Abstract
Transplantation of ovarian tissue for the preservation of fertility in oncological patients is becoming an accepted clinical practice. However, the risk of re-introducing tumour cells at transplantation has stirred an increased interest for complete in vitro folliculogenesis. This has not yet been achieved in humans possibly for the lack of knowledge on the environmental milieu that orchestrates folliculogenesis in vivo. The main aim of this study was to investigate the effect of oxygen availability on follicle health and growth during in vitro culture of ovarian tissue strips. To this end, a model was developed to predict the dissolved oxygen concentration in tissue under varying culture conditions. Ovarian cortical strips of bovine, adopted as an animal model, and human tissue were cultured in conventional (CD) and gas permeable (PD) dishes under different media column heights and gaseous oxygen tensions for 3, 6 and 9 days. Follicle quality, activation of primordial follicles to the primary stage, and progression to the secondary stage were analysed through histology. Follicle viability was assessed through a live-dead assay at the confocal scanning laser microscope. Findings showed a higher follicle quality and viability after culture of bovine ovarian strips in PD in adequate medium height and oxygen tensions. The best culture conditions found in the bovine were adopted for human ovarian strip culture and promoted a higher follicle quality, viability and progression. Overall, data demonstrated that modulation of oxygen availability in tissue plays a key role in maintaining follicles’ health and their ability to survive and progress to the secondary stage during ovarian tissue in vitro culture. Such culture conditions could increase the yield of healthy secondary follicles for subsequent dissection and individual culture to obtain competent oocytes.
Introduction
Advancements in anti-cancer therapy have improved the survival rates of cancer patients [1] and increased the focus on post-treatment quality of life and their future reproductive potential. Anti-cancer regimes, like chemotherapy and ionizing radiations, induce variable degrees of irreversible premature ovarian failure in 100% of patients, threatening their fertility even after complete cancer remission [2,3]. Ovarian tissue cryopreservation is a promising fertility preservation strategy and represents the only option available for pre-pubertal cancer patients due to their ovarian dormancy at this age [4–7]. Thus far, orthotopic ovarian tissue transplantation following cryopreservation has yielded more than 86 pregnancies worldwide [8] suggesting the future potential of this technique.
However, transplantation of ovarian tissue following cryopreservation is approached with extreme caution, particularly in the case of blood-borne and highly metastatic malignancies, where the possibility of re-introducing tumour cells back into the patient after cancer remission represents a serious risk [9,10]. Lately, the fact that the follicular basal lamina hinders cancer invasion into the oocyte and follicular cells [11] has triggered widespread interest in the development of culture systems for primordial ovarian follicles that constitute the major share of ovarian reserve in women of all age groups. Several strategies for in vitro follicular growth have been proposed over the years in which follicles were isolated either at the primordial stage or, after a first culture step of ovarian cortical strips, at the secondary stage, and then cultured until full maturation [12–16].
Ovarian strip in situ culture has tremendous advantages over isolated primordial follicle culture systems because follicles are maintained within their natural environment. The ovarian tissue itself is a regulating force, providing cells with an array of highly complex and dynamic bio-mechanical signals [17] which are very difficult to accurately mimic in vitro with an artificial matrix. Success of in vitro follicular culture in the murine model has confirmed the important role played by initiating primordial follicle growth in situ within the ovarian tissue in a two-step culture approach [12].
Unfortunately, the success of current ovarian tissue culture systems in the human is still unsatisfactory being limited by the low efficiency of long-term survival and growth of primordial and primary follicles in situ [18,19]. Over the years, several studies have investigated ovarian tissue nutritional and endocrine requirements with the aim to optimise media for cortical strip culture [20,21]. Nonetheless, the yield of secondary follicles seldom exceeded 10% of the total follicle population obtained post-culture [15,22]. It has been proposed that the inefficient transport of oxygen could be a major hurdle in establishing optimal ovarian tissue cultures because it may hinder oocyte/follicle development and cause tissue necrosis [23]. Only a few studies have investigated the importance of oxygen supply for follicular growth, and results are still controversial [23–27].
The main aim of this study was to investigate the influence of oxygen availability to ovarian cells (e.g. the dissolved oxygen concentrations at the surfaces of the ovarian strip, CO2,TS) during in vitro culture on quality, viability and progression of human follicles. To save precious human biological material, adequate culture conditions were identified in preliminary experiments performed in the bovine, and were then used for the culture of human ovarian tissue. To this end, CO2,TS was modulated by using conventional dishes (CD) or gas-permeable dishes (PD), by varying media column height above the strips, and by incubation in air or 5% O2.
Materials and methods
Chemicals and consumables
Lumox gas-permeable culture dishes and conventional culture dishes 50mm in diameter were from Sarstedt (Nümbrecht, Germany) and Falcon (Sigma-Aldrich, Milan, Italy) respectively. Leibovitz’s L-15 medium, α-MEM Glutamax medium (code number 32571), Insulin transferrin selenium (ITS) 100x, Live/dead Fixable far red stain were purchased from Invitrogen (Milan, Italy). Penicillin streptomycin 100x, Amphotericin B 250μg/ml, Bovine serum albumin (BSA), L-Ascorbic acid, L-Glutamine 200mM, Hoechst 33342, Fructose, α-thioglycerol and Eosin-Y were purchased from Sigma Aldrich (Milan, Italy). Mayers’s hematoxylin and paraffin wax were from Carlo Erba (Milan, Italy).
Collection and preparation of ovarian tissue
Bovine ovaries were collected at the Slaughterhouse Straccione (San Marcellino, Caserta, Italy; CEE accreditation number 1403/M) and transported to the laboratory in Leibovitz’s L-15, 1% penicillin-streptomycin (Pen-Strep), 1μg/ml Amphotericin-B, at 4°C, within 2h of slaughter.
The use of human tissue was approved by the Ethics Committee of Regione Campania (ASL NA1 Centro, Naples, Italy; reference number 57 CE 2–2017). After obtaining written informed consent, ovarian biopsies were collected from six women (age = 27.1 ± 6.9 years; range 18–34 years) during laparoscopic surgery for benign gynecologic conditions and transported to the lab as described above.
Bovine ovaries and human ovarian biopsies were transferred to handling medium (Leibovitz’s L-15, 2mM glutamine, 3mg/ml BSA, 1% Pen-Strep, 1μg/ml amphotericin B) and cortical slices (~ 0.5mm thick) were manually dissected at room temperature (RT) avoiding areas with visible antral follicles to ensure a predominant primordial follicle population. The cortical slices were further uniformly sliced into 1mm x 1mmx 0.5mm strips using a tissue chopper (Mcilwain, Mickle Laboratory Engineering Company, Ltd, Surrey, UK). The strips were pooled in a 10cm Petri dish, mixed by gentle agitation, washed twice in fresh handling medium, and 10 strips were randomly distributed into each culture dish. Fresh control strips from each ovary were processed for histology and viability assessment as a control.
Strip culture and modulation of oxygen availability
In each experiment, cortical strips from the same ovary were cultured in α-MEM, 3mM glutamine, 0.1% BSA, 1% Pen-Strep, 1% ITS (10 μg/ml Insulin, 5.5 μg/ml Transferrin, 6.7 ng/ml Selenium), 1μg/ml amphotericin-B, 50μg/ml ascorbic acid at 37°C, 5% CO2 and 95% humidity in air. Half medium was changed every 48h. At the end of culture, 5 strips from each dish were treated for histology and 5 strips for viability assessments.
To identify the culture conditions ensuring the most adequate oxygen availability (e.g. dissolved oxygen concentrations at the upper, CO2,TSU, and bottom surface, CO2,TSB, of the ovarian strip), oxygen supply was modulated by culturing ovarian strips in conventional (CD) or gas permeable dishes (PD) in a volume of medium yielding average column heights from the dish bottom of 1.4mm (HV: high volume, 5ml) or 0.7mm (LV: low volume, 2.5ml) in 5% CO2 in air or in 5% CO2, 5% O2, 90% N2. The most adequate culture conditions found in the bovine model were applied to human ovarian tissue culture.
Experimental design
In experiment I (n = 3), bovine ovarian strips (BOSs) from the same ovary were cultured as described above in 5ml of medium in permeable vs conventional dishes (conditions hereinafter referred to as PDHV and CDHV, respectively) for 3, 6 and 9 days in 5% CO2 in air.
Results of experiment I demonstrated a significantly higher follicle quality and viability in PD versus CD both at day 6 (D6) and 9 (D9). Hence, in experiment II (n = 3) BOSs from the same ovary were cultured in 5% CO2 in air for 6 days in high or low volumes of medium in PD vs CD (conditions hereinafter referred to as PDHV, PDLV, CDHV, CDLV, respectively).
In PD, the strip bottom surface is in direct contact with a gas-permeable polymer which ensures direct oxygen supply and also metabolic CO2 removal across such surface. Experiment III (n = 3) was designed to investigate whether the better outcome of BOSs cultured in PDHV in experiment I and II was caused by an adequate oxygen availability or an enhanced removal of metabolic CO2. To this end, BOSs were cultured in CDHV and PDHV for 6 days under 5% CO2 in air vs 5% CO2, 5% O2, 90% N2.
Experiment IV (n = 6) was aimed to investigate whether the most adequate culture condition found in bovine experiments could have similar effects on human follicle quality, activation, progression and viability. To this end, human ovarian strips (HOSs) from the same ovaries were cultured in PDHV vs CDHV under 5% CO2 in air for 6 and 9 days.
Model of oxygen transport in ovarian tissue
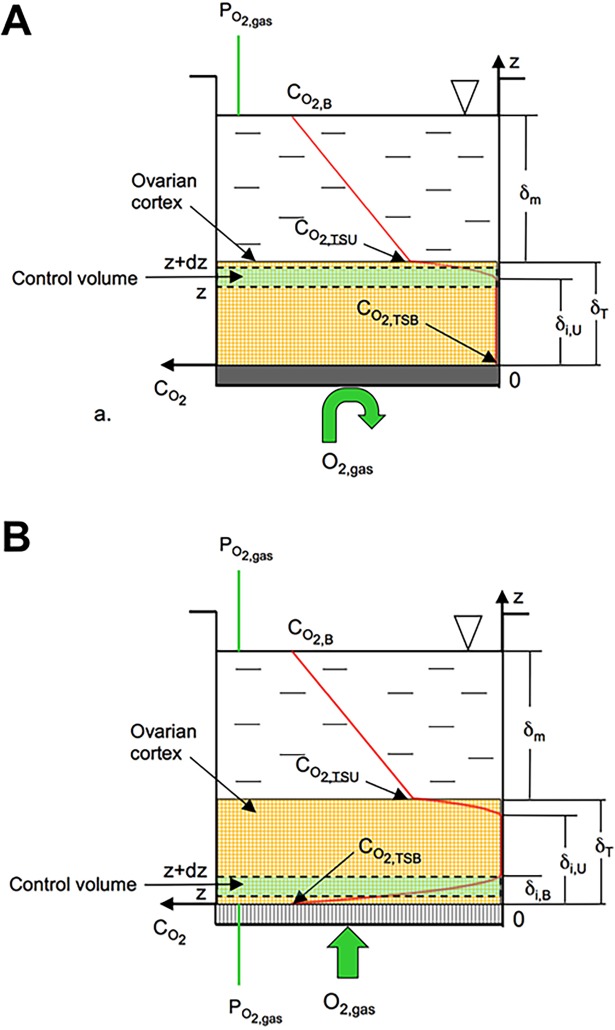
Dissolved oxygen concentration in ovarian cortical tissue during in vitro culture is generally much lower than that in air because of the poor solubility of oxygen in medium, and of the resistance to oxygen diffusion in medium and tissue combined with oxygen consumption by ovarian cells (Fig 1). To account for such effects and gather more realistic information than those derived from the incubator setting, dissolved oxygen concentration anywhere in tissue (CO2,T) under varying culture conditions was predicted with a model describing oxygen diffusive transport in medium and tissue at steady state. It was assumed that tissue has uniform properties, that oxygen enters tissue only across the upper surface (i.e. CD) or also from the bottom strip surface (i.e. PD), and that an anoxic zone forms amid the strip thickness. In CD, it is CO2,T >0 only in the uppermost strip part (i.e. from z>δi,U to z = δT), it decreases towards the strip bottom and levels off to zero at a fractional distance δi,U/ δT from it (i.e. CO2,T = 0 from z = 0 to z = δi,U). In PD, CO2,T at the strip bottom is assumed equal to the dissolved oxygen concentration in medium equilibrating the gaseous oxygen tension (CO2,B). As an effect, the dissolved oxygen concentration is CO2,T>0 also near the strip bottom but it decreases towards the upper strip surface and levels off to zero at a fractional distance δi,B/δT from the bottom (i.e. CO2,T = 0 from z = δi.B to z = δi,U). The bias associated to the lack of reliable estimates of ovarian cortex structural, transport and metabolic properties was minimized by lumping such properties in the dimensionless Thiele modulus ϕ and mass Biot number Bim, actually determining the dissolved oxygen concentration profile in tissue. The former compares the rate of oxygen metabolic consumption to diffusion in tissue. The latter compares the dissolved oxygen concentration drop in tissue to that in medium to sustain oxygen transport to cells. In CD, an oxygen mass balance about a tissue control volume infinitesimal in z yields the dimensionless dissolved oxygen concentration, CO2,T /CO2,B, in the uppermost strip part at a fractional distance z/ δT from the bottom as follows (S1 Appendix):
| (1) |
with
| (2) |
where: G’’’ is thse volumetric tissue oxygen consumption rate; DT and Dm are oxygen diffusivity in tissue and medium, respectively; δm and δT are the medium height above the strip and tissue thickness, respectively. In PD, CO2,T/ CO2,B close to the strip upper surface is the same as in Eqs 1 and 2. CO2,T/ CO2,B close to the strip bottom may be gathered with a similar mass balance by imposing that CO2,T equals CO2,B at z = 0 and that it levels off to zero at z = δi,B, to give (S2 Appendix):
| (3) |
Fig 1. Scheme of ovarian cortex culture in conventional (CD) and gas-permeable (PD) dishes.
The continuous red lines show exemplary dissolved oxygen profiles predicted in medium and tissue by the oxygen transport model described in Eqs 1–3 when strips of ovarian cortical tissue are cultured in: A) conventional dishes with gas-impermeable bottom (CD); B) dishes with gas-permeable bottom (PD). The green box identifies the control volume with respect to which the differential mass balance equations were written, as described in S1 Appendix and S2 Appendix for CD and PD, respectively. The meaning of the symbols may be found in text.
To gather qualitative information on oxygen availability in the ovarian strips for the conditions used for this work, exemplary profiles of the dimensionless dissolved oxygen concentration in tissue, CO2,T/CO2,B, were estimated from Eqs 1–3 for representative parameter values, as follows: G’’’ = 2x10-2 mol/(s m3); Dm = 3.5x10-9 m2/s; DT = 2.8x10-9 m2/s; δT = 0.4–0.5 mm; δm = 0.2 (LV) or 0.9 (HV) mm. CO2,B was set at 0.2 mol/m3 (i.e. pCO2gas = 21%), unless otherwise noted. These values yield the following dimensionless parameters values: ϕ = 2.5, Bim = 0.7 (HV) or 3 (LV). Oxygen availability to ovarian cells was characterized in terms of the dissolved oxygen concentrations at the upper (CO2,TSU) and bottom (CO2,TSB) strip surface, the average dissolved oxygen concentration in the strip (CO2,T,avg), and the percent strip volume operated under anoxic conditions.
Histology
To assess follicular quality, activation and progression, strips were fixed in Bouin’s, dehydrated in increasing ethanol concentrations, embedded in paraffin and 5μm serial sections were stained with hematoxylin and eosin. All follicles visualized in serial sections from each strip were graded and staged by two blinded expert observers. Follicles were evaluated only when the germinal vesicle was visible to minimize the chance of re-counting. Follicle quality was graded as previously reported [28]. Briefly: grade 1 follicles were spherical and had homogeneously distributed granulosa cells (GCs) and an oocyte with homogenous cytoplasm and slightly granular nucleus, in the center of which condensed chromatin in the form of a dense spherical structure is detected; grade 2 follicles had GCs pulled away from the edge of the follicle but still a spherical oocyte; and grade 3 follicles had GCs with pyknotic nuclei and misshapen oocyte with or without vacuolation. Follicle stages were scored according to Gougeon's criteria [29], as follows: primordial with a single layer of flat GCs; primary with a complete single layer of cuboidal GCs; secondary with two or more complete layers of cuboidal GCs.
Viability assessment
Strips were incubated in Dulbecco’s PBS with 1μg/ml Live/Dead Fixable Far Red Stain and 10μg/ml Hoechst 33342, 3 hours at 4°C under gentle agitation, fixed in 4% paraformaldehyde in PBS 2 hours at RT, washed in fresh PBS and incubated in PBS 10μg/ml Hoechst 33342 at 4°C overnight [30]. The live/dead probe is resistant to fixation, reacts with free amines both in the cell interior and on the cell surface and is excluded by cells with intact membranes. Strips were then optically cleared using See DB clearing protocol [31]. Briefly, samples were serially incubated in 5 mL of 20%, 40%, and 60% (wt/vol) fructose, each for 3 hours, 80% and 100% fructose (wt/vol) each for 12 hours, and finally in 115% (wt/vol) fructose for 24 hours with gentle shaking at RT. All fructose solutions were supplemented with 0.5%. α-thioglycerol. To avoid compression, strips were mounted in 115% fructose on a glass slide with 3 spacer coverslips (0.17mm) placed on each side and covered with a coverslip.
Analysis was carried out with a Leica TCS SP5 confocal scanning laser microscope (Leica Microsystems, Wetzlar, Germany) using a 405-nm diode laser for visualizing the nuclear label (Hoechst 33342) and a 633-nm helium neon laser for the live/dead probe. Each strip was traversed using the z-position control and fields to a depth of 300μm from the tissue surface were imaged using a 63x glycerol immersion objective.
Statistical analysis
For each experiment, data is presented as cumulative percentages. Overall, statistical analysis was performed by Fisher’s exact test for pairwise comparisons when overall significance was detected.
Results
Model-predicted oxygen availability
As shown in Table 1, when strips were cultured in air, oxygen availability increases with the culture conditions in the following order: CDHV<CDLV<PDHV<PDLV. When strips were cultured at pCO2gas = 5% in CDHV the model predicts that 99% of the strip volume is operated under anoxic conditions. Use of dishes with a gas-permeable bottom in PDHV slightly enhances oxygen availability, yet more than 85% of the strip volume is operated under anoxic conditions.
Table 1. Model-predicted oxygen availability in a tissue strip.
| Culture condition | CO2,TSB/CO2,B, % | CO2,TSU/CO2,B, % | CO2,T,avg/CO2,B, % | Anoxic strip volume fraction, % |
|---|---|---|---|---|
| CDHV | 0 | 3.6 | 0.053 | 89.3 |
| PDHV | 100 | 3.6 | 1.25 | 32.6 |
| CDLV | 0 | 32.6 | 0.3 | 67.7 |
| PDLV | 100 | 32.6 | 1.55 | 11.1 |
Model predictions were obtained for ϕ = 2.5 and Bim = 0.7 (HV) and 3 (LV): CO2,TSB−dissolved oxygen concentration at strip bottom surface; CO2,TSU−dissolved oxygen concentration at strip upper surface; CO2,T,avg−average dissolved oxygen concentration in the strip volume.
Experiment I
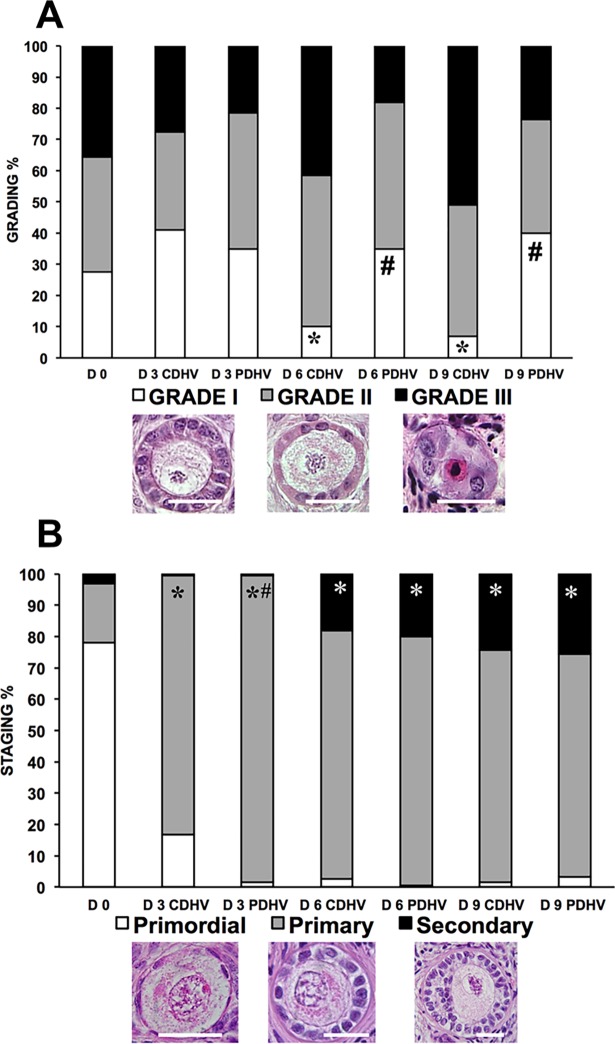
Histological analysis (n = 1837 follicles: D0, 307; D3 CDHV, 242; D3 PDHV, 265; D6 CDHV, 287; D6 PDHV, 223; D9 CDHV, 275; D9 PDHV, 238) showed that strips cultured in PDHV harboured significantly more grade I follicles both on D6 and 9. By D6 the proportion of grade I follicles in CDHV dropped significantly compared to day 0 (Fig 2A). Both groups (Fig 2B) showed a marked and significant decrease of primordial follicles and a corresponding significant increase of primary follicles at D3 as compared to D0. Interestingly, both groups showed a significantly higher proportion of secondary follicles at D6 and D9 than at D0.
Fig 2. Grading and staging of bovine follicle in strips cultured in air in PDHV vs CDHV.
Histological grading (A) and staging (B) of bovine follicles in strips cultured in PDHV and CDHV in air. Bar = 20μm. *P<0.01 vs D0; #P<0.01 vs corresponding treatments.
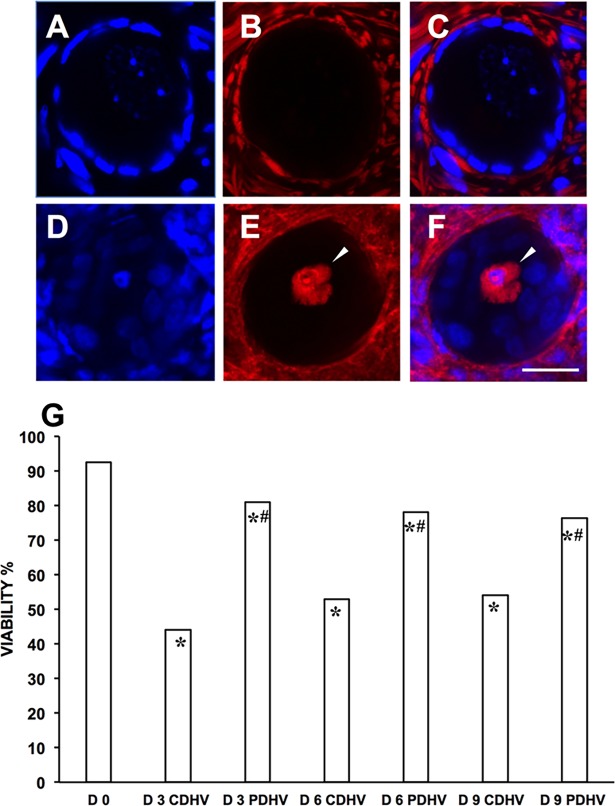
Follicle viability was obtained from a total of 1969 follicles (number of follicles: D0, 254; D3 CDHV, 278; D3 PDHV, 312; D6 CDHV, 323; D6 PDHV, 236; D9 CDHV, 202; D9 PDHV, 364). Fig 3 shows representative confocal micrographs of one viable (A-C) and one dead follicle (D-F). Viability significantly decreased during culture under both conditions. However, viability throughout the culture was about 80% in PDHV and only around 50% in CDHV (Fig 3G).
Fig 3. Viability of bovine follicles in strips cultured in air in PDHV vs CDHV.
Representative confocal micrographs of a live (A,B,C) and a dead (D,E,F) follicle. (A,D) Hoechst 33342–stained nuclei; (B,E) live–dead far-red probe; (C,F) merge. (G) Follicle viability in fresh and cultured strips. Arrowheads indicate a dead oocyte. Bar = 20μm. *P<0.01 vs d0; #P<0.01 vs corresponding treatments.
Taken together, this data (S1 Table) suggests that increased CO2,TS enhances follicular activation and preserves viability.
Experiment II
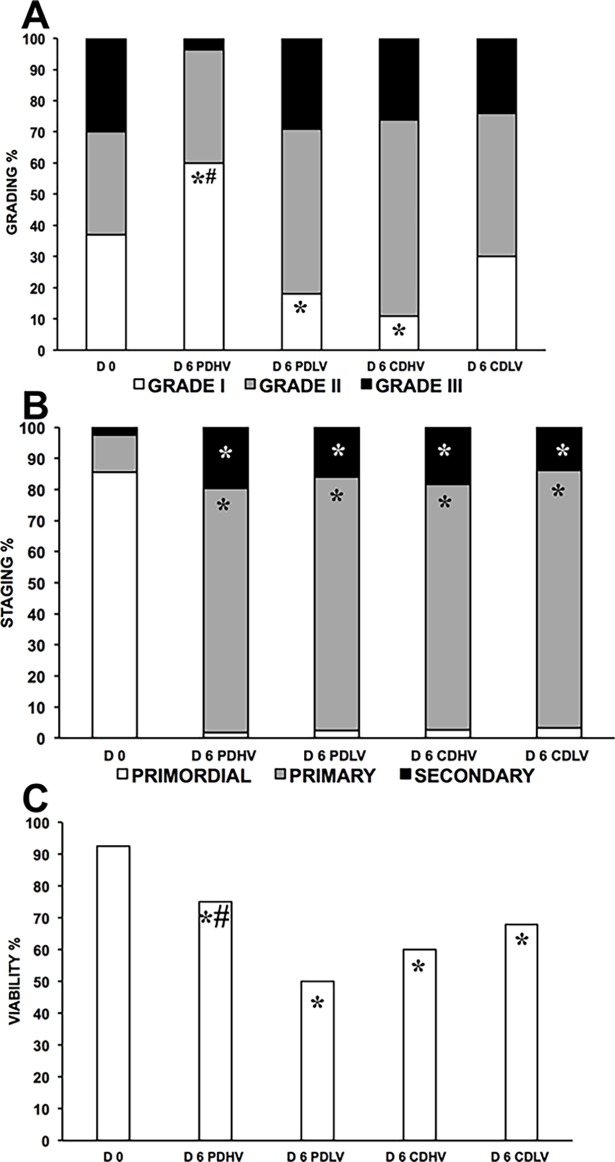
The transport model predicts that the combined use of different medium heights and CD or PD yielded progressively increasing oxygen availability in the following order: CDHV<CDLV<PDHV<PDLV (Fig 1 and Table 1). Histological analysis (n = 1243 follicles: D0, 273; D6 PDHV, 213; D6 PDLV, 287; D6 CDHV, 199; D6 CDLV, 271) showed that culture in PDHV yielded a significantly higher percentage of grade 1 follicles than both D0 and the other D6 samples (Fig 4A, S2 Table). The higher grading in PDHV compared to the fresh control could be due to the re-absorption of atretic follicles during culture coupled with no further addition of atretic follicles. Grading under the four conditions reflects the model-predicted oxygen availability in tissue. In fact, culture in CDHV and PDLV at the predicted lowest and highest oxygen availability yielded the worst follicle quality. Culture in CDLV outperformed CDHV in terms of grade I follicles possibly showing a positive effect of higher CO2,T caused by reduced media height.
Fig 4. Quality of bovine follicles in strips cultured under various conditions in air.
Histological grading (A), staging (B), and viability (C) of bovine follicles in strips cultured in PDHV, PDLV, CDHV and CDLV in air. *P<0.01 vs D0; #P<0.01 vs corresponding treatments.
By D6, the majority of the primordial follicles was activated to the primary stage and a significant progression to the secondary stage was observed compared to D0 with no significant differences among groups (Fig 4B, S2 Table).
Viability (n = 971 follicles: D0, 237; D6 PDHV, 173; D6 PDLV, 187; D6 CDHV, 148; D6 CDLV, 226) was 89.5% at D0 and significantly dropped in all groups at D6 (Fig 4C, S2 Table). Viability at D6 in PDHV was significantly better than in PDLV and CDHV, whereas in CDLV it was only slightly lower than in PDHV.
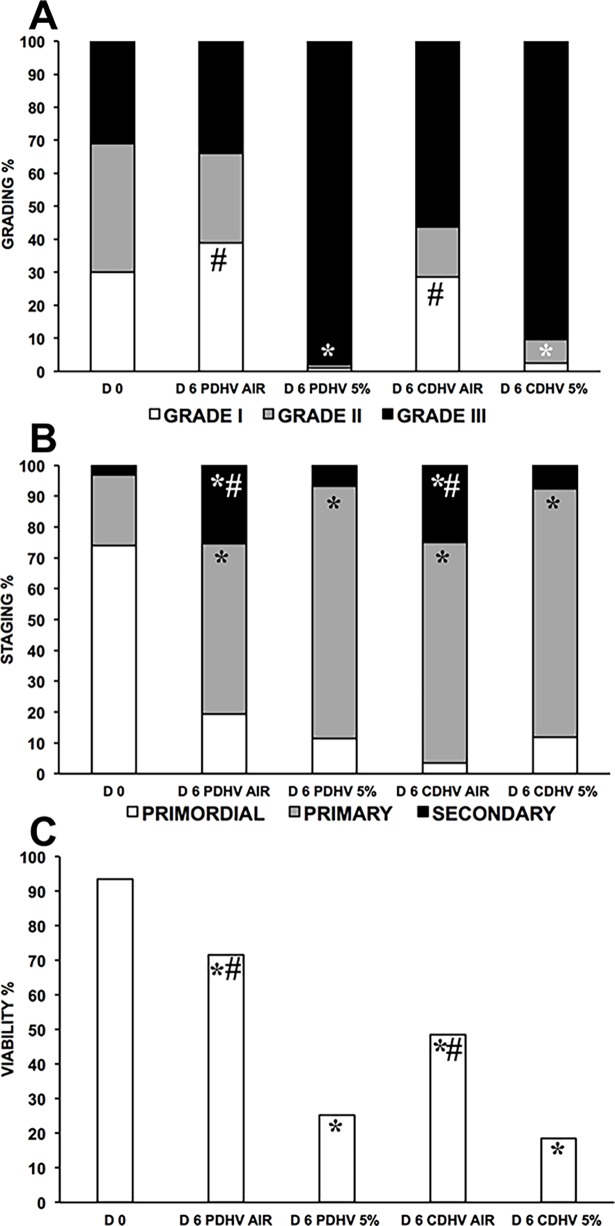
Experiment III
Histological analysis (n = 978 follicles: D0, 222; D6 PDHV AIR, 187; D6PDHV 5%, 148; D6 CDHV AIR, 265; D6 CDHV 5%, 156) showed that follicle quality was best in PDHV in air. In agreement with the large anoxic strip fraction predicted by the model, culture in 5% O2 markedly and significantly reduced follicle quality in both CDHV and PDHV (Fig 5A, S3 Table). All conditions supported high follicle activation, but culture under 5% O2 impaired progression to the secondary stage (Fig 5B, S3 Table). Viability analysis (n = 587 follicles: D0, 143; D6 PDHV AIR, 96; D6PDHV 5%, 109; D6 CDHV AIR, 121; D6 CDHV 5%, 118) indicates that PDHV in air provides the best culture conditions (Fig 5C, S3 Table).
Fig 5. Quality of bovine follicles in strips cultured in air vs. 5%O2.
Histological grading (A), staging (B), and viability (C) of bovine follicles in strips cultured in PDHV and CDHV in air versus 5% O2. *P<0.01 vs D0; #P<0.01 vs corresponding treatments.
Experiment IV
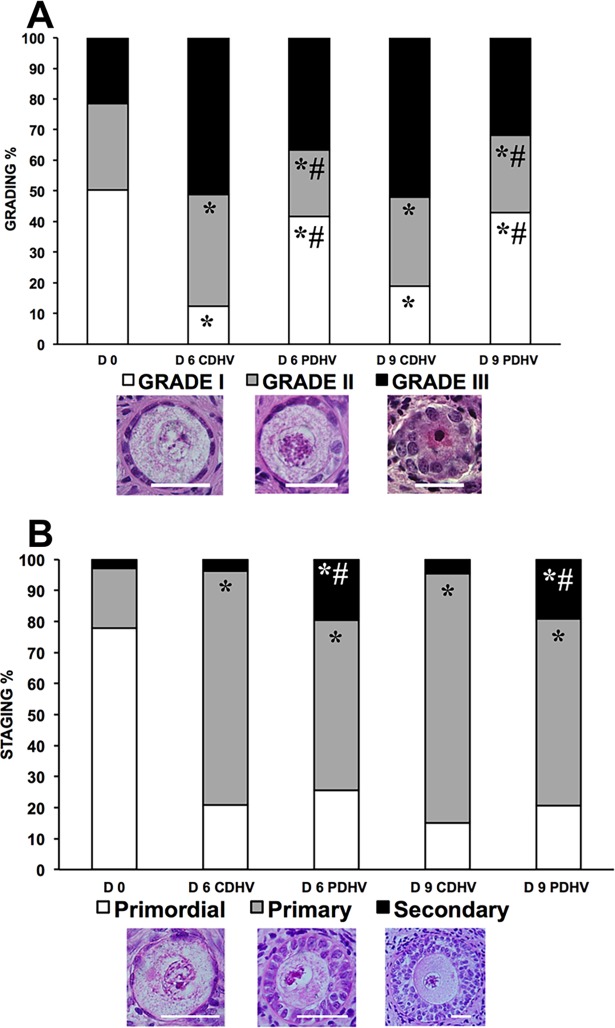
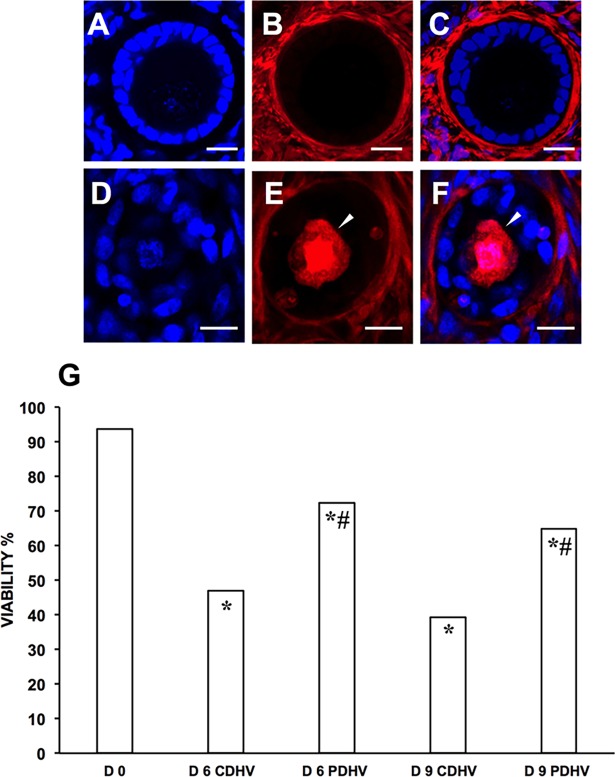
Histological analysis of HOSs (n = 1383 follicles: D0, 243; D6 CDHV, 327; D6 PDHV, 302; D9 CDHV, 206; D9 PDHV, 305) (Fig 6A, S4 Table) showed that follicle quality significantly dropped during culture as compared to D0 (Fig 6A). As seen in the bovine, culture in PDHV still provided the best conditions and yielded a greater proportion of grade 1 follicles than in CDHV at both D6 and 9 (Fig 6A). Culture under all conditions supported follicle activation but progression to the secondary stage was significantly higher in PDHV than in CDHV at both D6 and D9 (Fig 6B, S3 Table). In agreement with the histology findings, viability (n = 745 follicles: D0, 133; D6 CDHV, 125; D6 PDHV, 231; D9 CDHV, 119; D9 PDHV, 137) decreased during culture as compared to D0 (Fig 7A–7F). Culture in PDHV provided the best conditions yielding a significantly higher viability than in CDHV both at D6 and D9 (Fig 7G, S4 Table).
Fig 6. Grading and staging of human follicles in strips cultured in air in PDHV vs CDHV.
Histological grading (A) and staging (B) of human follicles in strips cultured in PDHV and CDHV. Bar = 20μm. *P<0.01 vs D0; #P<0.01 vs corresponding treatments.
Fig 7. Viability of human follicles in strips cultured in PDHV vs CDHV.
Representative confocal micrographs of a live (A,B,C) and a dead (D,E,F) follicle. (A,D) Hoechst 33342–stained nuclei; (B,E) live–dead far-red probe; (C,F) merge. (G) Follicle viability in fresh and cultured strips. Arrowheads indicate a dead oocyte. Bar = 15μm. *P<0.01 vs D0; #P<0.01 vs corresponding treatments.
Discussion
Human folliculogenesis in vivo is a complex process that stretches over a period of more than 290 days [32]. Although follicle growth in vitro is exceptionally accelerated [33], to achieve complete in vitro folliculogenesis it is necessary to develop culture strategies and systems that support long-term maintenance of tissue viability and follicle quality. The two-step culture strategy [12] is among the most promising techniques to realise complete in vitro folliculogenesis. Within this framework, the optimisation of cortical strip culture may play a pivotal role in the preservation of follicle health and in its activation and progression to the secondary stage. When ovarian tissue fragments are cultured in conventional culture dishes, it has been proposed that the poor availability of oxygen may hinder oocyte/follicle development and even cause tissue necrosis [23]. Few studies have investigated the importance of oxygen supply for follicular viability and growth but have not provided yet conclusive evidence [23–27]. In such studies, ovarian strips varying in geometry and thickness have been generally cultured at 100% vs 21% or 21% vs 5% pO2gas for various times. Tissue has also been cultured under uncontrolled heights of media differing for composition and supplements.
Albeit simple, the model proposed herein suggests that, in addition to pO2gas, the thickness of the ovarian strip, the metabolic rate at which oxygen is consumed (which changes with the tissue source and the culture medium), and the height of medium above the strip, all affect the resistance to oxygen transport from the gas phase, oxygen concentration in the strip, and ultimately oxygen availability to ovarian cells [34]. This suggests that the findings from the reported studies might be contradictory for the multitude of factors that were varied by different investigators and that could affect the actual oxygen availability to ovarian cells. To the best of our knowledge, this is the first time that the importance of oxygen for cortical ovarian tissue is investigated in culture experiments by systematically varying oxygen availability to ovarian cells. The oxygen transport model was instrumental to seeking those conditions that would permit ovarian cells culture in the strip at varying oxygen availability.
Our experiments investigated follicle quality, progression and viability in BOSs and HOSs cultured at varying oxygen availability by using CD or PD, or by changing medium height or gaseous oxygen tension. The overall results indicate that an adequate oxygen supply is required to maintain follicle quality and viability and to promote follicle progression both in bovine and human tissue. The significantly higher proportion of healthy and viable follicles in BOSs cultured in PDHV vs CDHV suggests that culture in CDHV under the conditions used established very low CO2,T‘s that compromise follicle health. Culture in PDHV yielded better oxygen availability and preserved follicle quality and viability. Culture under low medium height was used to increase CO2,T in BOSs and mimic the thin medium layer frequently used for strip culture on tissue inserts [34]. Culture in PDHV still offered the best conditions. Culture in CDHV and PDLV, that were predicted to establish the lowest and highest CO2,T respectively, impaired follicle health suggesting that bovine follicles require CO2,T within an optimal range for long-term in vitro culture.
Culture in PDs was expected to enhance oxygen supply by providing oxygen also through the tissue bottom (high CO2,TBS), but would also enhance removal of metabolic CO2. When BOSs were cultured in PDHV and CDHV in air vs. 5% O2, culture under reduced oxygen tension appeared to exert a dominant detrimental effect on follicle quality, viability and progression. This suggests that enhancing CO2 removal had less relevant effects on follicle health than enhancing the oxygen availability. This is in agreement with the increased growth, estradiol secretion, and decreased lactate production reported when oxygen availability to mouse preantral follicles was enhanced by culturing them at the medium/air interphase in inverted multiwells plates rather than in immersed upright condition [35]. Our findings are in disagreement with those of Jorssen et al. [24] that reported similar rates of follicle survival and progression in BOS cultured in 5% and 20% oxygen. Such a discrepancy is possibly due to the thinner strips used (0.2 vs 0.5mm in the present study) that may have not limited oxygen diffusion. It should be noted that the goodness of the model predictions is limited by the model assumptions and by the fact that model parameters had to be estimated from literature information for similar tissue under similar conditions to those used in this work for the lack of data. For the sake of the example, DT was assumed equal to that reported for ocular stroma [36] because it was thought that it could better account for the heterogeneous nature of the ovarian cortex. The value of G”‘ was extrapolated to 6 days of culture from the values reported in the only paper in which the oxygen consumption rate of ovarian cortical strips has been characterized [37].
The best and worst culture conditions identified for bovine tissue (i.e. PDHV and CDHV) were chosen to culture HOSs from six patients. A large number of follicles was analysed for histology and viability. Also for HOSs, culture in PDHV yielded better follicle quality and viability. Different from BOSs, culture in PDHV yielded also a better follicle progression in HOSs. The different culture outcome in the two species could possibly be due to variations in tissue anatomy, metabolism and resistance to oxygen diffusion, and underlies that, in spite of the fact that bovine tissue is considered a reliable model for human in vitro folliculogenesis [38], it may behave differently from human tissue at least in some respects.
Several studies have reported that supplementation of factors like FBS, FSH or activin was not particularly effective in augmenting the growth of primary follicles to the secondary stages [39–42]. It is worth mentioning that in PDHV we obtained one of the highest secondary follicles yield reported in literature by using basal serum-free medium without FSH or activin [43–46]. This suggests that adequate oxygen availability is a key factor to support follicle progression in HOSs culture.
Follicular oxygen requirement is a highly dynamic process in which follicle oxygen consumption increases as follicles progress from the primordial to the primary and secondary stage, outlining the role of both tricarboxylic acid cycle and oxidative phosphorylation in primordial follicle activation and progression [47]. Our results show that all culture conditions promoted follicle activation independent of the predicted oxygen concentration at the strip surfaces or interior, whereas progression to the secondary stage depended more strongly on oxygen availability. This agrees well with the increasingly higher oxygen depletion in tissue caused by the increasing oxygen consumption rates of human follicles as they progress from primordial to primary (2.5-fold) and from primary to the secondary stage (further 3.8-fold) reported by Ishikawa et al. [47].
In conclusion, culture of ovarian strips using gas-permeable dishes under optimal medium height enabled to maintain follicles health and promote their development. We believe that such an approach holds promise to increase the efficiency of a two-step culture strategy by improving quality and yield of secondary follicles for further isolated culture to achieve complete in vitro folliculogenesis.
Supporting information
Details on how the concentration profile in ovarian tissue was obtained when ovarian tissue is cultured in conventional dishes.
(DOCX)
Details on how the concentration profile in ovarian tissue was obtained when ovarian tissue is cultured in gas-permeable dishes.
(DOCX)
Percentages of bovine follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Percentages of bovine follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Percentages of bovine follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Percentages of human follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Acknowledgments
This study was supported by Merck Serono S.p.A. (Rome, Italy) with the grant “Optimizing ovarian tissue vitrification and subsequent in vitro culture using oxygen permeable dishes for sustaining in vitro follicle growth”.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Merck Serono S.p.A. (Rome, Italy), grant "Optimizing ovarian tissue vitrification and subsequent in vitro culture using oxygen permeable dishes for sustaining in vitro follicle growth". The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–1513. doi: 10.1016/j.fertnstert.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 3.Kondapalli LA, Dillon KE, Sammel MD, Ray A, Prewitt M, Ginsberg JP, et al. Quality of life in female cancer survivors: is it related to ovarian reserve? Qual Life Res. 2013;23:585–592. doi: 10.1007/s11136-013-0473-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032 [DOI] [PubMed] [Google Scholar]
- 5.Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Onco. 2005;6:209–218. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt KT, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG: An Int J Obstet Gynaecol. 2009;117:163–174. [DOI] [PubMed] [Google Scholar]
- 7.Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016; 33:1595–1603. doi: 10.1007/s10815-016-0814-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–336. doi: 10.1007/s10815-016-0843-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–2914. doi: 10.1182/blood-2010-01-265751 [DOI] [PubMed] [Google Scholar]
- 10.Abir R, Aviram A, Feinmesser M, Stein J, Yaniv I, Parnes D. Ovarian minimal residual disease in chronic myeloid leukaemia. Reprod Biomed Online. 2014;28:255–260. doi: 10.1016/j.rbmo.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Abir R, Nitke S, Haroush AB, Fisch B. In vitro maturation of human primordial ovarian follicles: Clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–898. doi: 10.14670/HH-21.887 [DOI] [PubMed] [Google Scholar]
- 12.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029 [DOI] [PubMed] [Google Scholar]
- 13.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–1810. doi: 10.1093/humrep/der468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014; 31:1013–1028. doi: 10.1007/s10815-014-0252-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070 [DOI] [PubMed] [Google Scholar]
- 16.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99:1523–1533. doi: 10.1016/j.fertnstert.2013.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West ER, Shea LD, Woodruff TK. Engineering the follicle microenvironment. Semin Reprod Med. 2007; 25:287–299. doi: 10.1055/s-2007-980222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzel Y, Oktem O. Understanding follicle growth in vitro: Are we getting closer to obtaining mature oocytes from in vitro-grown follicles in human? Mol Reprod Dev. 2017; 84:544–559. doi: 10.1002/mrd.22822 [DOI] [PubMed] [Google Scholar]
- 19.Yin H, Kristensen SG, Jiang H, Rasmussen A, Andersen CY. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum Reprod. 2016;31:1531–1539. doi: 10.1093/humrep/dew049 [DOI] [PubMed] [Google Scholar]
- 20.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. [DOI] [PubMed] [Google Scholar]
- 21.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 2010;139:971–978. doi: 10.1530/REP-10-0025 [DOI] [PubMed] [Google Scholar]
- 23.Morimoto Y, Oku Y, Sonoda M, Haruki A, Ito K, Hashimoto S, et al. High oxygen atmosphere improves human follicle development in organ cultures of ovarian cortical tissues in vitro. Hum Reprod. 2007;22:3170–3177. doi: 10.1093/humrep/dem314 [DOI] [PubMed] [Google Scholar]
- 24.Jorssen EP, Langbeen A, Fransen E, Martinez EL, Leroy JL, Bols PE. Monitoring preantral follicle survival and growth in bovine ovarian biopsies by repeated use of neutral red and cultured in vitro under low and high oxygen tension. Theriogenology. 2014;82:387–395. doi: 10.1016/j.theriogenology.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–1072. doi: 10.1093/humrep/der049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gook DA, Edgar DH, Lewis K, Sheedy JR, Gardner DK. Impact of oxygen concentration on adult murine pre-antral follicle development in vitro and the corresponding metabolic profile. Mol Hum Reprod. 2014;20:31–41. doi: 10.1093/molehr/gat062 [DOI] [PubMed] [Google Scholar]
- 27.Connolly JM, Kane MT, Quinlan LR, Dockery P, Hynes AC. Hypoxia limits mouse follicle growth in vitro. Reprod Fertil Dev. 2016; 28: 1570–1579. [DOI] [PubMed] [Google Scholar]
- 28.Talevi R, Barbato V, Mollo V, Fiorentino I, De Stefano C, Guarino FM, et al. Replacement of sodium with choline in slow-cooling media improves human ovarian tissue cryopreservation. Reprod Biomed Online. 2013;27:381–389. doi: 10.1016/j.rbmo.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 29.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121 [DOI] [PubMed] [Google Scholar]
- 30.Talevi R, Barbato V, Fiorentino I, Braun S, De Stefano C, Ferraro R, et al. Successful slush nitrogen vitrification of human ovarian tissue. Fertil Steril. 2016;105:1523–1531. doi: 10.1016/j.fertnstert.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 31.Ke MT, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013; 16:1154–1161. doi: 10.1038/nn.3447 [DOI] [PubMed] [Google Scholar]
- 32.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. [DOI] [PubMed] [Google Scholar]
- 33.Xu M, Barrett SL, West-Farrell ER, Kondipalli LA, Kieswetter SE, Shea LD, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009; 24:2531–2540. doi: 10.1093/humrep/dep228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higuchi CM, Maeda Y, Horiuchi T, Yamazaki Y. A simplified method for three-dimensional (3-D) ovarian tissue culture yielding oocytes competent to produce full-term offspring in mice. PLoS One. 2015; 10: e0143114 doi: 10.1371/journal.pone.0143114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wycherley G, Downey D, Kane MT, Hynes AC. A novel follicle culture system markedly increases follicle volume, cell number and oestradiol secretion. Reproduction. 2004;127:669–677. doi: 10.1530/rep.1.00040 [DOI] [PubMed] [Google Scholar]
- 36.Larrea X, Buchler P. A transient diffusion model of the cornea for the assessment of oxygen diffusivity and consumption. IOVS 2009;50:1076–1080. [DOI] [PubMed] [Google Scholar]
- 37.Kim SS, Yang HW, Kang HG, Lee HH, Lee HL, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004; 82:679–685. doi: 10.1016/j.fertnstert.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 38.Langbeen A, De Porte HF, Bartholomeus E, Leroy JL, Bols PE. Bovine in vitro reproduction models can contribute to the development of (female) fertility preservation strategies. Theriogenology. 2015;84:477–489. doi: 10.1016/j.theriogenology.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 39.Fortune JE, Kito S, Wandji S-A, Srsen V. Activation of bovine and baboon primordial follicles in-vitro. Theriogenology. 1998;49:441–449. [DOI] [PubMed] [Google Scholar]
- 40.Fortune JE, Kito S, Byrd DD. Activation of primordial follicles in-vitro. J Reprod Fertil. 1999;54:439–448. [PubMed] [Google Scholar]
- 41.Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol. 2000;163:53–60. [DOI] [PubMed] [Google Scholar]
- 42.Gigli I, Byrd DD, Fortune JE. Effects of oxygen tension and supplements to the culture medium on activation and development of bovine follicles in-vitro. Theriogenology. 2006;66:344–353. doi: 10.1016/j.theriogenology.2005.11.021 [DOI] [PubMed] [Google Scholar]
- 43.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070 [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin M, Kelsey TW, Wallace WH, Anderson RA, Telfer EE. Non-growing follicle density is increased following adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy in the adult human ovary. Hum Reprod. 2017;32:165–174 doi: 10.1093/humrep/dew260 [DOI] [PubMed] [Google Scholar]
- 45.Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97–106 doi: 10.1093/humrep/det388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlsson IB, Laitinen MP, Scott JE, Louhio H, Velentzis L, Tuuri T, et al. Kit ligand and c-Kit are expressed during early human ovarian follicular development and their interaction is required for the survival of follicles in long-term culture. Reproduction. 2006;131:641–649. doi: 10.1530/rep.1.00868 [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa T, Kyoya T, Nakamura Y, Sato E, Tomiyama T, Kyono K. Oxygen consumption rate of early pre-antral follicles from vitrified human ovarian cortical tissue. J Reprod Dev. 2014;60:460–467. doi: 10.1262/jrd.2014-009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details on how the concentration profile in ovarian tissue was obtained when ovarian tissue is cultured in conventional dishes.
(DOCX)
Details on how the concentration profile in ovarian tissue was obtained when ovarian tissue is cultured in gas-permeable dishes.
(DOCX)
Percentages of bovine follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Percentages of bovine follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Percentages of bovine follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Percentages of human follicle grading, staging and viability. H = Histology; V = Viability. Number of follicles analysed are indicated in brackets.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.