Abstract
Purpose
For the past 65 years, patient age at diagnosis has been widely used as a major mortality risk factor in the risk stratification of papillary thyroid cancer (PTC), but whether this is generally applicable, particularly in patients with different BRAF genetic backgrounds, is unclear. The current study was designed to test whether patient age at diagnosis is a major mortality risk factor.
Patients and Methods
We conducted a comparative study of the relationship between patient age at diagnosis and PTC-specific mortality with respect to BRAF status in 2,638 patients (623 men and 2,015 women) with a median age of 46 years (interquartile range, 35 to 58 years) at diagnosis and a median follow-up time of 58 months (interquartile range, 26 to 107 months). Eleven medical centers from six countries participated in this study.
Results
There was a linear association between patient age and mortality in patients with BRAF V600E mutation, but not in patients with wild-type BRAF, in whom the mortality rate remained low and flat with increasing age. Kaplan-Meier survival curves rapidly declined with increasing age in patients with BRAF V600E mutation but did not decline in patients with wild-type BRAF, even beyond age 75 years. The association between mortality and age in patients with BRAF V600E was independent of clinicopathologic risk factors. Similar results were observed when only patients with the conventional variant of PTC were analyzed.
Conclusion
The long-observed age-associated mortality risk in PTC is dependent on BRAF status; age is a strong, continuous, and independent mortality risk factor in patients with BRAF V600E mutation but not in patients with wild-type BRAF. These results question the conventional general use of patient age as a high-risk factor in PTC and call for differentiation between patients with BRAF V600E and wild-type BRAF when applying age to risk stratification and management of PTC.
INTRODUCTION
Thyroid cancer is a common endocrine malignancy, and its incidence has rapidly increased in recent decades.1-4 The most common histologic type is papillary thyroid cancer (PTC), accounting for > 85% of all thyroid malignancies, with conventional PTC (CPTC) being the dominant variant.5,6 Risk stratification is a critical component of standard management of thyroid cancer and is currently based mainly on clinicopathologic risk factors, among which patient age at diagnosis is a major factor. In 1953, Crile and Hazard7 described in detail the association between advanced patient age and unfavorable prognosis of thyroid cancer. Since then, numerous studies have confirmed this relationship. Thus, patient age has long been routinely applied as a major risk factor in risk stratification of thyroid cancer, which has profoundly impacted clinical practice in the management of thyroid cancer.8-10
The most important prognostic significance of patient age in thyroid cancer is its effect on patient mortality; older patient age is strongly associated with thyroid cancer–specific mortality.11,12 In fact, thyroid cancer is the only type of cancer for which patient age is a metric for disease staging in the American Joint Committee on Cancer (AJCC) and several other staging systems, reflecting the unique importance of patient age as a risk factor in thyroid cancer. The age of 45 years has been conventionally treated as a cutoff point demarcating the age-associated risk in thyroid cancer13; however, this has been recently changed to 55 years in the revised eighth edition of AJCC.14 Yet, some studies have suggested that the mortality risk of thyroid cancer continuously increases as patient age increases.15-18 A recent analysis by Adam et al19 of 31,802 patients with PTC in the SEER database demonstrated that age was associated with PTC-specific mortality in a continuous linear manner without an age cutoff point. However, critical questions remain unanswered as to why older patient age has such a remarkable adverse effect on PTC-specific mortality and whether age is a risk factor universally applicable to all patients with PTC.
The BRAF V600E mutation has been well known to be a main oncogenic driver of PTC, occurring in approximately 45% of patients.20-22 Many studies have demonstrated an association between BRAF V600E and older patient age as well as poor clinical outcomes, including recurrence of PTC23,24 and PTC-specific mortality.25,26 Given these data, we hypothesized that BRAF V600E might play an important role in the effect of patient age on PTC-specific mortality, and that, in the absence of BRAF V600E, patient age might not be a risk factor. We conducted this multicenter study to test this hypothesis.
PATIENTS AND METHODS
Study Medical Centers, Countries, and Patients
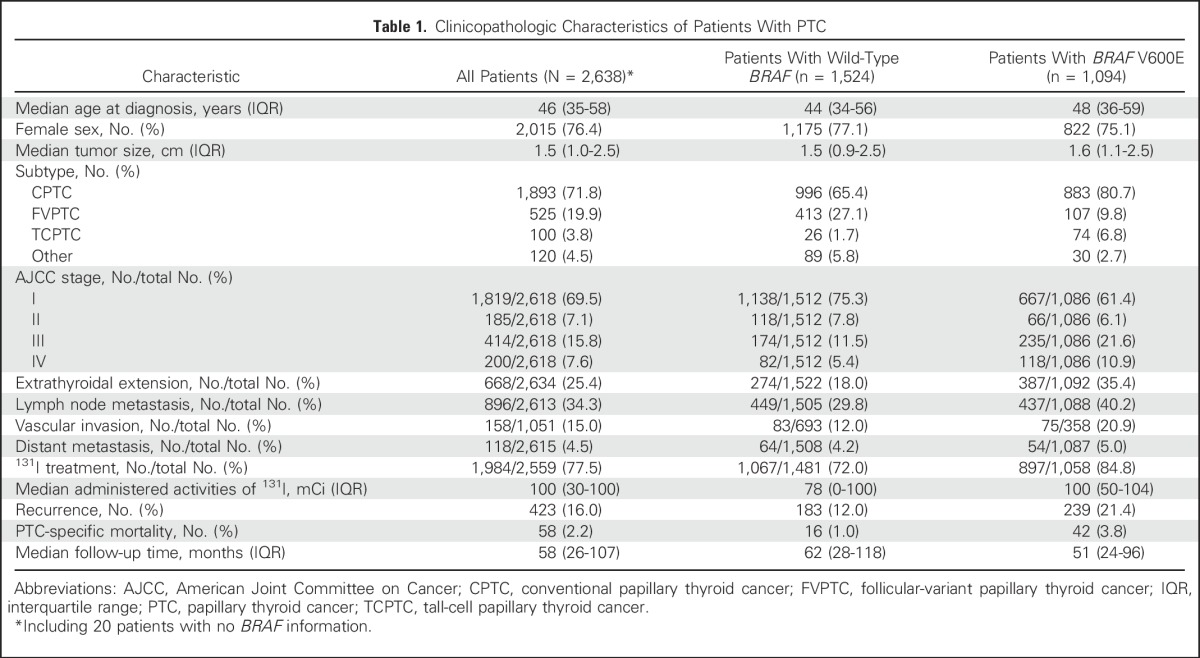
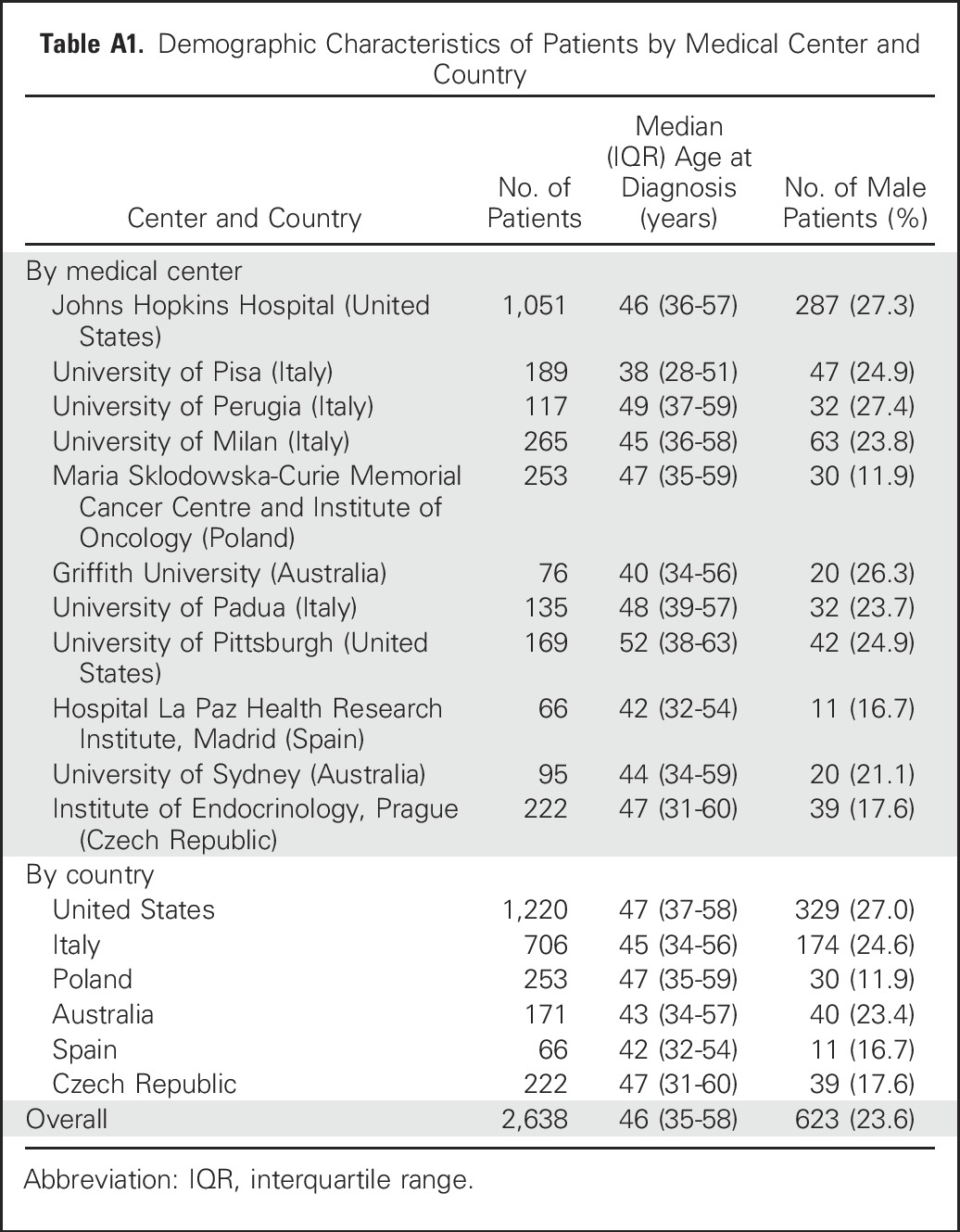
With the approval of the institutional review boards of the participating institutions and, where required, informed written patient consent, data from 2,638 patients with PTC on clinicopathologic characteristics and PTC-specific patient death were collected from 11 medical centers in six countries (Appendix Table A1, online only). These patients included 623 men (23.6%) and 2,015 women (76.4%) and had a median age of 46 years (interquartile range, 35 to 58 years) at diagnosis of PTC and a median clinical follow-up time of 58 months (interquartile range, 26 to 107 months) after the initial surgery. BRAF genetic testing failed in 20 patients, whereas 1,524 patients had wild-type BRAF and 1,094 patients had BRAF V600E mutation. Mortality analysis was focused on PTC-specific patient death, as previously defined (ie, death that occurred as a result of incurable PTC disease that invaded and compromised vital organs, causing the patient to die).25 Patient clinicopathologic characteristics that are well-known risk factors for PTC-specific mortality are listed in Table 1. For a separate analysis of patients with CPTC, a subset of 1,893 patients with CPTC was identified, and exclusion of 14 patients without BRAF information left 996 and 883 patients who had wild-type BRAF and BRAF V600E. All of these patients were consecutively selected and were treated with total or near-total thyroidectomy for PTC; other treatments, such as radioiodine ablation, were pursued as clinically indicated. Histopathologic diagnoses of thyroid cancer were established according to the WHO criteria.27 BRAF V600E mutation in primary PTC was examined and documented as previously described.23,25 BRAF V600E mutation status was determined after surgical and medical treatments in all patients and did not affect decision making regarding treatments.
Table 1.
Clinicopathologic Characteristics of Patients With PTC
Statistical Analyses
Spearman correlation coefficient was calculated to evaluate the association between patient age and PTC-specific mortality. Variance inflation factor to test multicollinearity was calculated for each clinicopathologic characteristic in the Cox hazards regression model; all variance inflation factors were low (ie, < 1.58), ensuring that multicollinearity was not a problem in the regression models. Multivariate Cox proportional hazards regression models with restricted cubic splines (RCS) and adaptive splines were used to demonstrate the continuous relationship between patient age and PTC-specific mortality.19 Hazard ratios (HRs) were natural logarithm-transformed and adjusted for multivariate clinicopathologic characteristics. The RCS model (knot number, 3) was used to estimate the HR and 95% CI of different ages compared with age 45 years. Comparing the statistical fitness of different number knots showed that the model with 3 knots had the lowest Akaike information criterion estimate, thus providing the best fit to the data. Adaptive splines are knot-free and do not rely on knot number. Statistical analyses were performed using the mgcv28 and rms29 packages in R (version 3.2.4; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Association Between Patient Age and PTC-Specific Mortality in Patients With BRAF V600E But Not Wild-Type BRAF
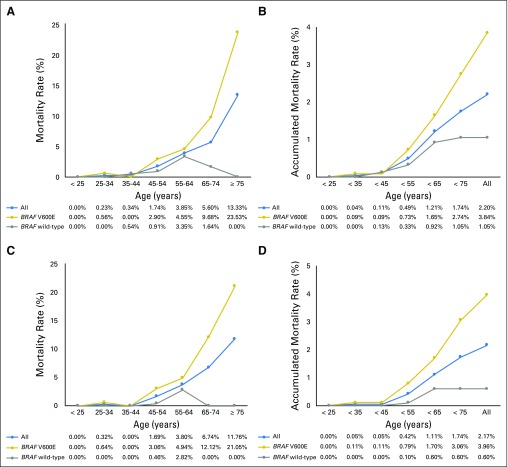
As shown in Figure 1, before the age of 45 years, the mortality rates (percentages of deaths in the cohort) were low in all of the patient groups. After the age of 45 years, mortality rates increased as patient age increased in all patients, and mortality rates increased even more rapidly in patients with BRAF V600E mutation. However, in striking contrast, there was no increase in mortality overall in patients with wild-type BRAF (Fig 1A). Accumulated mortality rates also increased continuously after age 45 years in all patients and increased even more rapidly and steeply in patients with BRAF V600E mutation, whereas there was only a marginal increase in accumulated mortality in patients with wild-type BRAF at age 45 to 64 years (Fig 1B). After age 65 years, the mortality rate began to decrease (Fig 1A) and the accumulated mortality rate stayed flat (Fig 1B) in patients with wild-type BRAF, whereas both the mortality rate and the accumulated mortality rate continuously and sharply increased as patient age increased in patients with BRAF V600E (Figs 1A and 1B). Spearman correlation analysis showed a strongly positive correlation between patient age and mortality rate in BRAF V600E patients (P = .002, r = 0.94), but the correlation was not significant in wild-type BRAF patients (P = .36, r = 0.41). Virtually identical results were obtained when only patients with CPTC were analyzed (Figs 1C and 1D). Spearman correlation analysis also showed a strongly positive correlation between patient age and mortality in patients with CPTC harboring BRAF V600E (P < .002, r = 0.94), but not in patients with CPTC harboring wild-type BRAF (P = .70, r = 0.18). These results suggest that the association between patient age and PTC-specific mortality depends on BRAF V600E status.
Fig 1.
Relationship between patient age and papillary thyroid cancer (PTC)–specific mortality in all patients, patients with BRAF V600E mutation, and patients with wild-type BRAF. (A) Mortality rates and (B) accumulated mortality rates by patient age in all patients with PTC. (C) Mortality rates and (D) accumulated mortality rates of patients with conventional PTC.
Rapidly Progressive Decline in Kaplan-Meier Survival Curve With Increasing Age in Patients With BRAF V600E But Not Wild-Type BRAF
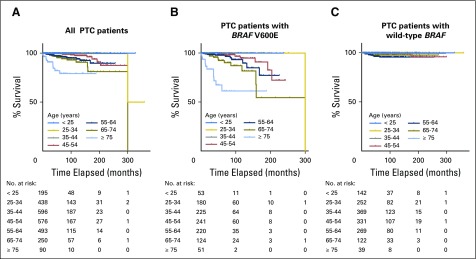
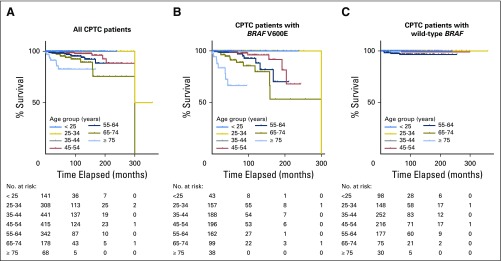
In the analysis of all patients, Kaplan-Meier survival curves progressively declined as patient age increased, particularly after age 45 years; decline was sharpest in patients ≥ 75 years old (Fig 2A). An even more rapidly progressive decline in survival curve was seen in patients with BRAF V600E as patient age increased (Fig 2B). In striking contrast, there was no progressive decline in survival curve in patients with wild-type BRAF as patient age increased (Fig 2C). Specifically, in patients with BRAF V600E, survival curves in patients younger than 45 years old were largely flat, and only one death occurred in the 25- to 34-year age group at a follow-up time of 300 months. Starting at age 45 years, the older the patients were, the more rapidly the survival curve declined and the most rapid decline occurred in patients ≥ 75 years old (Fig 2B). Similar results were observed when only patients with CPTC were analyzed (Fig 3). These results demonstrate a BRAF V600E–dependent association between decreasing PTC-specific patient survival and increasing patient age.
Fig 2.
Kaplan-Meier analysis of disease-specific survival curves of patients with papillary thyroid cancer (PTC) in various patient age groups: (A) all patients; (B) patients with BRAF V600E mutation; and (C) patients with wild-type BRAF.
Fig 3.
Kaplan-Meier analysis of disease-specific survival curves of patients with conventional papillary thyroid cancer (CPTC) in various patient age groups: (A) all patients; (B) patients with BRAF V600E; and (C) patients with wild-type BRAF.
Independent Linear Association Between Mortality Risk and Increasing Age in Patients With BRAF V600E But Not Wild-Type BRAF
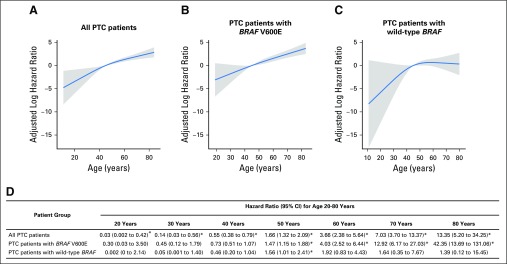
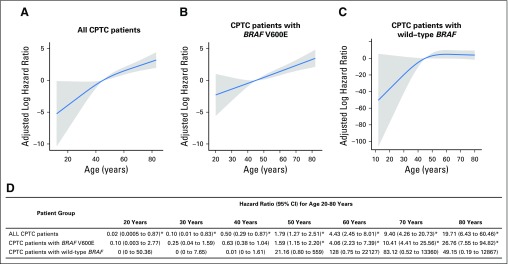
We used multivariate Cox proportional hazards regression models with RCS to further analyze the relationship between patient age and PTC-specific mortality with adjustment for the classic clinicopathologic characteristics of patient sex, tumor size, extrathyroidal extension, lymph node metastasis, distant metastasis, and administered activities of radioactive iodine (mCi), which are factors known to affect clinical outcomes of patients with PTC, as well as study center (Fig 4). To be comparable, for all RCS plots, patient age of 45 years, which was close to the median age of our cohort, was chosen as the reference for HR calculation. In all patients combined, RCS analysis demonstrated a nearly linear association between patient age and PTC-specific mortality risk, with the adjusted log HR continuously increasing as patient age increased (Fig 4A). In patients with BRAF V600E, an even stronger and steeper linear relationship between patient age and adjusted log HR of PTC-specific mortality risk was observed (Fig 4B). In contrast, in patients with wild-type BRAF, no significant relationship was observed between patient age and mortality risk; the mortality risk at various age segments generally did not show significant difference, and the line stayed flat as the patient age increased, even after age 75 years (Fig 4C). The increasing line before age 45 years is a result of the large variance from the low mortality rate in this young patient age range, which displayed insignificant HRs in reference to patient age of 45 years.
Fig 4.
Multivariate Cox proportional hazards regression analysis of papillary thyroid cancer (PTC)–specific mortality risk with restricted cubic splines (RCS). Continuous linear association between patient age and PTC-specific mortality was observed (A) in the analysis of all patients and (B) even more significantly in patients with BRAF V600E, but (C) not in patients with wild-type BRAF. The blue line represents the fitted line of the association between patient age and the estimated hazard ratio (HR) of mortality after adjustment; the shaded region represents the 95% CI. The models were adjusted for the following clinicopathologic characteristics: patient sex, tumor size, extrathyroidal extension, lymph node metastasis, distant metastases, administered activities of radioactive iodine, and study center. The RCS plots were performed with the age of 45 years as the reference for HR calculation. (D) Specific HRs and 95% CIs are presented for the indicated patient age points. (*) Significantly different HRs in reference to patient age of 45 years.
The adjusted specific HRs at different age points are presented in Fig 4D. HRs increased from age 20 to 80 years in the analysis of all patients. An even stronger upward trend in HRs was observed from age 20 to 80 years in patients with BRAF V600E, particularly after age 50 years. In contrast, in patients with wild-type BRAF, the HR was marginally significant only at age 50 years and was insignificant at all other age points (Fig 4D). Similar results were observed when only patients with CPTC were analyzed using RCS (Appendix Fig A1, online only).
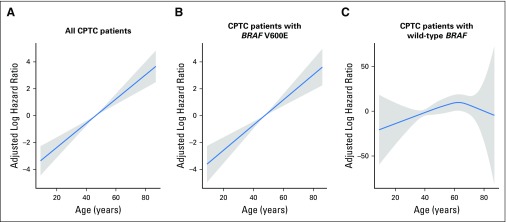
We also used adaptive smoother spline (Fig 5), used previously by Adam et al,19 to analyze the relationship between patient age and PTC-specific mortality and obtained similar results. Specifically, in analyses of all patients, a near-linear association between patient age and mortality risk was seen (Fig 5A). An even steeper linear association between patient age and mortality risk was seen in patients with BRAF V600E (Fig 5B). In contrast, no significant association between patient age and mortality risk was seen in patients with wild-type BRAF (Fig 5C). Similar results were obtained when only patients with CPTC were analyzed using the adaptive smoother spline (Appendix Fig A2, online only).
Fig 5.
Multivariate Cox proportional hazards regression analysis of papillary thyroid cancer (PTC)–specific mortality risk with adaptive smoother splines in (A) all patients with PTC, (B) patients with BRAF V600E mutation, and (C) patients with wild-type BRAF. The blue line represents the fitted line of the association between patient age and the estimated hazard ratio of mortality after adjustment; the shaded region represents the 95% CI. The models were adjusted for the following clinicopathologic characteristics: patient sex, tumor size, extrathyroidal extension, lymph node metastasis, distant metastases, administered activities of radioactive iodine, and study center.
DISCUSSION
Since Crile and Hazard described the association between advanced patient age and aggressiveness of thyroid cancer almost 65 years ago,7 numerous studies have confirmed this phenomenon. Today, patient age is a well-established mortality risk factor in the prognostication of thyroid cancer; various clinical guidelines and risk assessment models uniformly incorporate patient age as a major risk factor in the management of thyroid cancer.8-10,30,31 To further support the prognostic importance of patient age, a linear relationship between patient age and PTC-specific mortality was recently demonstrated, suggesting a continuous adverse impact on PTC prognosis as patient age increases.19 For thyroid cancer, the previous and recent editions of the AJCC staging system heavily emphasize the general risk of patient age.13,14 Thus, patient age has profoundly influenced the risk stratification and management of PTC. However, it remains to be determined whether patient age is a major risk factor for all patients with PTC.
This study explored the effect of BRAF V600E on age-associated mortality risk in patients with PTC. We reproduced the findings of Adam et al19 by demonstrating a similar linear association between patient age and PTC-specific mortality in the analysis of all patients combined. However, this linear relationship was even steeper in patients with BRAF V600E, particularly in patients older than age 45 years. In contrast, this association was lost in patients with wild-type BRAF, in whom the PTC-specific mortality risk remained flat with increasing patient age, even after age 45 years. Thus, the long-observed age-associated mortality risk in PTC is BRAF V600E dependent; patient age itself, in the absence of BRAF V600E, is not a significant risk factor. These findings challenge the conventional belief that older patient age is uniformly a mortality risk factor in PTC and question its universal application in risk stratification of PTC. Instead, the utility of patient age as a prognostic risk factor depends on BRAF V600E status. Specifically, in patients with BRAF V600E, age has a strong and continuous adverse effect on the prognosis of patients with PTC throughout the entire age spectrum examined, and in fact, the effect intensifies as patient age increases. Thus, in patients with BRAF V600E mutation, age is an important factor in risk stratification and management of PTC as conventionally applied. In contrast, in patients with wild-type BRAF, age is not a risk factor for poor prognosis; in these patients, both younger and older patients have a similar PTC-specific mortality risk and may be managed similarly. This new concept will likely have a major impact on the clinical management of PTC because the prevalence of BRAF V600E mutation in PTC is, on average, 45%.20 Thus, the majority of patients with PTC have wild-type BRAF, and in these patients, conventional use of patient age as a major risk factor is not valid. As such, many older patients will be able to avoid more aggressive treatment that would otherwise be administered as a result of the conventional concept of older patient age being a general high-risk factor. Our study calls for a BRAF genotype–based modification of the conventionally used risk assessment systems,8-10,30,31 as well as the recently developed quantitative risk assessment nomogram,32 which all incorporate patient age as a general risk factor for thyroid cancer. In addition, given this differentiating role of BRAF V600E status in patient age-related mortality risk of PTC, use of the conventional cutoff age of 45 years13 or the new cutoff age of 55 years14 in the risk stratification of PTC is inaccurate. Our study addressed the role of BRAF V600E mutation in PTC-specific mortality risk related to patient age at diagnosis. It would be interesting for future studies to investigate the role of the mutation in the dynamic effect, if any, of patient age on the prognosis of PTC as the age of the same patient increases after the diagnosis.
The large multicenter cohort of patients is a major strength of this study and is one of the largest cohorts of patients in BRAF mutation–related studies in thyroid cancer. The multicenter nature, however, is inherently associated with the potential limitation of data heterogeneity, as seen in population data such as the SEER data.19 Nevertheless, our study only looked at the single outcome parameter of PTC-specific patient death, which has a universally straightforward definition, and the binary data of BRAF mutation–positive and –negative status from each participating center were similarly included in the analysis. The participating centers are well-known thyroid cancer centers that actively follow contemporary standard practice guidelines in the management of thyroid cancer, minimizing the heterogeneity in the management of thyroid cancer. The fact that the overall analysis of all patients in the current study fully reproduced the findings of the linear effect of patient age on PTC-specific mortality in the study by Adam et al19 is consistent with the good generalizability of the current study. Another limitation is that TERT promoter mutation, which is also a prognostic genetic event in PTC, was not included in this study. However, TERT promoter mutations are relatively uncommon and mostly coexist with BRAF mutation in PTC.33,34 Moreover, TERT promoter mutation alone has limited or virtually no effect on PTC-specific mortality.35,36 Therefore, lack of information on TERT promoter mutation should not affect the clinical implications of this study on the use of BRAF V600E status in differentiating patient age–related mortality risk in PTC.
The molecular mechanism for the BRAF mutation–dependent effect of patient age on the prognosis of PTC remains to be defined. It is possible that certain age-associated genes, such as immune response–related genes,37 may cooperate with mutant BRAF in conferring poor prognosis because BRAF V600E was shown to be linked to abnormal immune responses in human cancers, including PTC.38-40 Another potential and more likely mechanism is the coexistence of BRAF V600E and TERT promoter mutations, which are synergistically associated with poor clinical outcomes in PTC, including disease recurrence and patient mortality.35,36 Both BRAF V600E20-22 and TERT promoter mutations34 occur in PTC more commonly in older patients. The present results are also consistent with a previous finding that BRAF V600E and older patient age had a synergistic effect on PTC-related mortality.25
In summary, in contrast to the long-held practice of treating patient age as a general risk factor for PTC, this large multicenter study demonstrates that age is a strong and continuous mortality risk factor only in patients with BRAF V600E mutation, and not in the more commonly seen patients with wild-type BRAF. These results call for differentiation between patients with wild-type BRAF and BRAF V600E when applying age to risk stratification and management of patients with PTC. This study has broad clinical implications.
Appendix
Fig A1.
Multivariate Cox proportional hazards regression analysis of mortality risk with restricted cubic splines (RCS) in patients with conventional papillary thyroid cancer (CPTC). (A) A continuous and nearly linear association between patient age and CPTC-specific mortality was observed in all patients. (B) The association was linear and even steeper in patients with BRAF V600E mutation. (C) A linear association was not seen in patients with wild-type BRAF. The blue line represents the fitted line of the association between patient age and the estimated hazard ratio (HR) of mortality risk after adjustment; the shaded region represents the 95% CI. The models were adjusted for the following clinicopathologic characteristics: patient sex, tumor size, extrathyroidal extension, lymph node metastasis, distant metastases, administered activities of radioactive iodine, and study center. The RCS plots were performed with the age of 45 years as the reference for HR calculation. (D) Specific HRs and 95% CIs were calculated for the indicated age points. (*) Significantly different HRs in reference to patient age of 45 years. Because of the small number of deaths in patients younger than age 45 years, there were large variations in log HRs in patients with CPTC harboring only wild-type BRAF in the young age ranges. Consequently, different y-axis scales are used for log HR for panels A, B, and C.
Fig A2.
Multivariate Cox proportional hazards regression analysis of conventional papillary thyroid cancer (CPTC)–specific mortality risk with adaptive smoother splines: (A) all CPTC patients; (B) CPTC patients with BRAF V600E mutation; and (C) CPTC patients with wild-type BRAF. The blue line represents the fitted line of the association between patient age and the estimated hazard ratio (HR) of mortality risk after adjustment; the shaded region represents the 95% CI. The models were adjusted for the following clinicopathologic characteristics: patient sex, tumor size, extrathyroidal extension, lymph node metastasis, distant metastases, administered activities of radioactive iodine, and study center. Because of the small number of deaths in patients younger than age 45 years, there were large variations in log HRs in patients with CPTC harboring only wild-type BRAF in the young age ranges. Consequently, different y-axis scales are used for log HR for panels A, B, and C.
Table A1.
Demographic Characteristics of Patients by Medical Center and Country
Footnotes
Supported by National Institutes of Health (NIH) Grants No. R01CA113507 and R01CA189224 (M.X.); Polish National Center of Research and Development MILESTONE Project Grant No. STRATEGMED2/267398/4/NCBR/2015 (Poland, A.C., B.J.); grants from Menzies Health Institute, Griffith University, Queensland Cancer Council and Queensland Smart State Fellowship (Australia; A.K.L.); Grants No. SAF2013-44709-R and SAF2016-75531-R (MINECO and FEDER), RD12/0036/0030, PI14/01980 (ISCIII), and GCB14142311CRES (AECC Foundation) (Spain; P.S. and G.R-E); Grants No. AZV 16-32665A and MH CZ-DRO (Institute of Endocrinology-EU, 00023761; Czech Republic; B.B., V.S.); grants from the New South Wales Cancer Institute (C.J.O.) and Cancer Council of New South Wales (Australia; R.C.-B.); NIH/National Institute on Aging Grant No. 5R03AG042334-02 (L.Y.); and grants from the Ministero della Istruzione Universitaria e Ricerca Scientifica, the Associazione Italiana per la Ricerca sul Cancro, the Istituto Toscano Tumori, and the Ministero della Salute (Italy; D.V., R.E.).
The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the article. The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH or the funding entities of the individual centers participating in this study.
See accompanying Editorial on page 433
AUTHOR CONTRIBUTIONS
Conception and design: Mingzhao Xing
Financial support: Mingzhao Xing
Administrative support: Mingzhao Xing
Provision of study materials or patients: David Viola, Rossella Elisei, Efisio Puxeddu, Laura Fugazzola, Carla Colombo, Agnieszka Czarniecka, Alfred K. Lam, Caterina Mian, Federica Vianello, Linwah Yip, Garcilaso Riesco-Eizaguirre, Pilar Santisteban, Christine J. O'Neill, Mark S. Sywak, Roderick Clifton-Bligh, Bela Bendlova, Vlasta Sýkorová, Mingzhao Xing
Collection and assembly of data: Guangwu Zhu, Rengyun Liu, David Viola, Rossella Elisei, Efisio Puxeddu, Laura Fugazzola, Carla Colombo, Barbara Jarzab, Agnieszka Czarniecka, Alfred K. Lam, Caterina Mian, Federica Vianello, Linwah Yip, Garcilaso Riesco-Eizaguirre, Pilar Santisteban, Christine J. O'Neill, Mark S. Sywak, Roderick Clifton-Bligh, Bela Bendlova, Vlasta Sýkorová, Mingzhao Xing
Data analysis and interpretation: Xiaopei Shen, Mingzhao Xing
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient Age–Associated Mortality Risk Is Differentiated by BRAF V600E Status in Papillary Thyroid Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Xiaopei Shen
No relationship to disclose
Guangwu Zhu
No relationship to disclose
Rengyun Liu
No relationship to disclose
David Viola
No relationship to disclose
Rossella Elisei
No relationship to disclose
Efisio Puxeddu
Research Funding: IBSA Italia S.R.L.
Travel, Accommodations, Expenses: IBSA Italia S.R.L.
Laura Fugazzola
No relationship to disclose
Carla Colombo
No relationship to disclose
Barbara Jarzab
Honoraria: Exelixis, AstraZeneca, Eisai, Bayer
Consulting or Advisory Role: SOBI, AstraZeneca
Speakers' Bureau: Novartis/Ipsen
Travel, Accommodations, Expenses: Novartis/Ipsen, Sanofi, Bayer
Agnieszka Czarniecka
No relationship to disclose
Alfred K. Lam
No relationship to disclose
Caterina Mian
No relationship to disclose
Federica Vianello
No relationship to disclose
Linwah Yip
No relationship to disclose
Garcilaso Riesco-Eizaguirre
No relationship to disclose
Pilar Santisteban
No relationship to disclose
Christine J. O'Neill
No relationship to disclose
Mark S. Sywak
No relationship to disclose
Roderick Clifton-Bligh
No relationship to disclose
Bela Bendlova
No relationship to disclose
Vlasta Sýkorová
No relationship to disclose
Mingzhao Xing
Patents, Royalties, Other Intellectual Property: Receiving royalties as co-holder of a licensed US patent related to BRAF V600E mutation in thyroid cancer
REFERENCES
- 1.Davies L, Welch HG: Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 295:2164-2167, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Noone A, Krapcho M, et al: SEER Cancer Statistics Review,1975-2013, National Cancer Institute. http://seer.cancer.gov/csr/1975_2013/
- 3.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 65:5-29, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Lim H, Devesa SS, Sosa JA, et al. : Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 317:1338-1348, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Y, Xing M: Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer 23:313-322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi X, Liu R, Basolo F, et al. : Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab 101:264-274, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crile G, Jr, Hazard JB: Relationship of the age of the patient to the natural history and prognosis of carcinoma of the thyroid. Ann Surg 138:33-38, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer PA, Cooper DS, Daniels GH, et al. : Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Intern Med 156:2165-2172, 1996 [PubMed] [Google Scholar]
- 9.Cooper DS, Doherty GM, Haugen BR, et al. : Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167-1214, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, et al. : 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1-133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran Cao HS, Johnston LE, Chang DC, et al. : A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 152:145-151, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazurat A, Torroni A, Hendrickson-Rebizant J, et al. : The age factor in survival of a population cohort of well-differentiated thyroid cancer. Endocr Connect 2:154-160, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edge SB, Byrd DR, Compton CC: AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010. [Google Scholar]
- 14. Amin MB, Edge S, Greene FL, et al: AJCC Cancer Staging Manual (ed 8). New York, NY, Springer, 2016. [Google Scholar]
- 15.Ganly I, Nixon IJ, Wang LY, et al. : Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid 25:1106-1114, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orosco RK, Hussain T, Brumund KT, et al. : Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid 25:125-132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi R-L, Qu N, Liao T, et al. : The trend of age-group effect on prognosis in differentiated thyroid cancer. Sci Rep 6:27086, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bischoff LA, Curry J, Ahmed I, et al. : Is above age 45 appropriate for upstaging well-differentiated papillary thyroid cancer? Endocr Pract 19:995-997, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Adam MA, Thomas S, Hyslop T, et al. : Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: Rethinking current staging systems. J Clin Oncol 34:4415-4420, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing M: BRAF mutation in thyroid cancer. Endocr Relat Cancer 12:245-262, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Xing M: BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev 28:742-762, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Xing M: Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184-199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing M, Alzahrani AS, Carson KA, et al. : Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33:42-50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing M, Westra WH, Tufano RP, et al. : BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373-6379, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Xing M, Alzahrani AS, Carson KA, et al. : Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493-1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elisei R, Ugolini C, Viola D, et al. : BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab 93:3943-3949, 2008 [DOI] [PubMed] [Google Scholar]
- 27. DeLellis RA, Lloyd R, Heitz PU, et al: WHO Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France, International Agency for Research on Cancer, 2004. [Google Scholar]
- 28. Wood SN: mgcv: Mixed GAM Computation Vehicle with GCV/AIC/REML Smoothness Estimation. R package version 1.8-17. 2017. https://CRAN.R-project.org/package=mgcv.
- 29. Harrell FE: rms: Regression Modeling Strategies. R package version 5.1‐0. 2017. https://CRAN.R-project.org/package=rms.
- 30. Hay ID, Bergstralh EJ, Goellner JR, et al: Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050-1057, 1993. [PubMed]
- 31.Pacini F, Castagna MG, Brilli L, et al. : Differentiated thyroid cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 19:ii99-ii101, 2008. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Shen W, Sakamoto N: Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 31:468-474, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Bishop J, Shan Y, et al. : Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer 20:603-610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R, Xing M: TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 23:R143-R155, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing M, Liu R, Liu X, et al. : BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 32:2718-2726, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Bishop J, Zhu G, et al. : Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: Genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol 3:202-208, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Haymart MR: Understanding the relationship between age and thyroid cancer. Oncologist 14:216-221, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Sumimoto H, Imabayashi F, Iwata T, et al. : The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 203:1651-1656, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai Y-T, Lakshmanan A, Lehman AM, et al. : BRAFV600E accelerates disease progression and increases immune suppression in a mouse model of B-cell leukemia. Blood 128:1206, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angell TE, Lechner MG, Jang JK, et al. : BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 24:1385-1393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]