Abstract
Deficits in glucose, impaired insulin signalling and brain insulin resistance are common in the pathogenesis of Alzheimer’s disease (AD); therefore, some scholars even called AD type 3 diabetes mellitus. Curcumin can reduce the amyloid pathology in AD. Moreover, it is a well-known fact that curcumin has anti-oxidant and anti-inflammatory properties. However, whether or not curcumin could regulate the insulin signal transduction pathway in AD remains unclear. In this study, we used APPswe/PS1dE9 double transgenic mice as the AD model to investigate the mechanisms and the effects of curcumin on AD. Immunohistochemical (IHC) staining and a western blot analysis were used to test the major proteins in the insulin signal transduction pathway. After the administration of curcumin for 6 months, the results showed that the expression of an insulin receptor (InR) and insulin receptor substrate (IRS)-1 decreased in the hippocampal CA1 area of the APPswe/PS1dE9 double transgenic mice, while the expression of phosphatidylinositol-3 kinase (PI3K), phosphorylated PI3K (p-PI3K), serine-threonine kinase (AKT) and phosphorylated AKT (p-AKT) increased. Among the curcumin groups, the medium-dose group was the most effective one. Thus, we believe that curcumin may be a potential therapeutic agent that can regulate the critical molecules in brain insulin signalling pathways. Furthermore, curcumin could be adopted as one of the AD treatments to improve a patient’s learning and memory ability.
Keywords: Alzheimer’s disease, APPswe/PS1dE9 double transgenic mice, curcumin, insulin receptor, insulin signal transduction pathway
Introduction
So far, a mass of evidence has suggested that the misfolding and the aggregation of amyloid beta (Aβ) and phosphorylated tau are the factors that lead to the pathogenesis of Alzheimer’s disease (AD).1 However, some references have revealed that in the initial stages of AD, it is the kind of disease accompanied with impairments in cerebral glucose utilisation.1 In recent years, emerging data have demonstrated that brain insulin resistance and insulin deficiency can cause AD-associated abnormalities in energy metabolism and further result in brain insulin signalling pathway injuries.1,2
The insulin signal pathway mediates normal biological effects by key molecules, including insulin receptor (InR), insulin receptor substrates (IRSs), phosphatidylinositol-3 kinase (PI3K), phosphorylated PI3K (p-PI3K), serine-threonine kinase (AKT), and phosphorylated AKT (p-AKT). Therefore, exploring the effects of drugs on insulin signal transduction contributes to the discovery of potential AD targets.
So far, the treatment of AD, especially by the long-time administration of drugs, is still unsatisfactory. Curcumin, a major yellow pigment extracted from curcuma, is extensively employed in Ayurvedic herbal remedies.3 Over the last 5 years, a number of studies have shown that curcumin is an effective agent for the amelioration of a wide variety of diseases, such as cancer, hepatic lesions, thrombogenesis, cardiac disease and arthritic, infectious and neurodegenerative diseases.3,4 Because of its low cost and lack of toxicity, curcumin is becoming a potential nutraceutical intervention in neurodegenerative diseases.5 Curcumin has anti-oxidant, anti-inflammatory and anti-proliferation effects and can fight against Aβ-induced neurotoxicity in vitro and in vivo.6–8 In our previous study, 3 months of curcumin treatment significantly improved spatial learning ability and memory, reduced Aβ40 and Aβ42 in the hippocampal CA1 area9 and regulated cerebral energy metabolism and the insulin signalling pathway. The spatial learning and memory disabilities and the structure and function of the synapses of the APPswe/PS1dE9 double transgenic mice could improve with 6 months of curcumin treatment.10,11 However, it is important to know whether or not curcumin can still mediate the insulin signalling pathway after a long-time administration and can take effect in mice with middle and advanced stages of AD. In this paper, we extended the observation by using APPswe/PS1dE9 double transgenic mice, which were treated with curcumin for 6 months, to investigate the expression of critical proteins, including InR, IRS-1, PI3K, p-PI3K, AKT and p-AKT. APPswe/PS1dE9 double transgenic mice could present AD pathological changes and learning and memory disorders earlier at 4 months.12 The CA1 region of the hippocampus is the core functional area of learning and memory; therefore, we chose this region of the APPswe/PS1dE9 double transgenic mice as the research object.13
Materials and methods
Materials
Curcumin (Cat. No. C1386) was purchased from Sigma-Aldrich. Rosiglitazone maleate (Cat. No. 09060108) was obtained from GlaxoSmithKline Ltd., Co. (Tianjin, PR China). All of the primary antibodies were rabbit anti-mice antibodies except β-actin (mouse monoclonal antibody), and all of them were purchased from Abcam (Hong Kong, PR China): InR (Cat. No. ab75998, diluted to 1:50 for IHC staining and 1:100 for western blot analysis), IRS-1 (Cat. No. ab52167, diluted to 1:50 for IHC staining and 1:500 for western blot analysis), PI3K (Cat. No. ab74136, diluted to 1:100 for IHC staining and 1:500 for western blot analysis), p-PI3K (Cat. No. ab61801, diluted to 1:50 for IHC staining and 1:500 for western blot analysis), AKT (Cat. No. ab8805, diluted to 1:100 for IHC staining and 1:500 for western blot analysis), β-actin (Cat. No. ab6276, diluted to 1:5000 for western blot analysis) and p-AKT (rabbit anti-mice, Cat. No. AB9234, diluted to 1:50 for IHC staining and 1:500 for western blot analysis). The SABC (Strept Actividin-Biotin Complex) IHC and 3,3’-diaminobenzidine (DAB) development kits were bought from Wuhan Boster Bio-engineering Ltd., Co. (Wuhan, PR China). The ECL western blot analysis substrate kit was purchased from Shangbo Beijing Biomedical Technology (Cat. No. WBKLS 0100). The PVDF membrane was received from Millipore (Cat. No. IDVH 00010). The Mini-PROTEAN® 3 gel electrophoresis instrument was purchased from Bio-Rad (USA). The images were analysed by the Motic Digital Medical Image Analysis System 6.0 (PR China).9
Animals
Sixty 3-month-old APPswe/PS1dE9 double transgenic mice and 12 wild-type C57/BL6J littermates were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (SCXK [Beijing] 2009-0004) and raised in the Barrier Environment Animal Lab in the Key Laboratory of Pharmacology of Dongzhimen Hospital, which is affiliated with Beijing University of Chinese Medicine (BUCM) (SYXK [Beijing] 2009-0028).9 The experiments were strictly performed abiding by the regulations and the guidelines of Beijing for the use of animals in research, and we received permission from the Animal Research Ethics Board of Dongzhimen Hospital. The mice were kept in a temperature-controlled environment with 12 h of light and 12 h of dark. The food and the purified water were freely available during the experiment. First, the mice spent 1 week acclimating themselves to the new environment. Then, the 60 APPswe/PS1dE9 double transgenic mice were randomly divided into five groups, with 12 mice in each group: the model group (VEH) (the APPswe/PS1dE9 double transgenic mice group without curcumin treatment), the rosiglitazone maleate (RSG) group, the high-dose curcumin (HDC) group, the medium-dose curcumin (MDC) group and the low-dose curcumin (LDC) group. The normal control (CON) group was composed of 12 wild-type C57/BL6J littermates.
Gavage
The dosage of the administration was prepared as follows for each treatment group: the RSG group, RSG 10 mg/kg/day; the HDC group, curcumin 400 mg/kg/day; the MDC group, curcumin 200 mg/kg/day; and the LDC group, curcumin 100 mg/kg/day. The curcumin and the RSG were dissolved in 0.5% sodium carboxymethyl cellulose (CMC) and fed to the mice at 0.1 mL/10 g body weight for 6 months, once per day, in a row. An equivalent amount of 0.5% CMC was used for the CON group and the VEH group.9
Tissue preparation
After testing their behaviour, all the mice were killed, and six mice in each group were selected at random for IHC staining. The other mice (n = 6/group) were chosen for western blot analysis. The mice that were selected for IHC staining were anaesthetised with 10% chloral hydrate first (400 mg/kg body weight, i.p.) and then quickly cardio-perfused with 50 mL 0.9% physiological saline, subsequently perfused with 60 mL of 4% paraformaldehyde. Then, the mice were decapitated; their brains were removed and placed in the same fixative solution. When the brains sank to the bottom of the jar, the fixative solution was replaced with a new fixative solution, and then paraffin embedding could be performed on the brains. Serial coronal sections of the hippocampus were cut at 4-μm intervals. After the mice that underwent western blot analysis were decapitated, the hippocampus tissues were dissociated immediately on ice and placed in cryovials. Then, we placed the cryovials into liquid nitrogen to freeze them and subsequently stored them at −80°C until the tissues were used for a western blot analysis.9
IHC staining and quantification
An IHC staining technique was used to examine the number of positive-stained neurons, including InR, IRS-1, PI3K, p-PI3K, AKT and p-AKT.14 The paraffin sections were kept in a 56°C oven for 1 h. Then, they were moved into p-xylene1 and p-xylene2 in sequence and stayed there for 20 min. Next, the slices were placed in gradient 100%, 95%, 80% and 70% alcohol for 3–5 min, and then they were placed in distilled water. Subsequently, the sections were washed with phosphate-buffered saline (PBS) three times (5 min each time). They were then incubated in 3% H2O2 (hydrogen peroxide) for 20 min and washed twice with distilled water. After they were placed in a citrate buffer solution (0.01 M, pH 6.0), the sections were put into a microwave oven and treated with moderate heat for 5 min. Once the sections cooled to an ambient temperature, we re-treated them with heat for 3 min. After that, the sections were treated with 5% goat serum for 30 min on an orbital shaker to block non-specific antibody binding. Next, the sections were placed in humidified boxes with a primary antibody for incubation at 4°C for a whole night.9 The second day, the sections were rinsed three times in PBST (5 min per time) and then were incubated with reagent 1 (Polymer Helper, Ready-to-Use PV-9001 or PV-9002, ZSGB-BIO, Beijing, PR China) at 37°C for 1 h. Afterward, they were rinsed for 5 min with PBST three times and then were incubated with reagent 2 (poly-horseradish peroxidase [HRP] anti-Rabbit IgG Ready-to-Use PV-9001, ZSGB-BIO, Beijing, PR China) at 37°C for 1 h. After rinsing the sections with PBST twice, 5 min each time, and rinsing them with Tris-Hcl for 5 min, we conducted staining by using a DAB substrate for 10–20 min. Then, the sections were dehydrated, cover-slipped and examined under a microscope. The primary antibodies are described in the Materials section of this article. Each time, two sections per mouse (a total of 12 sections for the six groups) were used for staining. To observe the number of positive-stained neurons, we chose five consecutive sections of the hippocampal CA1 region from each mouse and counted it at 20× magnification. Photographs were taken and analysed with Motic Med 6.0 Image software. The results were shown by the number of positive-stained cells in each group.
SDS-PAGE and western blot analysis
The western blot analysis procedures were performed as described previously.9 Hippocampus tissue was put into a whole cell lysis buffer (50 mL/g tissue) with the following composition: 50 mM Tris-HCl (pH 7.5), EDTA 0.1 mM, EGTA 0.1 mM, DTT 1 mM, 0.2% NP40, Leupeptin 5 mg/L, Aprotintin 2 ml/L and soybean trypsin inhibitor 50 mg/L. The cell lysates were homogenised and centrifuged at 12,000 rpm for 5 min at 4°C. The proteins were quantified by bicinchoninic acid assay.9 After that, a loading buffer was put into the protein samples and heated in boiling water for 5 min. The prepared samples were stored in a −20°C refrigerator for use. When the western blot analysis was performed, the protein samples were heated in boiling water for 5 min first and then separated and purified by SDS-PAGE under 90V (spacer gel) and 120V (separation gel). The band of target protein was excised from the gel and subsequently transferred electrophoretically to a polyvinylidene difluoride membrane by applying a 200-mA current on ice for 2 h. The membrane was prestained with Ponceau stain and then washed with PBS-Tween 20. After being sealed with 5% skimmed milk for 2 h, the membrane was hybridised with primary antibodies for a night at 4°C.9 Afterward, the membrane was rinsed with PBS-Tween 20 for 10 min three times and was incubated with HRP-conjugated IgG secondary antibody (1:5000–1:10,000, Jackson ImmunoResearch, Beijing, PR China) for 1 h. Then, it was rinsed with PBS-Tween 20 for 10 min three times. Finally, the membrane was examined by enhanced chemiluminescence (Beijing Dingguo Biotechnology Inc., Beijing, PR China) for 3 min. The protein bands were quantified with Image J software, and β-actin was used as the internal control.
Statistical analysis
All of the data were analysed with SPSS 15.0 software and were presented in the form of mean ± SD (standard deviation). A one-way ANOVA (non-parametric analysis of variance) was used for analysis, and post-hoc comparisons were made using a Mann–Whitney U-test. The data were considered statistically significant at P <0.05.
Results
InR expression in the hippocampal CA1 region
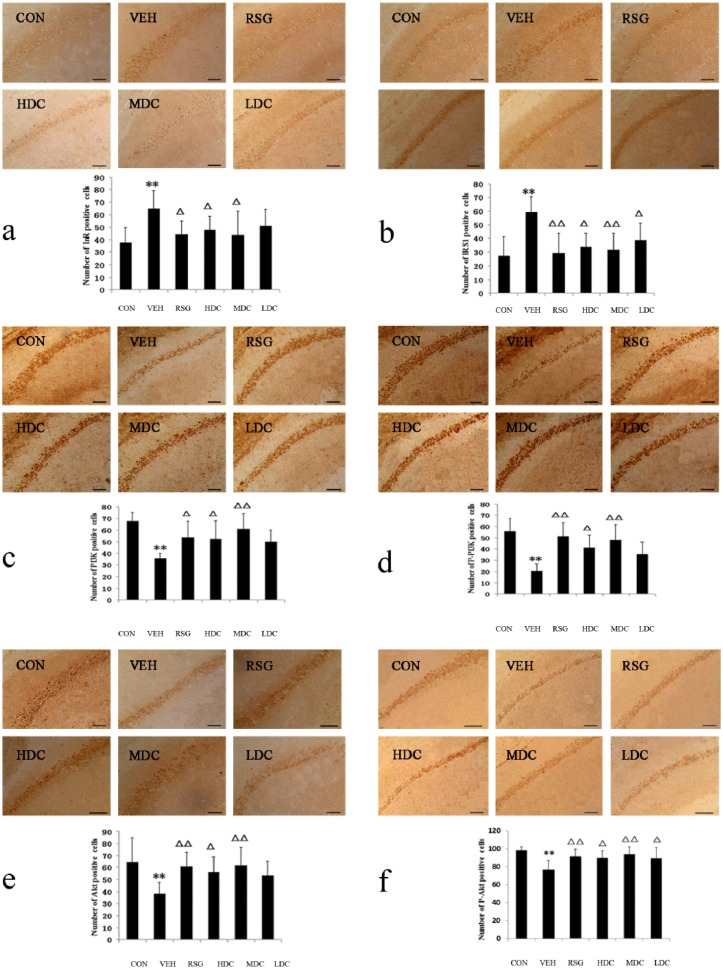
The quantification analysis of the IHC staining showed that the number of InR positive-stained cells in the VEH group was significantly higher than the number in the CON group (P <0.01). Compared with the VEH group, the RSG, HDC and MDC groups had fewer InR positive-stained cells (P <0.05), whereas the LDC group showed no difference (P >0.05) (Figure 1a).
Figure 1.
The number of positive-stained cells of InR, IRS-1, PI3K, p-PI3K, AKT and p-AKT in IHC staining. CON, normal control group; HDC, high-dose curcumin group; LDC, low-dose curcumin group; MDC, medium-dose curcumin group; RSG, rosiglitazone maleate group; VEH, model group. Compared with the CON group, **P <0.01, *P <0.05; compared with the VEH group, ΔΔP <0.01, ΔP <0.05. (a) The number of InR positive-stained cells in IHC. (b) The number of IRS-1 positive-stained cells in IHC. (c) The number of PI3K positive-stained cells in IHC. (d) The number of p-PI3K positive-stained cells in IHC. (e) The number of AKT positive-stained cells in IHC. (f) The number of p-AKT positive-stained cells in IHC.
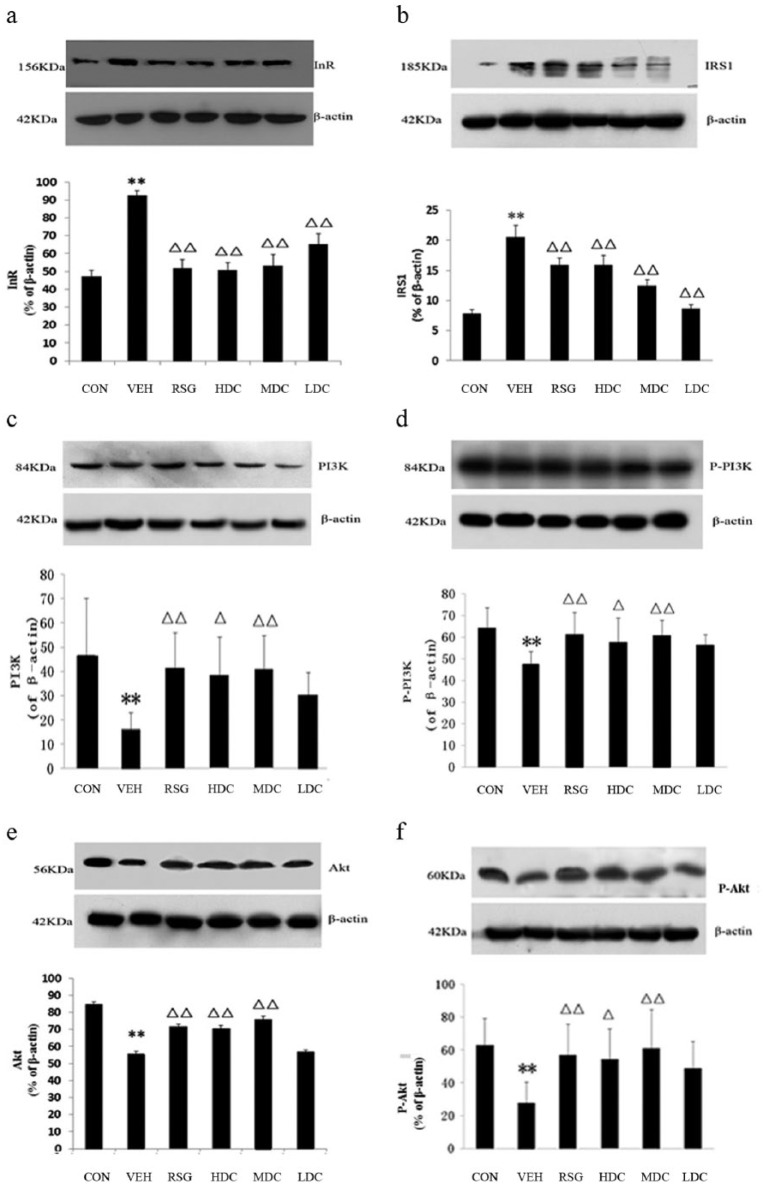
The western blot analysis indicated that the protein expression of InR in the VEH group was significantly increased compared with the CON group (P <0.01). All of the treated groups showed a significant reduction in the InR expression when compared to the VEH group (P <0.01) (Figure 2a).
Figure 2.
The expression of InR, IRS-1, PI3K, p-PI3K, AKT and p-AKT in western blot analysis. CON, the normal control group; HDC, high-dose curcumin group; LDC, low-dose curcumin group; MDC, medium-dose curcumin group; RSG, rosiglitazone maleate group; VEH, model group. Compared with the CON group, **P <0.01, *P <0.05; compared with the VEH group, ΔΔP <0.01, ΔP <0.05. (a) The expression of InR protein in western blot analysis. (b) The expression of IRS-1 protein in western blot analysis. (c) The expression of PI3K protein in western blot analysis. (d) The expression of p-PI3K protein in western blot analysis. (e) The expression of AKT protein in western blot analysis. (f) The expression of p-AKT protein in western blot analysis.
IRS-1 expression in the hippocampal CA1 region
In contrast with the CON group, there was a significant increase in the number of positive cells and the protein expression of IRS-1 in the VEH group (P <0.01).
When compared to the VEH group, the number of IRS-1 positive cells in all of the treated groups were decreased (P <0.05). In addition, there was a significant difference in the RSG and MDC groups (P <0.01). The protein expression of IRS-1 revealed a similar result to the IHC results. All of the treated groups showed a significant reduction in the IRS-1 expression compared with the VEH group (P <0.01) (Figures 1b and 2b).
PI3K and p-PI3K expression in the hippocampal CA1 region
The IHC staining and western blot analysis results showed that the number of PI3K and p-PI3K positive-stained cells and the protein expression in the VEH group were significantly lower than those in the CON group (P <0.01).
In comparison with the VEH group, the PI3K positive-stained cells in the RSG and HDC groups had an obvious increase (P <0.05) and in the MDC group had a significant increase (P <0.01). There was no difference between the LDC group and the VEH group (P >0.05). The protein expression of PI3K and the IHC staining results were very much alike; all of the treated groups except for the LDC group showed a significant increase compared with the VEH group (P <0.01 for the RSG and MDC groups; P <0.05 for the HDC group) (Figures 1c and 2c).
The IHC results of p-PI3K were the same as the western blot analysis results. In contrast to the VEH group, the positive cells and the protein expression of p-PI3K in the RSG and MDC groups were significantly increased (P <0.01). Meanwhile, the HDC group also had an obvious increase (P <0.05), whereas no difference was found in the LDC group (P >0.05) (Figures 1d and 2d).
AKT and p-AKT expression in the hippocampal CA1 region
The number of AKT and p-AKT positive-stained cells and the protein expression in the VEH group were significantly reduced compared to those in the CON group (P <0.01).
Compared with the VEH group, the positive-stained cells and the expression of AKT showed different degrees of increase in each group except for the LDC group. The increase was significant in the RSG and MDC groups (P <0.01) and was also noticeable in the HDC group (P <0.05 for IHC staining; P <0.01 for western blot analysis) (Figures 1e and 2e).
The results of the IHC staining for p-AKT were consistent with those of the western blot analysis. Compared with the VEH group, the increase in the HDC group was notable (P <0.05), and the increase was also significant in the RSG and MDC groups (P <0.01). In addition, an obvious rise in the number of positive cells was found in the LDC group (P <0.05), but the western blot analysis saw no apparent growth of the protein expression (P >0.05) (Figures 1f and 2f).
Discussion
In AD, impaired insulin or IGF-1 signalling contributes to the inhibition of PI3K/AKT and increases the activation of GSK-3β. This process results in the hyper-phosphorylation of tau,15 which then contributes to neurodegeneration by enhancing oxidative stress and increasing apoptosis, mitochondrial dysfunction and necrosis.1 Apart from this, the inhibition of insulin/IGF-1 signalling also impairs the Wnt pathway, which reduces GSK-3β via a PI3K/AKT-independent mechanism.16–18 The IRS family plays an important role in the function of insulin signal transduction connected with its upstream insulin receptor and its downstream PI3K protein. In this way, the IRS family serves to maintain the normal effects of an insulin signalling pathway, inhibit cell apoptosis and stimulate cell growth, survival, metabolism and plasticity. Studies demonstrate that insulin sensitisers can be a therapy for diabetes, but they can also improve central insulin resistance and ameliorate cognitive impairment and synaptic plasticity.19,20 RSG, an insulin sensitiser drug, improves cognitive function in both AD model mice21 and AD patients.22 That is why RSG is used as the positive control drug.
In this study, we used IHC staining and a western blot analysis to test the critical proteins in insulin signalling pathways. The results showed that after 6 months of administration, curcumin decreased the expression of InR and IRS-1 and increased the expression of PI3K, p-PI3K, AKT and p-AKT in the hippocampal CA1 area of APPswe/PS1dE9 double transgenic mice. The western blot analysis results were consistent with the IHC observation. In the pathology of APPswe/PS1dE9 double transgenic mice, the increase of InR probably results from brain insulin resistance. The experiments on the 9-month-old AD mice and 6-month-old AD mice gave a similar result. In light of this, we consider that curcumin can improve learning and memory abilities by regulating the critical molecules of the insulin signalling pathway and can also make the improvement last longer. The PI3K/AKT/mTOR signalling pathway, which regulates autophagy, is downregulated.23 The other study about the PI3K/AKT/GSK-3β signalling pathway showed that it can improve glucose homeostasis when upregulated.24 Our results also showed that curcumin can upregulate the PI3K/AKT signalling pathway to reduce insulin resistance and improve cerebral glucose metabolism.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the 111 Project (B08006), the National Natural Science Foundation of China (Nos. 81073076 and 81573927).
References
- 1. de la Monte SM. (2012) Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Current Alzheimer Research 9: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Y, Deng Y, Zhang B, et al. (2014) Deregulation of brain insulin signaling in Alzheimer’s disease. Neuroscience Bulletin 30: 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marchiani A, Rozzo C, Fadda A, et al. (2014) Curcumin and curcumin-like molecules: From spice to drugs. Current Medicinal Chemistry 21: 204–222. [DOI] [PubMed] [Google Scholar]
- 4. Brondino N, Re S. (2014) Curcumin as a therapeutic agent in dementia: A mini systematic review of human studies. Scientific World Journal 2014: 174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darvesh AS, Carroll RT, Bishayee A, et al. (2012) Curcumin and neurodegenerative diseases: A perspective. Expert Opinion on Investigative Drugs 21: 1123–1140. [DOI] [PubMed] [Google Scholar]
- 6. Chin D, Huebbe P, Pallauf K, et al. (2013) Neuroprotective properties of curcumin in Alzheimer’s disease–merits and limitations. Current Medicinal Chemistry 20: 3955–985. [DOI] [PubMed] [Google Scholar]
- 7. Goozee KG, Shah TM, Sohrabi HR, et al. (2016) Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. British Journal of Nutrition 115(3): 449–465. [DOI] [PubMed] [Google Scholar]
- 8. Thapa A, Jett SD, Chi EY. (2016) Curcumin attenuates amyloid-β aggregate toxicity and modulates amyloid-β aggregation pathway. ACS Chemical Neuroscience 7(1): 56–68. [DOI] [PubMed] [Google Scholar]
- 9. Wang P, Su C, Li R, et al. (2014) Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. Journal of Neuroscience Research 92: 218–231. [DOI] [PubMed] [Google Scholar]
- 10. Fan H, Wei P, Chen X, et al. (2013) Effect of curcumin on the ethology of APPswe /PS1dE9 transgenic mice. Chinese Journal of Comparative Medicine 23(6): 33–37. [Google Scholar]
- 11. He Y, Wang P, Wei P, et al. (2016) Effects of Curcumin on Synapses in APPswe/PS1dE9 Mice. International Journal of Immunopathology and Pharmacology 29: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neha, Sodhi RK, Jaggi AS, et al. (2014) Animal models of dementia and cognitive dysfunction. Life Sciences 109(2): 73–86. [DOI] [PubMed] [Google Scholar]
- 13. Overk Cassia R., Masliah Eliezer. (2014) Pathogenesis of synaptic degeneration in Alzheimer’s disease and Lewy body disease. Biochemical Pharmacology 88: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillies RM, Robinson SP, McPhail LD, et al. (2011) Immunohistochemical assessment of intrinsic and extrinsic markers of hypoxia in reproductive tissue: Differential expression of HIF1α and HIF2α in rat oviduct and endometrium. Journal of Molecular Histology 42: 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim B, Feldman EL. (2012) Insulin resistance in the nervous system. Trends in Endocrinology and Metabolism 23: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grote CW, Morris JK, Ryals JM, et al. (2011) Insulin receptor substrate 2 expression and involvement in neuronal insulin resistance in diabetic neuropathy. Experimental Diabetes Research 2011: 212571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Khoury NB, Gratuze M, Papon MA, et al. (2014) Insulin dysfunction and Tau pathology. Frontiers in Cellular Neuroscience 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma T. (2014) GSK3 in Alzheimer’s disease: Mind the isoforms. Journal of Alzheimers Disease 39: 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu SH, Jiang T, Yang SS, et al. (2013) Pioglitazone ameliorates intracerebral insulin resistance and tau-protein hyperphosphorylation in rats with type 2 diabetes. Experimental and Clinical Endocrinology & Diabetes 121: 220–224. [DOI] [PubMed] [Google Scholar]
- 20. Yoon HM, Jang KJ, Han MS, et al. (2013) Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-kappaB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells. Experimental and Therapeutic Medicine 5: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedersen WA, McMillan PJ, Kulstad JJ, et al. (2006) Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Experimental Neurology 199: 265–273. [DOI] [PubMed] [Google Scholar]
- 22. Risner ME, Saunders AM, Altman JF, et al. (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics Journal 6: 246–254. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Zhang X, Teng Z, et al. (2014) Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. European Journal of Pharmacology 740: 312–320. [DOI] [PubMed] [Google Scholar]
- 24. Jimenez S, Torres M, Vizuete M. (2011) Age-dependent accumulation of soluble amyloid β (aβ) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-α (sAPPα) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3β pathway in Alzheimer mouse model. Journal of Biological Chemistry 286(21): 18414–18425. [DOI] [PMC free article] [PubMed] [Google Scholar]