Abstract
Background
Mouse models of heart disease are extensively employed. The echocardiographic characterization of contractile function is usually focused on systolic function with fewer studies assessing diastolic function. Furthermore, the applicability of diverse echocardiographic parameters of diastolic function that are commonly used in humans has not been extensively evaluated in different pathophysiological models in mice.
Methods and results
We used high resolution echocardiography to evaluate parameters of diastolic function in mouse models of chronic pressure overload (aortic constriction), volume overload (aorto-caval shunt), heart failure with preserved ejection fraction (HFpEF; DOCA-salt hypertension), and acute sarcoplasmic reticulum dysfunction induced by thapsigargin - all known to exhibit diastolic dysfunction. Left atrial area increased in all three chronic models while mitral E/A was difficult to quantify at high heart rates. Isovolumic relaxation time (IVRT) and Doppler E/E′ increased significantly and the peak longitudinal strain rate during early filling (peak reverse longitudinal strain rate) decreased significantly after aortic constriction, with the changes being proportional to the magnitude of hypertrophy. In the HFpEF model, reverse longitudinal strain rate decreased significantly but changes in IVRT and E/E′ were non-significant, consistent with less severe dysfunction. With volume overload, there was a significant increase in reverse longitudinal strain rate and decrease in IVRT, indicating a restrictive physiology. Acute thapsigargin treatment caused significant prolongation of IVRT and decrease in reverse longitudinal strain rate.
Conclusion
These results indicate that the combined measurement of left atrial area plus reverse longitudinal strain rate and/or IVRT provide an excellent overall assessment of diastolic function in the diseased mouse heart, allowing distinction between different types of pathophysiology.
Keywords: Mouse, Echocardiography, Diastolic function, Hypertrophy
Highlights
-
•
Several echocardiographic indices of diastolic function are applicable to mouse models.
-
•
Isovolumic relaxation time (IVRT) and peak strain during filling are easily quantified.
-
•
Left atrial area increases with pressure and volume overload as well as HFpEF.
-
•
Changes in IVRT and strain during filling distinguish restrictive physiology.
-
•
Combined left atrial area and diastolic strain provide an ideal diagnostic framework.
1. Introduction
Systolic heart failure is invariably accompanied by left ventricular (LV) diastolic dysfunction but the latter often also occurs in the absence of (or precedes) significant systolic dysfunction in many human diseases. Conditions that may be associated with isolated diastolic dysfunction include hypertensive cardiac hypertrophy, diabetes mellitus, obesity, metabolic syndrome and aging [1], [2]. Many patients with overt heart failure have predominantly diastolic dysfunction with relatively well-preserved systolic function - termed "heart failure with preserved ejection fraction (HFpEF)".[2] The prevalence of HFpEF has been suggested to be up to 50% of all heart failure cases and the condition imposes significant morbidity and mortality.[2], [3]
Echocardiography is immensely valuable in the non-invasive evaluation of human LV diastolic function. The term diastolic function encompasses active myocardial relaxation, which significantly influences early ventricular filling, as well as passive end-diastolic ventricular stiffness, which impacts on late ventricular filling. Different echocardiographic parameters assess different aspects of diastolic function, its consequences and/or determinants. An impairment of relaxation is readily detected by a prolongation of the isovolumic relaxation time (IVRT, assessed from aortic and mitral Doppler recordings) or the mitral Doppler inflow E-wave deceleration time (DT).[4] The ventricular filling pattern on transmitral Doppler, often reported as the ratio between early and late filling (E/A ratio), is a deceptively simple measure that needs to be carefully interpreted because it is influenced not only by myocardial relaxation and ventricular stiffness but also by the left atrial pressure.[4], [5] Adding to the complexity, the left atrial pressure is itself affected by left ventricular properties (it tends to rise as the ventricle becomes stiffer). The E/A ratio decreases when relaxation is significantly impaired but rises again with the progression of diastolic dysfunction when left atrial pressure increases (termed “pseudo-normalization”). A further increase in LV passive stiffness may lead to a predominantly early filling pattern with abrupt termination of filling - a so-called “restrictive” pattern.[4] In this case, there is a marked decrease in IVRT and DT along with an increase in E/A ratio and an accurate interpretation of the physiology requires the measurement of multiple parameters. Given the importance of left atrial pressure for diastolic function, parameters that provide information about left atrial properties are very useful. The E/E′ ratio (mitral inflow E wave/tissue Doppler mitral annulus velocity) correlates with left atrial pressure while the left atrial volume provides a useful indication of chronically elevated left atrial pressure.[5], [6]
Genetic, surgical and pharmacological mouse models of cardiovascular disease are widely used in experimental research. The mouse is often the experimental model of choice due to the availability of homogenous strains, the ease of genome manipulation and breeding, and the extensive range of gene-modified models available to researchers. Additionally, there are multiple established surgical and pharmacological procedures to model human cardiac conditions in mice. The non-invasive assessment of cardiac function in such models is, however, largely focused on systolic function using standard views whereas diastolic properties tend to be quantified in a rudimentary manner. The main reason for this is the technical challenge in assessing diastolic function at physiologically high heart rates (400–600 bpm) in a very small organ.
The aim of this study was to assess which of the several echocardiographic parameters of diastolic function that are commonly used in humans can be reliably applied in mice and whether their applicability differs according to the specific pathophysiological mouse model of cardiovascular stress. We then wanted to design a simple, technically straightforward algorithm to guide detection and distinction between different patterns of diastolic dysfunction in mice.
2. Material and methods
2.1. Animals
Studies were conducted in C57Bl/6 mice, in accordance with the UK Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act, 1986 and with institutional ethics approval.
2.2. Experimental models
Surgical procedures were either performed under 1.5% isoflurane (TAC, Shunt) or injectable anesthesia with ketamine (75 mg/kg) plus medetomidine hydrochloride (1 mg/kg) (AAB, DOCA-salt hypertension). We used 2 models of chronic pressure overload, namely suprarenal abdominal aortic banding (AAB) and transverse aortic constriction (TAC). AAB was performed in mice weighing 16-18 g using a 28-gauge band.[7] Sham constriction involved identical surgery apart from band placement. Animals were studied six weeks post-surgery. Minimally invasive TAC surgery was performed using a 27-gauge needle to constrict the aorta between the brachiocephalic and left common carotid arteries.[8] Sham operated mice underwent the same procedure apart from constriction. Mice were studied two weeks after TAC.
Chronic volume overload was induced by producing an aortocaval fistula (Shunt).[9] Briefly, the aorta and inferior vena cava were dissected free, the aorta was clamped just above the renal arteries, and was then punctured with a 23-gauge needle passing through to the inferior vena cava in an infra-renal position. The lateral puncture site of the aorta was sealed with cyanoacrylate glue. Sham animals underwent the same procedure except for vessel puncture. Animals were studied one week post-surgery.
The deoxycorticosterone acetate (DOCA)-salt model of hypertension, which causes primarily diastolic rather than systolic impairment,[10] was studied. A unilateral nephrectomy was performed under i.p. ketamine and medetomide anesthesia, followed by subcutaneous implantation of a DOCA pellet (0.7 mg/day). Animals additionally received a 1% NaCl drinking water solution. The Sham group underwent surgery without nephrectomy, DOCA or salt. Mice were studied by echocardiography 21 days post-surgery, followed by cardiac catheterization for LV pressure-volume analysis.
As an acute model of diastolic dysfunction, mice were treated with a single dose of thapsigargin (0.75 mg/kg i.p.; Sigma-Aldrich, UK), an inhibitor of the sarcoplasmic reticulum Ca2 +-ATPase.[11] Echocardiography was performed prior to and five hours following thapsigargin.
2.3. Heart weight measurement
After sacrifice of animals, the heart was excised and dissected into its four chambers. Organs were weighed and the heart or left ventricular weight was normalized by the total body weight (BW).
2.4. Echocardiography
Mice were anesthetised with 1.5% isoflurane and imaged in the supine position using a Vevo 2100 Imaging System with a 40-MHz linear probe (Visualsonics, Canada). Core temperature was maintained at 37 °C. Heart rates were kept consistent between experimental groups (400–500 bpm). ECG monitoring was obtained using limb electrodes. A standard 2D echocardiographic study was initially performed in the parasternal long-axis view for assessment of LV dimensions and systolic function. Image depth, width and gain settings were used to optimise image quality. Frame rates were > 150 Hz with a mean of 201 Hz (± 24).
Diastolic parameters were evaluated as follows:
Doppler flow profiles were acquired using pulsed wave Doppler in the apical 4-chamber view. The sample volume was placed close to the tip of the mitral leaflets in the mitral orifice parallel to the blood flow in order to record maximal transmitral flow velocities. To assess IVRT, a simultaneous mitral inflow and aortic outflow profile was recorded, allowing measurement of the time interval between aortic valve closure and mitral valve opening.
Tissue Doppler imaging in the apical 4-chamber view was performed by placing the pulsed wave Doppler at the septal corner of the mitral annulus. E´ was determined as peak mitral annular velocity during early filling.
Left atrial area was quantified in the apical 4-chamber view by tracing the border of the left atrium. Measurement was performed in end-systole before opening of the mitral valve.
Speckle tracking was performed as described previously.[12] Briefly, a number of tracking points were placed on the endocardial and epicardial border in the parasternal long axis view using the Vevo2100 Imaging Software 1.5.0. Subsequent frame by frame tracking through the cardiac cycle allowed automatic calculation of strain and strain rates. The software divides the LV into six segments and calculates longitudinal and radial strain and strain rates for each segment as well as overall mean values. The data presented in this study are the mean global longitudinal strain rate values. To quantify the peak longitudinal strain rate during early LV filling, the “reverse peak” option in the Vevo2100 1.5.0 Image Software was used (Supplementary Fig. 1A). We term this parameter the peak reverse longitudinal strain rate (rLSR).
All views were digitally stored in cine loops consisting of 300 frames. Subsequent analyses were performed off-line by an experienced sonographer who was blinded to the type of mouse model. LV volumes and ejection fraction (EF) were obtained using the standard 2D quantification software. Inter-observer and intra-observer variability for indices of diastolic function were evaluated from 10 randomly selected echocardiographic studies, analyzed in a blinded manner on two separate occasions by the same observer (intra-observer variability) and by two independent observers (inter-observer variability).
2.5. Cardiac catheterization
LV pressure-volume analysis was performed using a 1.2F microconductance catheter system (Scisense Inc.) inserted via the right carotid artery and positioned at the LV apex.[13] Data were collected using the ADVantage™ system (Scisence Inc.) coupled to a Powerlab/8SP with Chart Software (ADinstruments). Analyses were performed using Labscribe (IWork Systems Inc.) software.
2.6. Statistics
Data are presented as mean ± SEM. Two group comparisons were performed by unpaired Student's t-test or a paired Student's t-test, as appropriate. Correlations were assessed using linear regression and coefficients of determination (r2). p < 0.05 was considered significant.
3. Results
3.1. Echocardiographic features during chronic pressure overload
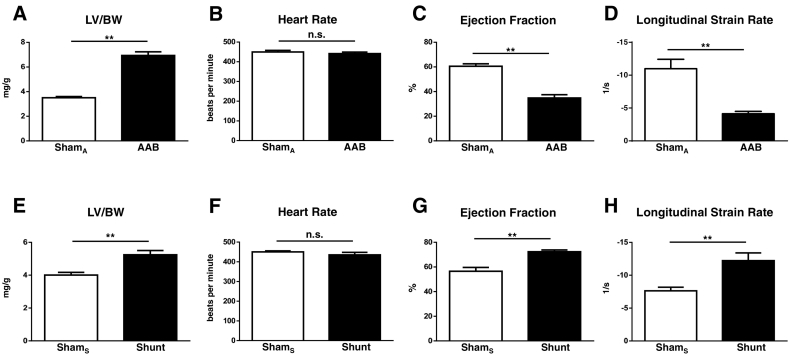
Six weeks after AAB, there was significant LV hypertrophy (LVH) as assessed by the LV/body weight ratio (Fig. 1A). Heart rates were similar in the banded and sham groups (Fig. 1B). There was significant systolic dysfunction in the AAB group as evidenced by reductions in EF and the mean global longitudinal strain rate (Fig. 1C–D).
Fig. 1.
LV hypertrophy and echocardiographic assessment of LV function in murine models of pressure and volume overload. Left ventricular weight versus body weight ratio (LV/BW), heart rate, ejection fraction and longitudinal strain rate were compared in mice after AAB (A–D) and Shunt (E–H) or the respective control procedures (ShamA and ShamS). n = 6–7/group; ** p < 0.01; n.s., not significant.
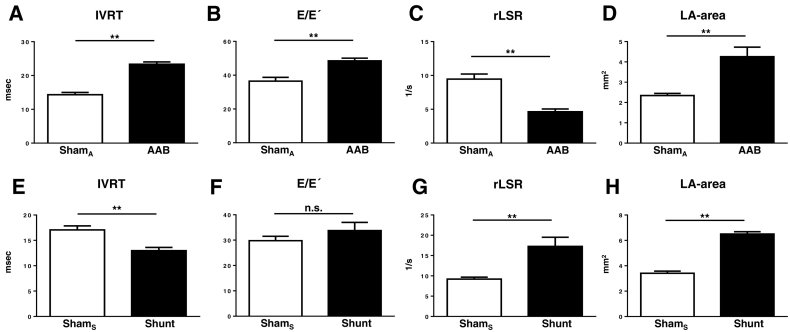
Turning to diastolic parameters, IVRT was significantly prolonged in AAB versus Sham, indicating impaired relaxation (Fig. 2A). The E/E′ ratio was significantly higher in AAB compared to Sham (Fig. 2B). The peak reverse longitudinal strain rate, quantified as an index of the peak early LV filling rate, was significantly decreased in AAB compared to Sham (Fig. 2C). Left atrial area or volume is a sensitive marker of chronic diastolic dysfunction and elevated left atrial pressure in humans.[14] In mice, left atrial area was found to be significantly higher in AAB versus Sham (Fig. 2D). Representative examples of the IVRT traces, apical 4-chamber images and tissue Doppler traces are shown in Fig. 3A–B and Supplementary Fig. 2. The E/A ratio and the E-wave deceleration time (DT) could not be evaluated consistently at high heart rates, which were associated in over 50% of cases with fusion of the E and A waves.
Fig. 2.
LV diastolic function in mouse models of pressure and volume overload. Isovolumic relaxation time (IVRT), E/E´, reverse longitudinal strain rate (rLSR) and left atrial (LA) area were measured after AAB (A–D) or Shunt (E–H). ShamA and ShamS denote the respective control groups. n = 6–7/group; ** p < 0.01; n.s., not significant.
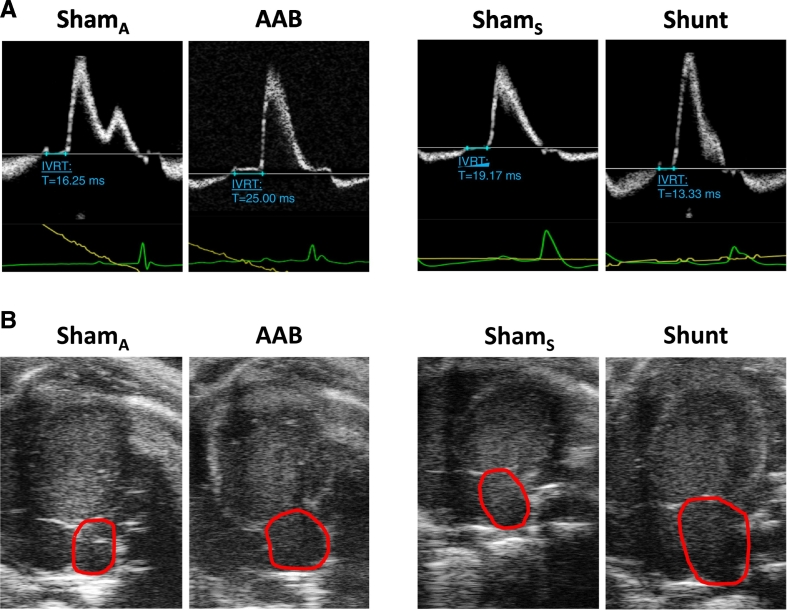
Fig. 3.
Representative Doppler flow profiles for measurement of IVRT and apical 4-chamber views for measurement of left atrial area. A. IVRT measurement (in blue) from the aortic outflow and mitral inflow profiles in the AAB and Shunt groups. B. Left atrial area measurement (red lines) in the AAB and Shunt groups compared to their respective controls (ShamA and ShamS). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A similar pattern of changes was observed in the TAC model of chronic pressure overload (Supplementary Fig. 3). There was evidence of systolic dysfunction (reduced EF) and LVH (increased LV/body weight ratio), as expected. Diastolic dysfunction was evident from a prolonged IVRT, elevated E/E′ ratio, decreased reverse longitudinal strain rate, and an increased left atrial area.
The inter- and intra-observer variability for diastolic parameters are shown in Table 1.
Table 1.
Intra- and interobserver variability of diastolic parameters. The coefficient of variation is shown for measurements of the isovolumic relaxation time (IVRT), E/E′, reverse longitudinal strain rate (rLSR) and left atrial (LA) area.
| Parameter | Intra-observer coefficient of variation | Inter-observer coefficient of variation |
|---|---|---|
| IVRT | 1.51% | 5.05% |
| E/E´ | 1.86% | 7.88% |
| rLSR | 4.77% | 4.71% |
| LA area | 2.31% | 3.19% |
3.2. Inter-relationship of diastolic parameters during cardiac hypertrophy
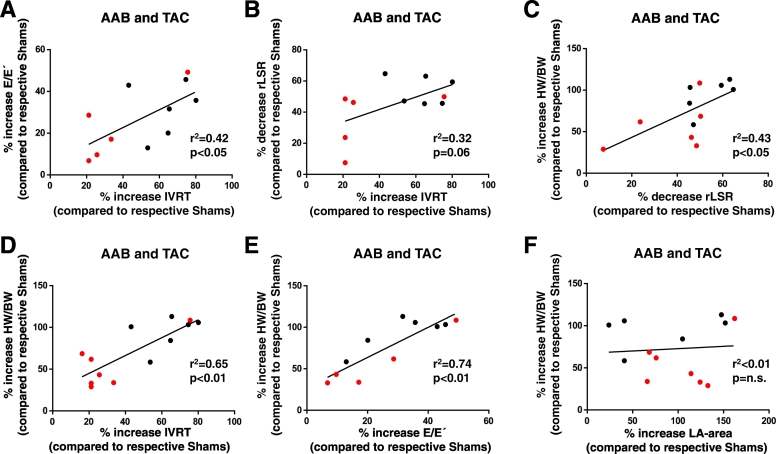
The relative change in IVRT correlated significantly and positively with that in the E/E´ ratio in the combined group of TAC and AAB pressure overload models (Fig. 4A). The correlation between relative IVRT and reverse longitudinal strain rate changes was not significant (p = 0.06) (Fig. 4B). There was no correlation between the changes in reverse longitudinal strain rate and E/E´, and changes in left atrial area also did not correlate with any of the other three parameters (Supplementary Fig. 4). We assessed the relationship between the magnitude of change in these parameters and the extent of cardiac hypertrophy. This analysis showed that the percentage changes in reverse longitudinal strain rate, IVRT and E/E′ were each significantly and positively correlated with the extent of hypertrophy (Fig. 4C–E). However, there was no correlation between the relative increase in left atrial area and extent of hypertrophy (Fig. 4F).
Fig. 4.
Correlation of diastolic parameters and cardiac hypertrophy in models of pressure overload. The % change in IVRT relative to the control Sham group was computed and plotted against the % change in E/E′ (A) or reverse longitudinal strain rate (rLSR) (B). The AAB (black symbols) and TAC (red symbols) groups were combined. (C–F). Correlation of changes in diastolic function parameters (rLSR, IVRT, E/E´ and left atrial (LA) area) with the relative increases in heart weight/body weight ratio. n = 11–13/group. The coefficients of determination (r2) from linear regression analysis and p-values are shown on the graphs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Diastolic dysfunction during chronic volume overload
One week after generation of an aortocaval fistula, the Shunt group showed evidence of eccentric LVH as assessed by increases in echocardiographic left ventricular end-diastolic dimensions (4.7 ± 0.09 vs 4.0 ± 0.10 mm, p ≤ 0.01) and LV/body weight ratio (Fig. 1E). Heart rates were similar between groups (Fig. 1F). The Shunt group was in a hypercontractile stage with a significantly higher EF and mean global longitudinal strain rate than Sham (Fig. 1G–H).
Left atrial area was significantly increased in Shunt compared to Sham (Fig. 2H; Fig. 3B). In marked contrast to chronic pressure overload, IVRT was significantly reduced in Shunt (Fig. 2E; Fig. 3A) and the peak reverse longitudinal strain rate was significantly increased compared to Sham (Fig. 2G). There was no significant difference in the E/E′ ratio between Shunt and Sham (Fig. 2F).
3.4. Assessment of a mouse model of HFpEF
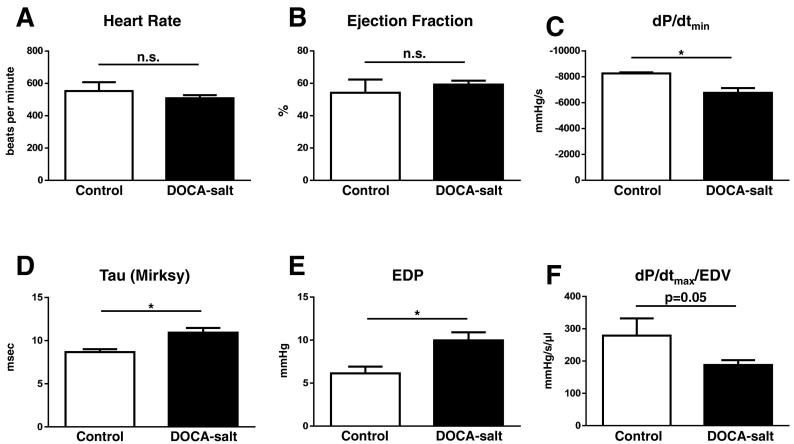
The DOCA-salt model has been proposed as a mouse model of HFpEF.[10] Unlike AAB, TAC and shunt, this model has not been extensively studied in the mouse and we therefore first performed invasive LV pressure-volume analysis. This confirmed a preserved EF with evidence of impaired relaxation (a reduced dP/dtmin and a prolonged isovolumic relaxation time constant, Tau) and increased LV end-diastolic pressure (Fig. 5). There was moderate LVH, as assessed by the HW/body weight ratio (5.08 ± 0.05 vs 4.87 ± 0.07 mg/g, p = 0.04), and evidence of mild systolic dysfunction as assessed by dP/dtmax normalized for end-diastolic volume (EDV).
Fig. 5.
Invasive LV pressure-volume analysis of systolic and diastolic function in a mouse DOCA-salt model of HFpEF. A. Heart rate; B. Ejection fraction; C. LV dP/dtmin; D. Isovolumic relaxation time constant, Tau (Mirsky); E. LV end-diastolic pressure (EDP); F. LV dP/dtmax/normalized by end-diastolic volume (EDV). n = 3–7/group; * p < 0.05.
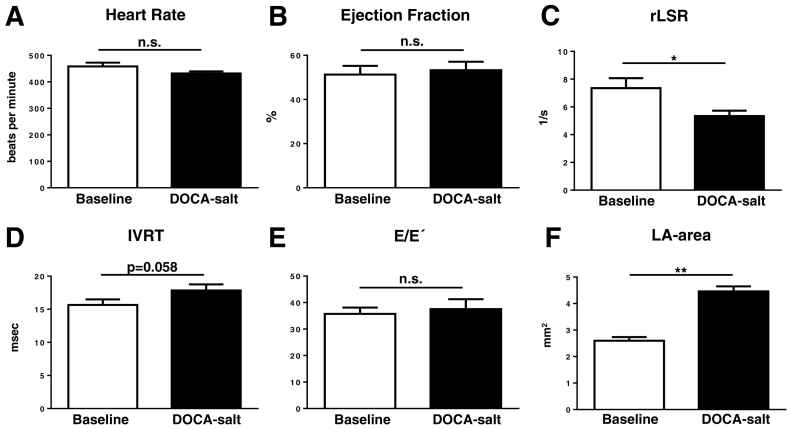
Assessment by echocardiography also demonstrated a preserved EF (Fig. 6). There was a significant decrease in the reverse longitudinal strain rate with a borderline increase in IVRT and no change in E/E´ in the DOCA-salt group compared to control (Fig. 6C–E). Left atrial area showed a significant increase in the DOCA-salt group (Fig. 6F).
Fig. 6.
Non-invasive echocardiographic assessment of diastolic function in a mouse DOCA-salt model of HFpEF. A. Heart rate; B. Ejection fraction; C. Reverse longitudinal strain rate (rLSR); D. Isovolumic relaxation time (IVRT); E. E/E´ ratio; F. Left atrial (LA) area. n = 6–8/group; * p < 0.05, ** p < 0.01.
3.5. Acute response to the sarcoplasmic reticulum Ca2 +-ATPase inhibitor thapsigargin
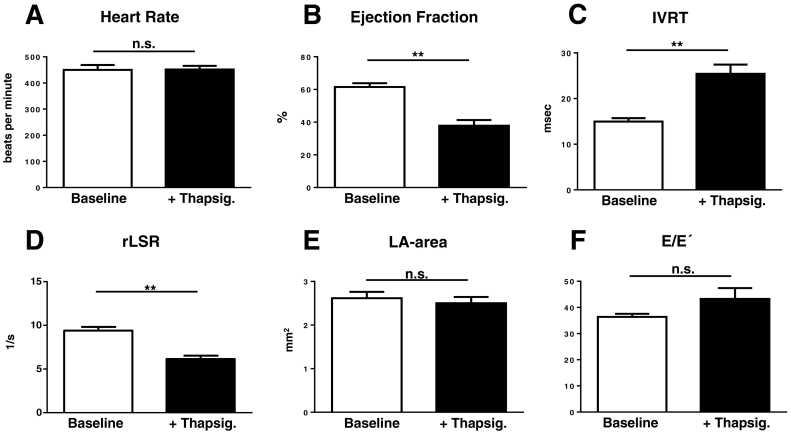
Treatment with thapsigargin did not affect heart rates, but caused a significant decrease in systolic function (Fig. 7A–B). It resulted in a significant increase in IVRT and a significant decrease in the reverse longitudinal strain rate (Fig. 7C–D), consistent with impairment of relaxation and early LV filling. However, there were no changes in the left atrial area or the E/E′ ratio (Fig. 7E–F).
Fig. 7.
Effect of acute thapsigargin treatment on diastolic function. A–F. Effect of thapsigargin (Thapsig.; 0.75 mg/kg intraperitoneally) on heart rate, ejection fraction (EF), IVRT, reverse longitudinal strain rate (rLSR), left atrial (LA) area and E/E′ respectively. n = 5–6/group; ** p < 0.01.
4. Discussion
The echocardiographic criteria for the assessment of LV diastolic function in humans are well established.[5], [15] A central parameter for the diagnosis of diastolic dysfunction that is recommended by the European Society of Cardiology is the E/E′ ratio, which requires tissue Doppler and mitral pulse wave Doppler measurements.[16] The E/E′ ratio correlates with LV filling pressure, which increases as diastolic dysfunction progresses, and an E/E′ ratio > 15 is considered abnormal in humans.[6] If the E/E′ ratio is between 8 and 15 and therefore potentially suggestive of diastolic dysfunction, it is recommended to also assess the mitral E/A ratio, E-wave deceleration time and the left atrial volume index. IVRT and diastolic LV strain analysis are not part of the European Society of Cardiology algorithm,[16] but have been used in many clinical studies. No analogous algorithm is available for the assessment of LV diastolic function in the mouse heart, and the few studies that do assess diastolic function do not appear to have a clear rationale for the choice of measured parameter. Furthermore, the parameters that are used in human studies cannot necessarily be extrapolated to mouse studies, given the substantial differences in heart rate and organ size between species. The current study therefore evaluated the applicability of several different parameters to assess LV diastolic function in mice. In addition, we investigated these parameters in diverse experimental models since the pattern of diastolic dysfunction may vary according the type of hemodynamic or other stress and different parameters may be useful in different settings. Accordingly, we included models of chronic pressure overload with combined systolic and diastolic dysfunction (AAB and TAC), a model of chronic volume overload with hypercontractile function (Shunt), a model of HFpEF (DOCA-salt), and a model of acute contractile dysfunction (thapsigargin). Based on this wide range of models, we aimed to develop a simple algorithm to guide the characterization of diastolic dysfunction that could be applied to diverse mouse models. To the best of our knowledge, this is the first study to systematically undertake such an analysis.
Although IVRT is not routinely quantified in human studies, it is a well-established and sensitive marker of impaired LV relaxation[4] and has been employed in some previous mouse studies.[17], [18] We found that IVRT was technically quite straightforward to quantify by echocardiography in the mouse heart at physiological heart rates. IVRT increased significantly in both chronic pressure overload models (AAB and TAC) as well as during acute sarcoplasmic reticulum dysfunction induced by thapsigargin. This is in line with the knowledge that pressure overload-induced LVH is accompanied by impaired relaxation and that sarcoplasmic reticulum function is crucial for relaxation. Since a significant impairment of LV isovolumic relaxation will slow down early ventricular filling, we reasoned that this would be reflected in the peak myocardial longitudinal strain rate during early filling (the reverse longitudinal strain rate). This parameter has only rarely been used in human or animal studies[19] but it is potentially attractive because it is technically very straightforward to estimate with systems that use speckle tracking to quantify myocardial strain. We found that the reverse longitudinal strain rate decreased in both surgical pressure overload models as well as after thapsigargin treatment. Changes in reverse longitudinal strain rate paralleled those in IVRT during pressure overload, suggesting that they may provide similar information. Furthermore, the magnitude of change in both parameters was significantly correlated to the extent of hypertrophy. Analyses in the HFpEF model, where an impairment in LV relaxation was confirmed by invasive measurement of LV dP/dtmin and Tau, showed that the reverse longitudinal strain rate was significantly decreased compared to Sham but that there was only a borderline increase in IVRT. This suggests that while IVRT and reverse longitudinal strain rate respond to similar pathophysiology, the latter may be a more sensitive marker.
The volume overload (Shunt) model causes a markedly greater increase in LV end-diastolic volume and end-diastolic wall stress than pressure overload, resulting in a “restrictive” LV filling pattern with an increased early filling rate and an abrupt termination of filling.[9], [20] This was readily detected by a significant decrease in IVRT and a significant increase in the reverse longitudinal strain rate in this model. It is notable that the changes in IVRT and reverse longitudinal strain rate were in opposite directions in volume overload versus pressure overload, therefore highlighting that these echocardiographic parameters easily enable a distinction to be made between the different types of diastolic physiology; i.e. impaired relaxation in pressure overload versus restrictive filling in volume overload LVH.
The E/E′ ratio is one of the parameters that is most widely used to assess diastolic dysfunction in humans,[16] and has also been used in many murine studies.[21], [22], [23] The E/E′ increased significantly in both our chronic pressure overload models as compared to Sham. However, we found no increase in E/E′ in the DOCA-salt model despite invasive evidence of an increased LV filling pressure (LVEDP). Likewise, no increase in E/E′ was observed in the Shunt model, which is known to have a greatly elevated filling pressure.[9] The same was true after acute treatment with thapsigargin. These results suggest that the value of measurement of the E/E′ ratio is highly model-dependent, a finding probably related to the differences in pathophysiology among models.
Left atrial area or volume is a very useful parameter to assess diastolic function in humans, reflecting a chronic increase in LV filling pressures.[14], [16], [24] However, it is rarely measured in murine studies.[25] We found that measurement of left atrial area in the mouse heart was relatively straightforward in the apical 4-chamber view. Furthermore, left atrial area was significantly increased in all four chronic models, i.e. AAB, TAC, Shunt and DOCA-salt models, in line with increased filling pressures in all models. It was however unchanged in the acute thapsigargin model. Therefore, left atrial area appears to be a very good marker of chronically elevated filling pressures across different murine cardiac models, in contrast to the E/E′ ratio. This is consistent with a human study that demonstrated a higher sensitivity for left atrial dimensions as compared to E′-related parameters in detecting LV diastolic dysfunction in patients with a normal EF.[26] It should be noted, however, that the relative increase in left atrial area was not significantly correlated with the extent of hypertrophy; this may reflect a high sensitivity of left atrial dilatation to elevated filling pressure but a non-linear response.
The E/A ratio and the E-wave deceleration time (DT) are commonly used in human echocardiography[16] and have also been used in many mouse studies.[21], [27] We found that it was very challenging or impossible to consistently measure these values in mice at heart rates > 450 bpm due to fusion of the E- and A-waves, as previously noted by others. A consistent measurement of E/A ratio in the mouse heart is probably only feasible if heart rate is artificially reduced, for example with deeper anesthesia, but this is undesirable due to the likelihood of associated cardiac depression.[28] Therefore, the routine assessment of these parameters is unlikely to be useful in mouse echocardiography.
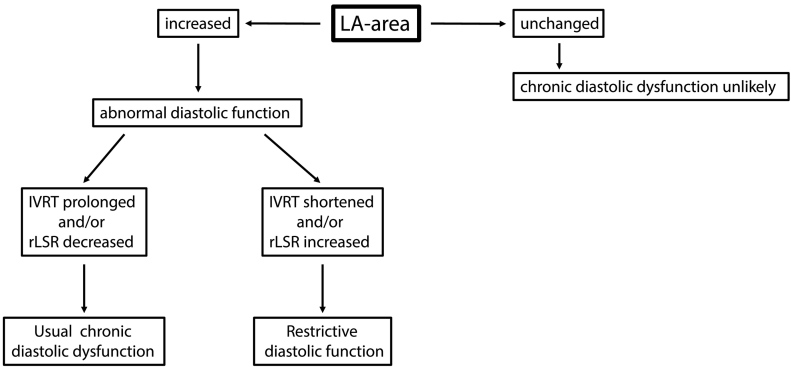
The pathophysiology of LV diastolic dysfunction is of increasing interest given the focus on identifying effective therapies for HFpEF and the increasing prevalence of conditions such as hypertensive LVH and metabolic syndrome that predispose to HFpEF. However, the application of well characterized methods to carefully study LV diastolic dysfunction by echocardiography in experimental mouse models has lagged behind. The results of the present study provide a framework for the systematic analysis of LV diastolic function in the mouse heart (Fig. 8). We propose that the left atrial area (a marker of LV filling pressure) is an optimal parameter for the detection of chronic LV diastolic dysfunction. The subsequent quantification of either reverse longitudinal strain rate or IVRT enables distinction between “usual” diastolic dysfunction and a restrictive physiology. The E/E′ ratio appears to be very useful in pressure overload models but less so in volume overload or HFpEF models in the mouse. Additional echocardiographic indices of diastolic function are probably unnecessary in the majority of cases.
Fig. 8.
Algorithm for assessment of chronic LV diastolic dysfunction in mice. If the left atrial (LA) area is unchanged compared to control mice, chronic diastolic dysfunction is unlikely. If the LA area is increased, subsequent assessment of the isovolumic relaxation time (IVRT) and reverse longitudinal strain rate (rLSR) can be used to distinguish between “usual” and “restrictive” diastolic dysfunction.
Nomenclature
Disclosures
None.
Financial support
This work was supported by the British Heart Foundation (RG/13/11/30384, CH/1999001/11735) and a DFG Joint PhD Studentship to MS (IRTG1816).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yjmcc.2017.10.006.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Zile M.R., Baicu C.F., Gaasch W.H. Diastolic heart failure - abnormalities in active relaxation and passive stiffness of the left ventricle. N. Engl. J. Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 2.Udelson J.E. Heart failure with preserved ejection fraction. Circulation. 2011;124:e540–e543. doi: 10.1161/CIRCULATIONAHA.111.071696. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 4.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., Dokainish H., Edvardsen T. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Oh J.K., Appleton C.P., Hatle L.K., Nishimura R.A., Seward J.B., Tajik A.J. The noninvasive assessment of left ventricular diastolic function with two-dimensional and doppler echocardiography. J. Am. Soc. Echocardiogr. 1997;10:246–270. doi: 10.1016/s0894-7317(97)70062-2. [DOI] [PubMed] [Google Scholar]
- 6.Ommen S.R., Nishimura R.A., Appleton C.P., Miller F.A., Oh J.K., Redfield M.M. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 7.Byrne J.A., Grieve D.J., Bendall J.K., Li J.M., Gove C., Lambeth J.D. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ. Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 8.Martin T.P., Robinson E., Harvey A.P., MacDonald M., Grieve D.J., Paul A. Surgical optimization and characterization of a minimally invasive aortic banding procedure to induce cardiac hypertrophy in mice. Exp. Physiol. 2012;97:822–832. doi: 10.1113/expphysiol.2012.065573. [DOI] [PubMed] [Google Scholar]
- 9.Toischer K., Rokita A.G., Unsold B., Zhu W., Kararigas G., Sossalla S. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silberman G.A., Fan T.H., Liu H., Jiao Z., Xiao H.D., Lovelock J.D. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thastrup O., Cullen P.J., Drobak B.K., Hanley M.R., Dawson A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2 + stores by specific inhibition of the endoplasmic reticulum Ca2 +-ATPase. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhan A., Sirker A., Zhang J., Protti A., Catibog N., Driver W. High-frequency speckle tracking echocardiography in the assessment of left ventricular function and remodeling after murine myocardial infarction. Am. J. Phys. 2014;306:H1371–H1383. doi: 10.1152/ajpheart.00553.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M., Prosser B.L., Bamboye M.A., Gondim A.N., Santos C.X., Martin D. Contractile function during angiotensin-II activation: increased Nox2 activity modulates cardiac calcium handling via phospholamban phosphorylation. J. Am. Coll. Cardiol. 2015;66:261–272. doi: 10.1016/j.jacc.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abhayaratna W.P., Seward J.B., Appleton C.P., Douglas P.S., Oh J.K., Tajik A.J. Left atrial size: physiologic determinants and clinical applications. J. Am. Coll. Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Oh J.K., Park S.J., Nagueh S.F. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ. Cardiovasc. Imaging. 2011;4:444–455. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 16.Paulus W.J., Tschope C., Sanderson J.E., Rusconi C., Flachskampf F.A., Rademakers F.E. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Charles P.Y., Nan C., Pinto J.R., Wang Y., Liang J. Correcting diastolic dysfunction by Ca2 + desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J. Mol. Cell. Cardiol. 2010;49:402–411. doi: 10.1016/j.yjmcc.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Zhang L., Jean-Charles P.Y., Nan C., Chen G., Tian J. Dose-dependent diastolic dysfunction and early death in a mouse model with cardiac troponin mutations. J. Mol. Cell. Cardiol. 2013;62:227–236. doi: 10.1016/j.yjmcc.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Khoury D.S., Thohan V., Torre-Amione G., Nagueh S.F. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M.S., Spevack D., Burkhoff D., Kronzon I. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J. Am. Coll. Cardiol. 2004;44:1543–1549. doi: 10.1016/j.jacc.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Fraysse B., Weinberger F., Bardswell S.C., Cuello F., Vignier N., Geertz B. Increased myofilament Ca2 + sensitivity and diastolic dysfunction as early consequences of MyBPC3 mutation in heterozygous knock-in mice. J. Mol. Cell. Cardiol. 2012;52:1299–1307. doi: 10.1016/j.yjmcc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Jaswal J.S., Ussher J.R., Sankaralingam S., Wagg C., Zaugg M. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ. Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. [DOI] [PubMed] [Google Scholar]
- 23.Szardien S., Nef H.M., Voss S., Troidl C., Liebetrau C., Hoffmann J. Regression of cardiac hypertrophy by granulocyte colony-stimulating factor-stimulated interleukin-1beta synthesis. Eur. Heart J. 2012;33:595–605. doi: 10.1093/eurheartj/ehr434. [DOI] [PubMed] [Google Scholar]
- 24.Tsang T.S., Barnes M.E., Gersh B.J., Bailey K.R., Seward J.B. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am. J. Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Quesada C., Cavalera M., Biernacka A., Kong P., Lee D.W., Saxena A. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ. Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida C., Nakao S., Goda A., Naito Y., Matsumoto M., Otsuka M. Value of assessment of left atrial volume and diameter in patients with heart failure but with normal left ventricular ejection fraction and mitral flow velocity pattern. Eur. J. Echocardiogr. 2009;10:278–281. doi: 10.1093/ejechocard/jen234. [DOI] [PubMed] [Google Scholar]
- 27.Erkens R., Kramer C.M., Luckstadt W., Panknin C., Krause L., Weidenbach M. Left ventricular diastolic dysfunction in NRF2 knock out mice is associated with cardiac hypertrophy, decreased expression of Serca2a, and preserved endothelial function. Free Radic. Biol. Med. 2015;89(215):906–917. doi: 10.1016/j.freeradbiomed.2015.10.409. [DOI] [PubMed] [Google Scholar]
- 28.Roth D.M., Swaney J.S., Dalton N.D., Gilpin E.A., EA Ross J., Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am. J. Phys. 2002;282:H2134–H 2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures