Abstract
Background and purpose
We report a systematic review of lung radiation doses from breast cancer radiotherapy.
Methods and materials
Studies describing breast cancer radiotherapy regimens published during 2010–2015 and reporting lung dose were included. Doses were compared between different countries, anatomical regions irradiated, techniques and use of breathing adaptation.
Results
471 regimens from 32 countries were identified. The average mean ipsilateral lung dose (MLDipsi) was 9.0 Gy. MLDipsi for supine radiotherapy with no breathing adaption was 8.4 Gy for whole breast/chest wall (WB/CW) radiotherapy, 11.2 Gy when the axilla/supraclavicular fossa was irradiated, and 14.0 Gy with the addition of internal mammary chain irradiation; breathing adaptation reduced MLDipsi by 1 Gy, 2 Gy and 3 Gy respectively (p < 0.005). For WB/CW radiotherapy, MLDipsi was lowest for tangents in prone (1.2 Gy) or lateral decubitus (0.8 Gy) positions. The highest MLDipsi was for IMRT in supine position (9.4 Gy).
The average mean contralateral lung dose (MLDcont) for WB/CW radiotherapy was higher for IMRT (3.0 Gy) than for tangents (0.8 Gy).
Conclusions
Lung doses from breast cancer radiotherapy varied substantially worldwide, even between studies describing similar regimens. Lymph node inclusion and IMRT use increased exposure, while breathing adaptation and prone/lateral decubitus positioning reduced it.
Keywords: Breast cancer, Lung dose, Imrt, Breathing adaptation
Radiotherapy improves survival in several categories of women with early breast cancer [1], [2]. When irradiating the breast or chest wall with or without the regional lymph nodes exposing the lungs is unavoidable and this incidental exposure may increase the risk of subsequent primary lung cancer [3], [4], [5], [6], pneumonitis [7] and lung fibrosis [8]. The risk of these side-effects increases with lung radiation exposure, so knowledge of the doses received by the lungs with modern breast cancer radiotherapy is important. This topic is particularly relevant today, as three large studies recently demonstrated that in women with high risk breast cancer, radiotherapy to the regional lymph nodes including the internal mammary chain (IMC) significantly reduces breast cancer recurrence [9], [10], [11]. These nodes are close to the lungs, so irradiating them can increase lung radiation exposure.
For lung cancer, radiation approximately multiplies a woman’s pre-existing risk, so the absolute risk of primary lung cancer from radiotherapy will be much higher for a smoker than for a non-smoker. In an individual patient data meta-analysis of 75 randomised trials of breast cancer radiotherapy, the risk of radiation-related lung cancer increased by ∼11 per cent (95% confidence interval 6–19) per Gy mean lung dose [6]. The estimated absolute risk of radiation-related lung cancer from 5 Gy mean whole lung dose was ∼4% for a smoker but <1% for a non-smoker. Hence particular attention needs to be paid to minimising lung doses in smokers [6], [12].
Pneumonitis is another possible complication of breast cancer radiotherapy, occurring within three months of radiotherapy [7] and leading to lung fibrosis several months later [8]. As with radiation-related lung cancer, the risk of pneumonitis increases with increasing lung radiation dose [7], [13]. It is also more common in women who receive chemotherapy in addition to radiotherapy [7], [14], [15], [16].
In order to assist with weighing the benefit against the risks of different breast cancer radiotherapy regimens, we present a systematic review of lung doses from breast cancer radiotherapy dosimetry studies published during 2010–2015. We describe how lung exposure varies according to country, anatomical regions irradiated, technique, treatment position and the use of breathing adaptation. In addition, we discuss the likely future absolute risks of radiation-related lung cancer for women irradiated recently.
Methods
Study identification
Studies were identified following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. Embase and Scopus were queried using search terms (“dos∗ AND breast∗ AND cancer∗/carcinom∗/tumor∗/tumour∗ AND radiation/radiotherapy∗”) to retrieve breast cancer radiotherapy dosimetry studies published between 1 January 2010 and 18 September 2015. All studies reporting any measure of lung dose were eligible, regardless of whether the plans were actually delivered. Reference sections of eligible papers were scanned to identify additional eligible studies. Studies reporting only dose from a tumour bed boost were excluded because this is nearly always given alongside whole breast radiotherapy, which delivers higher lung doses. Studies of bilateral breast cancer were also excluded.
Data abstraction
Eligible studies were categorised according to radiotherapy technique (Table E1-E2). The information abstracted from each study included: author, year, country of first author, patient position, radiotherapy planning technique (e.g. conformal vs IMRT), field type (e.g. static vs rotational), beam energy, breathing adaptation, whether or not the radiotherapy plans were delivered, region(s) irradiated, prescription dose to the target and number of fractions, number of CT planning scans per regimen included in the study, the type of dose calculation algorithm, the presence of unfavourable anatomy and cancer laterality. Lung dose measures abstracted were: mean ipsilateral lung dose (MLDipsi), mean contralateral lung dose (MLDcont), mean whole lung dose (i.e. where both lungs were considered a single organ) (MLDwhole), V5ipsi (i.e. percent ipsilateral lung volume irradiated to 5 Gy or more), V10ipsi, V20ipsi, V30ipsi and V40ipsi. Dose calculation algorithms [18] were categorised as “type A” (no/poor modelling of lateral electron transport), “type B” (some modelling of lateral electron transport), Monte Carlo and “other”. Each regimen was classified as to whether or not it was reported for a high income country [19].
Data analyses
Analyses considered the average of the lung dose measures from the CT plans included for each regimen described in each study. We term these “average lung doses”. Average lung doses were compared between countries, region(s) irradiated, techniques used, patient treatment positions and whether breathing adaption was used. Variation in the average doses within each of these categories was assessed using chi-squared tests for heterogeneity, difference or trend, as appropriate.
Results
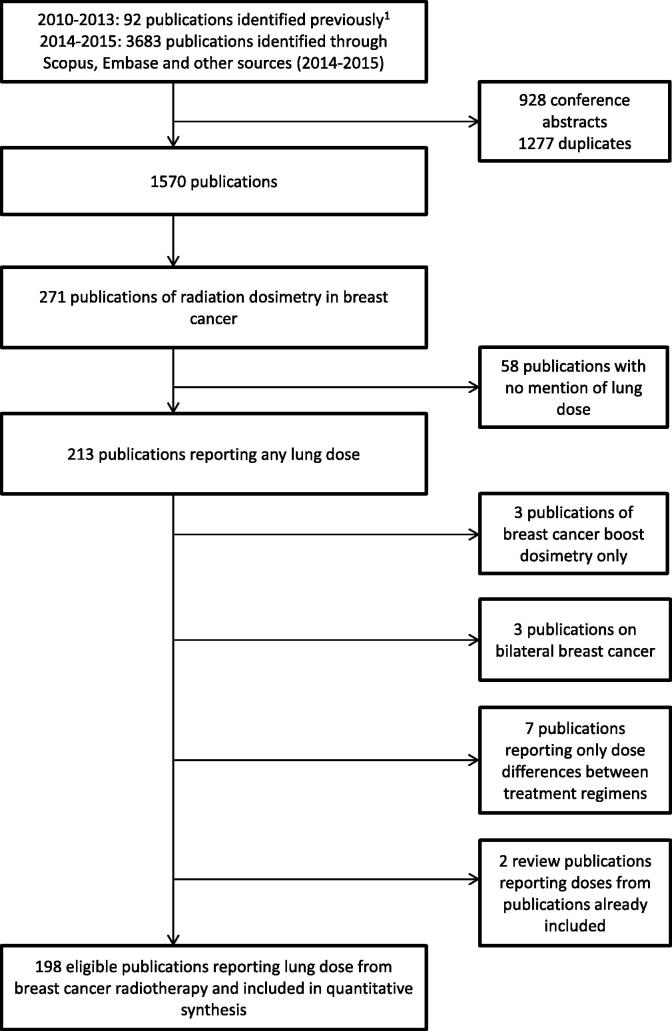
Radiation doses to the lungs from breast cancer radiotherapy were reported in 198 studies from 32 countries including 579 regimens (Fig. 1, Table 1, Tables E3,E4). The year in which the CT plans were generated was reported in 64/198 studies: it was before 2005 in four studies, 2005–09 in 29 studies and 2010–15 in 31 studies. 3D–treatment planning was used in all 198 studies; one study simulated a 2D regimen on 3D CT scans with field borders based on bony structures and midline wire marking [20]. MLDipsi was available for 471 regimens in 153 studies and was the commonest dose measure reported (Table 1). Among those 153 studies, 94 reported MLDipsi separately for left-sided and right-sided breast cancer radiotherapy regimens. The difference in average MLDipsi according to laterality was small and not significant (left-sided 9.5 Gy in 287 regimens, right-sided 8.7 Gy in 38 regimens, p for difference = 0.94). Therefore, left-sided and right-sided regimens were considered together in our analyses.
Fig. 1.
The process of study identification for the review.
Table 1.
Studies reporting lung doses from breast cancer radiation therapy regimens and published during 2010–2015.
| Breast cancer laterality | Dose measure | Number of Studies* | Number of regimens† | CT plans per regimen‡ |
Lung dose |
||
|---|---|---|---|---|---|---|---|
| Average | Range | Average | Range | ||||
| Left | MLDipsi | 90 | 287 | 16 | 1–148 | 9.5 Gy | 0.3–27.5§ |
| MLDcont | 39 | 147 | 11 | 1–31 | 2.5 Gy | 0–15.0§ | |
| V20ipsi | 81 | 258 | 17 | 1–148 | 16.8% | 0–44.5 | |
| V5ipsi | 52 | 162 | 18 | 1–148 | 41.8% | 0.8–94 | |
| Right | MLDipsi | 16 | 38 | 7 | 1–45 | 8.7 Gy | 1.2–16.1 |
| MLDcont | 6 | 13 | 3 | 1–14 | 2.7 Gy | 0.2–6.5 | |
| V20ipsi | 12 | 25 | 11 | 1–45 | 16.1% | 4.7–35.6 | |
| V5ipsi | 6 | 14 | 6 | 1–47 | 42.4% | 1.5–88.7 | |
| Unspecified | MLDipsi | 60 | 146 | 33 | 6–494 | 7.9 Gy | 0.3–22.9 |
| MLDcont | 21 | 58 | 24 | 6–246 | 1.6 Gy | 0–6.4 | |
| V20ipsi | 58 | 135 | 40 | 6–494 | 13.9% | 0.1–44.5 | |
| V5ipsi | 27 | 57 | 34 | 8–431 | 32.2% | 0.7–99.3 | |
| All studies reporting MLDipsi | 153 | 471 | 21 | 1–494 | 9.0 Gy | 0.3–27.5‡ | |
| All studies reporting MLDcont | 62 | 218 | 14 | 1–246 | 2.3 Gy | 0–15.0‡ | |
| All studies reporting V20ipsi | 139 | 417 | 19 | 6–494 | 15.8% | 0–44.5 | |
| All studies reporting V5ipsi | 78 | 233 | 21 | 1–431 | 39.5% | 0.7–99.3 | |
| All studies | 198| | 579 | – | – | – | – | |
Definitions: MLDipsi: mean dose to the ipsilateral lung, MLDcont: mean dose to the contralateral lung, V20ipsi: percent volume of the ipsilateral lung receiving 20 Gy or more, V5ipsi: percent volume of the ipsilateral lung receiving 5 Gy or more.
Some studies reported doses for both left-sided and right-sided regimens and so contribute more than once.
Some regimens reported several dose measures (e.g. both MLDipsi and V20ipsi).
For four regimens in one study the number of CT planning scans was not reported.
Four regimens (three from Al-Rabhi 2013, one from Ares 2010, see full references in Table E4) were excluded because doses reported were inconsistent with values presented elsewhere in the publication.
This total represents the number of unique studies, without the multiple contributions from studies which reported doses for both left-sided and right-sided regimens.
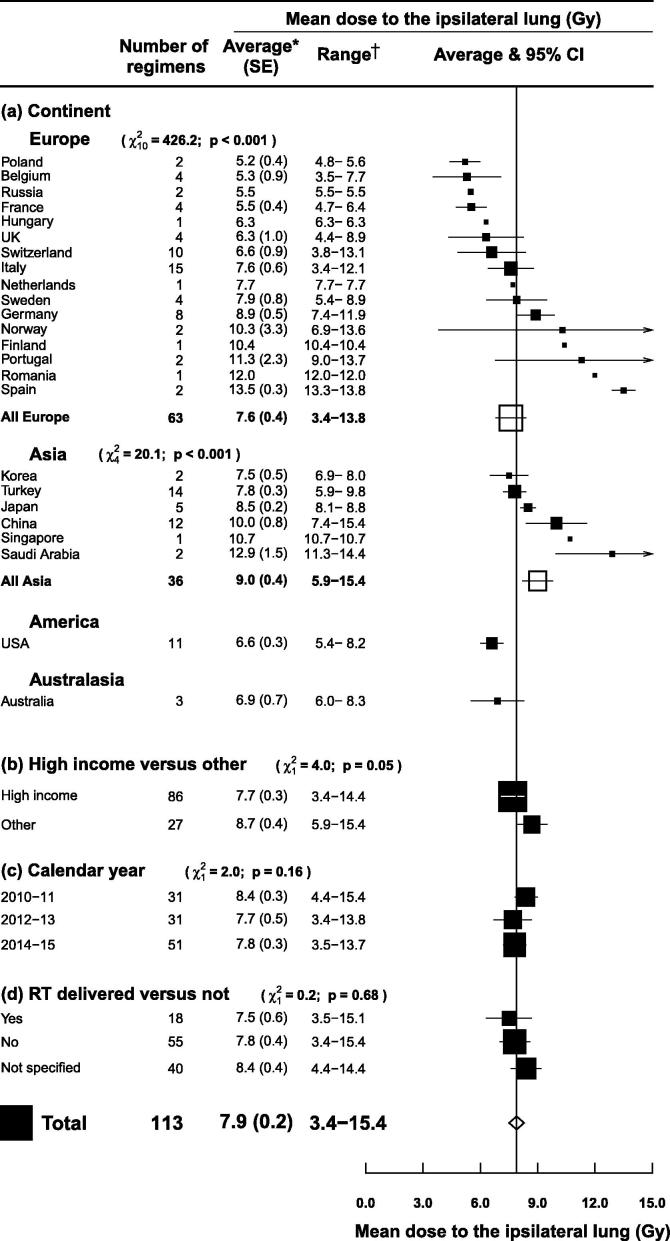
Tangential regimens
Whole breast/chest wall tangential radiotherapy in supine position with no breathing adaption was the most commonly reported combination of technique and regions irradiated (113 regimens in 70 studies) (Fig. 2). The average MLDipsi for all 113 regimens was 7.9 Gy (SE 0.2) and it varied significantly according to continent (p for heterogeneity <0.001). There was also substantial variation according to country within Europe and Asia (p for heterogeneity <0.001 for both). The average MLDipsi was lowest for Poland and Korea (5.2 and 7.5 Gy respectively) and highest for Spain and Saudi Arabia (13.5 and 12.9 Gy respectively). The average MLDipsi in “high income” countries was lower than in other countries (7.7 versus 8.7 Gy, p for difference = 0.05), but it did not vary significantly according to calendar year of publication (p for trend = 0.16) or whether the treatment plans were actually delivered (p for difference = 0.68).
Fig. 2.
Mean ipsilateral lung dose (MLDipsi) from left or right tangential breast cancer radiotherapy according to country, gross national income per person in the country concerned, calendar year, and whether the radiotherapy plans were actually delivered or just planned. Regimens that irradiated the internal mammary chain, partial breast, axilla, or supraclavicular fossa were excluded, as were regimens with breathing adaptation, prone or lateral decubitus positioning and studies of women with unfavourable anatomy. *Average of mean ipsilateral lung doses for reported regimens. †Range of mean ipsilateral lung doses for reported regimens. χ2 and p values are for: heterogeneity (a), difference (b, d) or trend (c). For (d), the category “Not specified” was omitted from the χ2. Abbreviations: SE: standard error; CI: confidence interval.
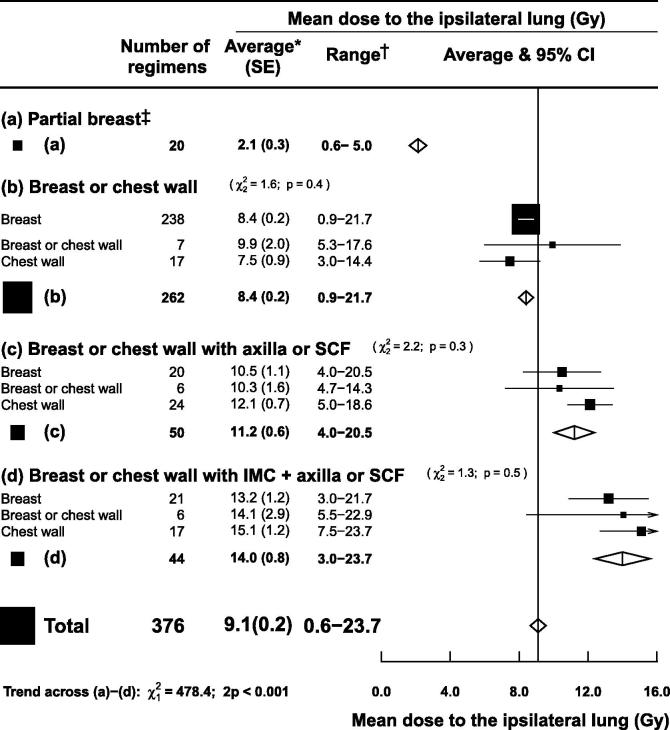
Regions irradiated
After excluding studies of women with unfavourable anatomy and regimens in the prone or lateral decubitus positions, 376 of the 471 regimens remained, with an average MLDipsi of 9.1 Gy (SE 0.2). The average MLDipsi was highly correlated with the extent of the regions irradiated (2p for trend <0.001, Fig. 3). For partial breast irradiation (20 regimens) average MLDipsi was 2.1 Gy (SE 0.3) and for whole breast/chest wall irradiation (262 regimens) it was 8.4 Gy (SE 0.2). The addition of axilla/supraclavicular fossa irradiation (50 regimens) increased it to 11.2 Gy (SE 0.6) and inclusion of the internal mammary chain (IMC) (44 regimens) further increased it to 14.0 Gy (SE 0.8).
Fig. 3.
Mean ipsilateral lung dose (MLDipsi) from left or right breast cancer radiotherapy according to regions irradiated. Studies of women with unfavourable anatomy were excluded as were regimens using prone or lateral decubitus positioning, and regimens irradiating the internal mammary chain (IMC) but not the axilla or the supraclavicular fossa (SCF). *Average of mean ipsilateral lung doses for reported regimens. †Range of mean ipsilateral lung doses for reported regimens. χ2 and p values are for heterogeneity. Abbreviations: SE: standard error; CI: confidence interval.
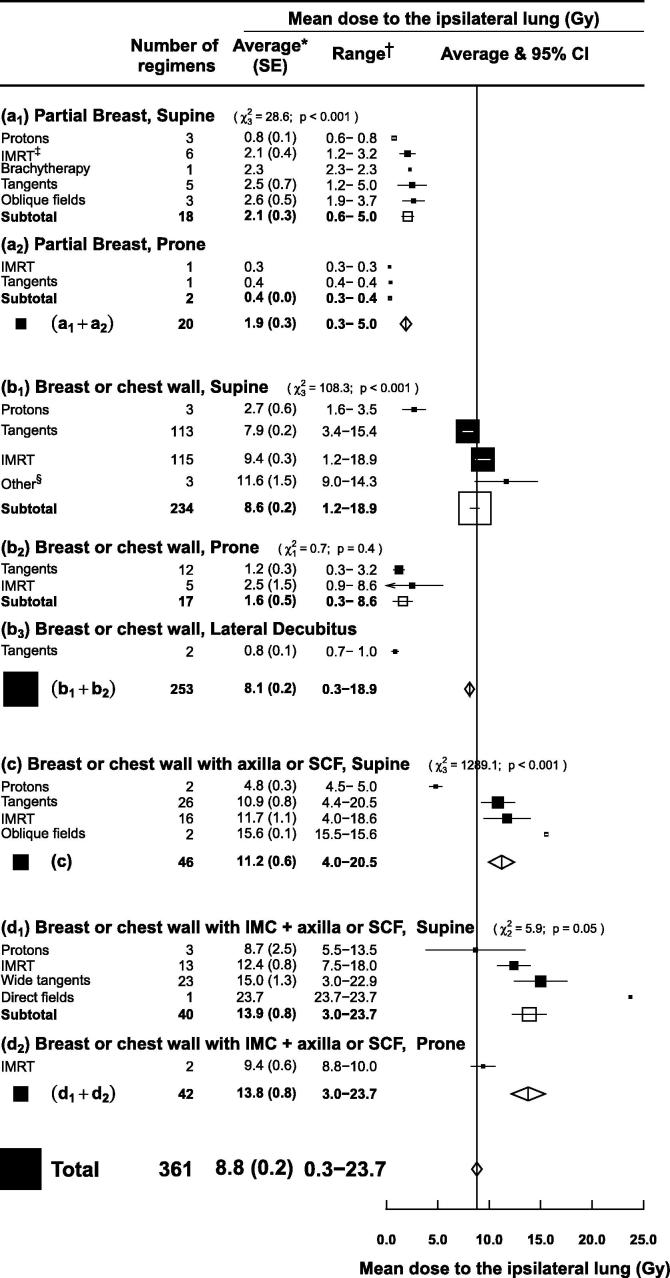
Technique and treatment position
For each combination of region(s) irradiated, the average MLDipsi varied according to both technique and treatment position (Fig. 4). The commonest treatment position was supine (338 regimens). The commonest techniques were tangents (159 regimens) and intensity modulated radiotherapy (IMRT) (156 regimens). Average MLDipsi was similar for static field and rotational IMRT so these two types of IMRT were considered together.
Fig. 4.
Mean ipsilateral lung dose (MLDipsi) from left or right breast cancer radiotherapy according to regions irradiated and technique used. Regimens using breathing adaptation (e.g. deep inspiration breath hold) were excluded, as were studies of women with unfavourable anatomy and regimens irradiating the internal mammary chain (IMC) but not the axilla or the supraclavicular fossa (SCF). *Average of mean ipsilateral lung doses for reported regimens. †Range of mean ipsilateral lung doses for reported regimens. ‡Static field IMRT and rotational IMRT are included jointly as “IMRT”. § “Other” techniques included two dynamic conformal arc therapy regimens and one unspecified 3D conformal regimen. χ2 and p values are for heterogeneity. Abbreviations: SE: standard error; CI: confidence interval; IMRT: intensity modulated radiotherapy. IMC: internal mammary chain. SCF: supraclavicular fossa.
For 20 partial breast regimens (Fig. 4, panels a1 and a2), the average MLDipsi for the different techniques and treatment positions varied from 0.3 to 2.6 Gy. For 234 whole breast/chest wall regimens in supine position (Fig. 4, panel b1) the MLDipsi was 7.9 (SE 0.2) from tangents and 9.4 (SE 0.3) from IMRT (p for difference <0.001). Doses for these techniques from 17 whole breast/chest wall regimens in prone position were lower: MLDipsi 1.6 (SE 0.5). The lowest doses were from two whole breast/chest wall regimens in lateral decubitus position: MLDipsi = 0.8 (SE 0.1).
When the axilla/SCF was also irradiated (Fig. 4, panel c), the commonest regimens were tangents (26 regimens) which delivered MLDipsi = 10.9 (SE 0.8) and IMRT (16 regimens), which delivered MLDipsi = 11.7 (SE 1.1). When the IMC was also included (Fig. 4, panels d1 and d2), there were 23 wide tangential regimens which delivered MLDipsi = 15.0 (SE 1.3) and 13 IMRT regimens, which delivered MLDipsi = 12.4 (SE 0.8). The highest reported MLDipsi values were for rare techniques that included oblique or direct fields and were not actually delivered to patients.
Doses from proton therapy were reported for 11 regimens. Within each category of regions irradiated, proton therapy resulted in the lowest average MLDipsi: partial breast 0.8 Gy (SE 0.07), whole breast/chest wall 2.7 Gy (SE 0.6), whole breast/chest wall and axilla/supraclavicular fossa 4.8 Gy (SE 0.3), whole breast/chest wall and axilla/supraclavicular fossa with IMC 8.7 Gy (SE 2.5) (Fig. 4, panels a1, b1, c1 and d1).
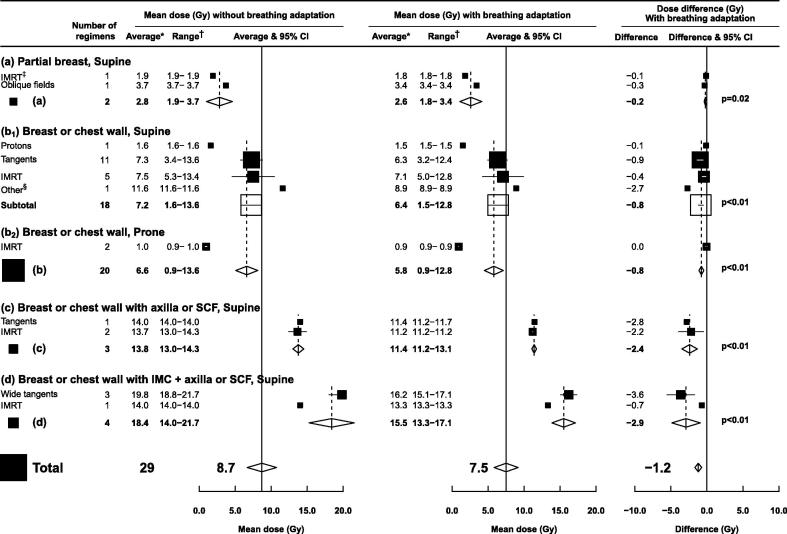
Breathing adaptation
For 29 regimens (21 studies) the average MLDipsi was reported for a given regimen both with and without breathing adaption in the same women (Fig. 5). Breathing adaptation reduced the average MLDipsi significantly in all regimens, with the magnitude of the reduction depending on the extent of the regions irradiated. For partial breast irradiation the average reduction was 0.2 Gy; for whole breast/chest wall radiotherapy it was 0.8 Gy; for regimens that included the axilla/supraclavicular fossa it was 2.5 Gy; and for regimens that also included the IMC it was 2.9 Gy. These reductions were seen regardless of radiotherapy technique or patient positioning.
Fig. 5.
Mean ipsilateral lung doses (MLDipsi) from left or right breast cancer radiotherapy with or without breathing adaptation according to regions irradiated and regimens used. Only studies providing doses with and without breathing adaptation in the same woman were included. Studies of women with unfavourable anatomy were excluded. *Average of mean ipsilateral lung doses for reported regimens. †Range of mean ipsilateral lung doses for reported regimens. ‡Static field IMRT and rotational IMRT are included jointly as “IMRT”. § “Other” includes one unspecified 3D conformal regimen. p values are calculated using a paired t-test. Abbreviations: CI: confidence interval; IMRT: intensity modulated radiotherapy; IMC: internal mammary chain; SCF: supraclavicular fossa.
Other measures of lung dose
The average V20ipsi (311 regimens, 139 studies) was 15.9% and the average V5ipsi (174 regimens, 77 studies) was 40.9% (Fig. E1, E2). For treatment in the supine position not including the IMC, there were no material differences in V20ipsi between IMRT and tangents (Fig. E1 panels a, b1, c). When the IMC was included, IMRT led to a substantially lower V20ipsi than wide tangents (p < 0.001) (Fig. E1 panel d1). Prone positioning was effective at reducing V20ipsi, and for each region category for which it was reported average V20ipsi for protons was lower than for other techniques. V5ipsi was higher for IMRT than for tangential regimens regardless of the regions irradiated (Fig. E2). Other volumetric dose measures such as V30ipsi and V40ipsi were each reported in fewer than 50 studies.
The average MLDcont (168 regimens, 62 studies) was 2.2 Gy (Fig. E3). MLDcont was higher for IMRT than for tangential regimens, regardless of the regions irradiated. For partial breast radiotherapy, the average MLDcont was 0.2 Gy. For the other region categories, average MLDcont varied from 1.9 to 2.6 Gy, and did not increase according to the extent of the ipsilateral regions irradiated. The lowest contralateral lung doses within each region category were from proton therapy.
The average MLDwhole (218 regimens, 88 studies) was 6.0 Gy (Fig. E4). Variation according to regions irradiated, technique, and patient position was similar to MLDipsi (Fig. 4).
Dose calculation algorithm
The dose calculation algorithm used for external beam photon radiotherapy was reported in 147/198 (74%) of the studies. Of these, 108 studies used a type B algorithm, 30 studies used type A and 9 studies used Monte Carlo. No significant differences in MLDipsi, V20ipsi, V5ipsi, MLDcont or MLDwhole were observed according to algorithm (p for heterogeneity >0.8 for all dose measures) (data not shown).
Discussion
This systematic review of lung doses from breast cancer radiotherapy regimens published during 2010–2015 shows that lung exposure varied substantially worldwide but that several factors influenced it systematically. Lung doses increased with more extensive regions irradiated and reduced with the use of breathing adaptation or prone or lateral decubitus positioning. Lung doses also depended on the technique used, with the highest doses from IMRT and lowest doses from proton therapy.
Methods to reduce lung exposure
There are several ways of reducing lung dose in breast cancer radiotherapy today. First, in our study, limiting the extent of irradiated regions reduced MLDipsi (Fig. 3). This is often done in current practice, for example regional nodal irradiation is only usually recommended in women at high risk of cancer recurrence, while in some women at very low risk of recurrence, radiotherapy may be omitted altogether [21]. Second, lung-sparing techniques may be used. The lowest ipsilateral lung doses for breast/chest wall irradiation were from proton therapy (<2.7 Gy) or from prone or lateral decubitus positioning (<2.5 Gy) (Fig. 4). These techniques also reduce mean heart doses [22] but are not yet widely used: proton therapy is expensive and available in only a few centres [23], while among the papers included in this review only one reported lung doses for lateral decubitus positioning and 15 reported doses for prone positioning. The limited implementation of new positioning techniques is due to the need for specialised equipment and concerns about the reproducibility of daily patient set-up [24]. For regimens that did not include the IMC, tangents spared the lungs better than IMRT (Fig. 4). When the IMC was irradiated, IMRT resulted in lower mean ipsilateral lung dose than tangents (Fig. 4, Fig. E1) but it increased contralateral lung dose (Fig. E2, Fig. E3). In clinical practice, contralateral lung dose may be reduced by limiting the number of beams – or using a partial arc in rotational treatments – to avoid beam entry through the contralateral lung. In addition, strict optimisation constraints and strategic beam placement may minimise low doses in the ipsilateral lung. Finally, lung dose can be reduced using breathing adaptation [25]. Historically this has been used to reduce cardiac exposure [24] but our study suggests that it also reduces lung exposure, with the magnitude of the gain increasing with more extensive regions irradiated (Fig. 5).
Strengths and limitations
Our study has several strengths. First, this comprehensive systematic review included 471 regimens and 153 studies, with the main analyses based on the most commonly reported measure of lung exposure, MLDipsi, Second, our doses are likely to represent normal radiotherapy practice, since most included studies focussed on target volume coverage or heart dose and lung doses were reported only for completeness rather than as a primary endpoint. As such, it is unlikely that the groups who published these doses paid more attention to minimising lung exposure than other groups who did not publish their lung doses. Third, this systematic review was carried out by both a radiation oncologist and a physicist to ensure that all regimens were reliably categorised.
Our study also has some limitations. For a given regimen, there was variation in the lung doses received by individual patients. Kim et al [26] reported lung doses for a tangential field-in-field regimen delivered to 157 women and found that the MLDipsi for individual women ranged from 2.8-18.7 Gy. Therefore, although our study provides a summary of the average doses from various regimens, lung doses also vary substantially from patient to patient. In addition, lung doses were presented separately for women with left-sided and right-sided breast cancer in only 38 regimens (16 studies), which limited our investigation of the potential differences between left- and right-sided regimens.
Absolute benefits and risks
Our results may be helpful in weighing the benefit against the risks of different breast cancer regimens. Large meta-analyses have shown that in most women irradiated for breast cancer according to current guidelines, the absolute benefits of radiotherapy outweigh the risks [1], [2], [26]. The risk of lung cancer increases with lung dose (i.e. MLDwhole). In our study, the typical MLDwhole from modern radiotherapy was around 5 Gy from whole breast or chest wall regimens (Fig E4). Estimates of absolute risk based on data from 40,000 women in 75 randomised trials suggest the absolute 30-year risk of radiation-related lung cancer from MLDwhole 5 Gy for a typical non-smoker aged 50 years at irradiation would be only ∼0.3%. For a long-term continuing smoker it is considerably higher, at ∼4% [6], although this would be reduced if the woman stopped smoking [12], [27]. In our study, regimens that irradiated the axilla/supraclavicular fossa and IMC increased the MLDwhole to around 9 Gy, which would increase the typical absolute 30-year risk of radiation-related lung cancer to ∼0.6% for a non-smoker and ∼10% for a long-term continuing smoker. Hence, for some long-term smokers, the risks of irradiating the SCF, axilla and IMC may outweigh the benefits, especially if they continue to smoke [6]. In applying these estimated risks, it should be remembered that they are for typical modern radiotherapy in typical populations of women. But, as we have shown, lung doses in breast cancer regimens vary from patient to patient, as do population disease-rates, so the absolute risks, and the effect of smoking cessation for individual women will vary around these typical values.
Conclusions
Lung exposure in breast cancer radiotherapy varied substantially between different countries and regimens, so the radiation-related risks of lung cancer, pneumonitis and lung fibrosis will also vary. Exposure may be reduced by minimising the extent of the irradiated region, using breathing adaptation and using prone or lateral decubitus patient positioning or proton therapy. These lung doses may help oncologists tailor treatment for women who have high estimated risks of radiation-related lung cancer and fibrosis.
Acknowledgements
This work was funded by the Danish Cancer Society – Denmark, the Danish Society for Clinical Oncology – Denmark, the Eva and Henrik Fraenkel memorial fund – Denmark, the UK Medical Research Council – UK, Cancer Research UK – United Kingdom (grant C8225/A21133), and core funding to the Clinical Trial Service Unit (from Cancer Research UK, Medical Research Council, British Heart Foundation – UK). FD was supported by a Medical Research Council UK Clinical & Research Fellowship.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.radonc.2017.11.022.
Appendix A. Supplementary data
References
- 1.McGale P., Taylor C., Correa C. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darby S., McGale P., Correa C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Henson K.E., McGale P., Taylor C., Darby S.C. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grantzau T., Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: A systematic review and meta-analysis of 762,468 patients. Radiother Oncol. 2015;114:56–65. doi: 10.1016/j.radonc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Taylor C., Correa C., Duane F.K. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lind P.A., Marks L.B., Jamieson T.A. Predictors for pneumonitis during locoregional radiotherapy in high-risk patients with breast carcinoma treated with high-dose chemotherapy and stem-cell rescue. Cancer. 2002;94:2821–2829. doi: 10.1002/cncr.10573. [DOI] [PubMed] [Google Scholar]
- 8.Omarini C., Thanopoulou E., Johnston S.R.D. Pneumonitis and pulmonary fibrosis associated with breast cancer treatments. Breast Cancer Res Treat. 2014;146:245–258. doi: 10.1007/s10549-014-3016-5. [DOI] [PubMed] [Google Scholar]
- 9.Thorsen L.B.J., Offersen B.V., Danø H. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2015;34:1–8. doi: 10.1200/JCO.2015.63.6456. [DOI] [PubMed] [Google Scholar]
- 10.Poortmans P.M., Collette S., Kirkove C. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 11.Whelan T.J., Olivotto I.A., Parulekar W.R. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaitelman S., Howell R.M., Smith B. Effects of smoking on late toxicity from breast radiation. J Clin Oncol. 2017;35:1633–1635. doi: 10.1200/JCO.2017.72.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks L.B. Dosimetric predictors of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2002;54:313–316. doi: 10.1016/s0360-3016(02)02928-0. [DOI] [PubMed] [Google Scholar]
- 14.Vogelius I.R., Bentzen S.M. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51:975–983. doi: 10.3109/0284186X.2012.718093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taghian A.G., Assaad S.I., Niemierko A. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001;93:1806–1811. doi: 10.1093/jnci/93.23.1806. [DOI] [PubMed] [Google Scholar]
- 16.Hanna Y.M., Baglan K.L., Stromberg J.S., Vicini F.A., Decker D.A. Acute and subacute toxicity associated with concurrent adjuvant radiation therapy and paclitaxel in primary breast cancer therapy. Breast J. 2002;8:149–153. doi: 10.1046/j.1524-4741.2002.08306.x. tbj08306 [pii] [DOI] [PubMed] [Google Scholar]
- 17.PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines 2015. http://www.prisma-statement.org (accessed July 2, 2015).
- 18.Knöös T., Wieslander E., Cozzi L. Comparison of dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Phys Med Biol. 2006;51:5785–5807. doi: 10.1088/0031-9155/51/22/005. [DOI] [PubMed] [Google Scholar]
- 19.World Bank. Open Data. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=XD&page=1. Accessed 2016-12-16. n.d.
- 20.Barsoum M., Mostafa M., El Hossieny H. Dosimetric prospective study comparing 2D and 3D planning for irradiation of supraclavicular and infraclavicular regions in breast cancer patients. J Egypt Natl Canc Inst. 2015;27:25–34. doi: 10.1016/j.jnci.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Kunkler I.H., Williams L.J., Jack W.J.L., Cameron D.A., Dixon J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 22.Taylor C.W., Zhe W., Macaulay E. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 23.Mishra M.V., Aggarwal S., Bentzen S.M. Establishing evidence-based indications for proton therapy: an overview of current clinical trials. Int J Radiat Oncol Biol Phys. 2016;97:228–235. doi: 10.1016/j.ijrobp.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett F.R., Colgan R.M., Donovan E.M. The UK HeartSpare Study (Stage IB): Randomised comparison of a voluntary breath-hold technique and prone radiotherapy after breast conserving surgery. Radiother Oncol. 2015;114:66–72. doi: 10.1016/j.radonc.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Tanguturi S., Lyatskaya Y., Chen Y. Prospective assessment of deep inspiration breath-hold using 3-dimensional surface tracking for irradiation of left-sided breast cancer. Pract Radiat Oncol. 2015;5:358–365. doi: 10.1016/j.prro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Kim H., Bae H.S., Lee M.Y. Analysis of predictive factors for lung injury after forward-planned intensity-modulated radiotherapy in whole breast irradiation. J Breast Cancer. 2014;17:69–75. doi: 10.4048/jbc.2014.17.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budach W., Bölke E., Kammers K. Adjuvant radiation therapy of regional lymph nodes in breast cancer – a meta-analysis of randomized trials-an update. Radiat Oncol. 2015;10:1–7. doi: 10.1186/s13014-015-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.