aspect of the present study is that the fluticasone/formoterol association allows a greater control on BHR compared to fluticasone alone. Our study confirms that a low ICS dose associated with formoterol induces a greater effect than higher ICS doses: Group 1 (fluticasone 125 µg twice/day) versus Group 3 (fluticasone 250 µg twice/day). In fact, after 4 weeks from the discontinuation of the treatment, there were significant differences between Group 1 and Groups 2 and 3 in the PD20 MCH.
The importance of verifying the efficacy of ICS/LABA association other than in symptoms and exacerbations also in BHR is justified by the fact that changes in PC20 MCH are related to changes in airway inflammation and caliber.19 The treatment able to reduce BHR also induces a reduction of airway inflammation.20
In conclusion, our data show that the association between ICS with LABA improves respiratory performance in mild asthma, administering the lowest dose of corticosteroid able to control, as much as possible, both fluctuation in lung function and BHR. Therefore, the development of fixed combinations containing both substances in one device is a logic consequence simplifying asthma therapy.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Dissanayake S, Jain M, Grothe B, et al. (2015) An evaluation of comparative treatment effects with high and low dose fluticasone propionate/formoterol combination in asthma. Pulmonary Pharmacology & Therapeutics 35: 19–27. [DOI] [PubMed] [Google Scholar]
- 2. Foresi A, Morelli MC, Catena E. (2000) Low-dose budesonide with the addition of an increased dose during exacerbations is effective in long-term asthma control. Chest 117: 440–446. [DOI] [PubMed] [Google Scholar]
- 3. Szefler SJ, Martin RJ, King TS, et al. (2002) Significant variability in response to inhaled corticosteroids for persistent asthma. Journal of Allergy and Clinical Immunology 109: 410–418. [DOI] [PubMed] [Google Scholar]
- 4. Ram FSF, Cates CJ, Ducharme FM. (2006) Long-acting beta-2agonist versus antileukotrienes as addontherapy to inhaled corticosteroids for chronic asthma. Cochrane Database of Systemic Reviews 25: CD003137. [Google Scholar]
- 5. Aalbers R, Vogelmeier C, Kuna P. (2016) Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respiratory Medicine 111: 1–7. [DOI] [PubMed] [Google Scholar]
- 6. Sin DD, Man SF. (2006) Corticosteroids and adrenoceptor agonists: The compliments for combination therapy in chronic airways diseases. European Journal of Pharmacology 533: 28–35. [DOI] [PubMed] [Google Scholar]
- 7. Chetta A, Foresi A, Del Donno M, et al. (1996) Bronchial responsiveness to distilled water and methacholine and its relationship to inflammation and remodeling of the airways in asthma. American Journal of Respiratory and Critical Care Medicine 153: 910–917. [DOI] [PubMed] [Google Scholar]
- 8. Riccioni G, Castronuovo M, De Benedictis M, et al. (2001) Zafirlukast versus budesonide on bronchial reactivity in subjects with mild-persistent asthma. International Journal of Immunopathology and Pharmacology 14: 87–92. [PubMed] [Google Scholar]
- 9. Koenig SM1, Murray JJ, Wolfe J, et al. (2008) Does measuring BHR add to guideline derived clinical measures in determining treatment for patients with persistent asthma? Respiratory Medicine 102: 665–73. [DOI] [PubMed] [Google Scholar]
- 10. Riccioni G, Di Stefano F, De Benedictis M, et al. (2001) Seasonal variability of non-specific bronchial responsiveness in asthmatic patients with allergy to house dust mites. Allergy and Asthma Proceedings 22: 5–9. [DOI] [PubMed] [Google Scholar]
- 11. Thamrin C, Taylor DR, Jones SL, et al. (2010) Variability of lung function predicts loss of asthma control following withdrawal of inhaled corticosteroid treatment. Thorax 65: 403–408. [DOI] [PubMed] [Google Scholar]
- 12. Koenig SM, Murray JJ, Wolfe J, et al. (2008) Does measuring BHR add to guideline derived clinical measures in determining treatment for patients with persistent asthma? Respiratory Medicine 102: 665–673. [DOI] [PubMed] [Google Scholar]
- 13. Crapo RO, Casaburi R, Coates AL, et al. (2000) Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. American Journal of Respiratory and Critical Care Medicine 161: 309–329. [DOI] [PubMed] [Google Scholar]
- 14. Thamrin C, Zindel J, Nydegger R, et al. (2011) Predicting future risk of asthma exacerbations by using individual conditional probabilities. Journal of Allergy and Clinical Immunology 127: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 15. Ducharme FM, Ni Chroinin M, Greenstone I, et al. (2010) Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 5: CD005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cates CJ, Karner C. (2013) Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 4: CD007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes PJ. (2002) Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. European Respiratory Journal 19: 182–191. [DOI] [PubMed] [Google Scholar]
- 18. Usmani OS, Ito K, Maneechotesuwan K, et al. (2005) Glucocorticoid receptor nuclear translocation in airway cells following inhaled combination therapy. American Journal of Respiratory and Critical Care Medicine 172: 704–712. [DOI] [PubMed] [Google Scholar]
- 19. Cockcroft DW, Davis BE. (2006) Mechanisms of airway hyperresponsiveness. Journal of Allergy and Clinical Immunology 118: 551–559. [DOI] [PubMed] [Google Scholar]
- 20. Brannan JD. (2010) Bronchial hyperresponsiveness in the assessment of asthma control: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138(2 Suppl): 11–17. [DOI] [PubMed] [Google Scholar]