Abstract
Ischemia-reperfusion (IR) injury to the small intestine following clamping of the superior mesenteric artery results in an intense local inflammatory response characterized by villous damage and neutrophil infiltration. IL-17A, a cytokine produced by a variety of cells in response to inflammatory cytokines released following tissue injury, has been implicated in IR injury. Using Il17a−/−, Il23r−/− and Rorγt−/− mice and administration of anti-IL17A and anti-IL-23 neutralizing antibodies to wild-type mice, we demonstrate that intestinal IR injury depends on IL17A and that IL-17A is downstream of the binding of autoantibody to ischemia-conditioned tissues and subsequent complement activation. Using bone marrow chimeras, we demonstrate that the IL17A required for intestinal IR injury is derived from hematopoietic cells. Finally, by transferring autoantibody-rich sera into Rag2γc−/− and Rag2−/− mice we demonstrate that innate lymphoid cells (ILCs) are the main producers of IL-17A in intestinal IR injury. We propose that local production of IL-17A by ILCs is crucial for the development of intestinal IR injury and may provide a therapeutic target for clinical exploitation.
INTRODUCTION
Intestinal ischemia-reperfusion (IR) injury occurs when the blood supply to the intestine is re-established following a period of transient disruption of blood flow. Intestinal IR injury occurs in a variety of clinical conditions including shock, trauma, sepsis, aortic surgery, and acute mesenteric artery occlusion (1, 2). Intestinal IR injury triggers a cascade of events that result in both local and remote organ injury. Intestinal damage after IR injury is characterized by severe villus destruction with disruption and dysfunction of the intestinal epithelium (3). Re-establishment of the blood supply to the intestine initiates an intense local inflammatory response with neutrophil infiltration (4). IR injury depends on elements of both the innate and the adaptive immune response (5). Contributors to the tissue damage include IgM (6), natural antibodies (7), complement (8, 9), neutrophils (4), platelets (10, 11), B lymphocytes (12), and T (13) lymphocytes.
IL-17A is a proinflammatory cytokine that causes epithelial cells to secrete neutrophil chemoattractant chemokines such as CXCL1, CXCL2 and IL-8 (14). Cells that produce IL-17A include TCRα/β T cells (15, 16), TCRγ/δT cells (17) and CD45+CD4+TCR−IL-7R+ type 3 innate lymphoid cells (ILC3s) (18, 19), which reside at mucosal surfaces. Other sources of IL-17A include dendritic cells (DCs), macrophages, neutrophils and natural killer cells (NK) (20). The IL17A receptor is a heterodimer of the IL-17RA and IL-17RC chains that bind IL-17A and its homologue IL-17F, and is expressed predominantly on epithelial cells (21, 22). Because of its ability to mobilize neutrophils, IL-17A is important in the pathogenesis of autoimmune diseases and in chronic steroid resistant asthma characterized by neutrophil predominance (23, 24). It is also important for host defense against candida infection, as illustrated by the susceptibility to candida infection of patients who carry mutations in IL17A or genes encoding IL-17A receptor chains (25). Naïve TCRα/β cells differentiate into T helper 17 (Th17) cells following TCR ligation in the presence of the inflammatory cytokines IL-1, IL-6 and TGFβ(26). The differentiation of TCR CD4+ Th17 cells is promoted by the cytokine IL-23 (20), whereas the rapid production of IL-17A by TCRγ/δT cells and ILC3s is directly driven by IL-23 (27). IL-23 is a heterodimer of the 40 kD chain (p40), shared with IL-12, and the IL-23 specific 19 kD chain p19 (28), and is produced by epithelial cells and DCs (29, 30). The IL-23R is a heterodimer of the IL12Rβ1 chain, shared with the IL-12R, and an IL-23R specific chain (30, 31).
We have previously shown, using immunofluorescence microscopy, an increase in IL-17A expression in the intestine after IR injury (13). Importantly, IL-17A, via its role in neutrophil recruitment, has been shown to play a critical role in intestinal IR injury (32, 33), as well as in IR injury in other organ systems including heart (34, 35), liver (36, 37), lung (38), kidney (39, 40), brain (41, 42). It has been claimed that Paneth cells store IL-17A and are the major source of IL-17A in intestinal IR injury (33). However, because published gene array analyses do not show that Paneth cells express Il17a (43, 44); we set out to analyze the source of IL-17A relevant to intestinal IR injury. We have confirmed the role of IL-17A and demonstrated a role for IL-23 in intestinal IR injury, and we provide evidence that ILCs are the relevant source of IL-17A in intestinal IR injury.
METHODS
Mice
Il17a−/− and Il23r−/− mice on a C57BL/6J (B6) background were generated as described (45) (46). Age-matched C57BL/6J WT (Jackson Laboratory, Bar Harbor, ME) wild type (WT) CD45.2 mice were used as controls. CD45.1 C57BL/6J WT mice (Jackson Laboratory, Bar Harbor, ME) were used in making the bone marrow chimeras. Rag2−/− mice and Rag2−/−γc−/− mice on C57BL/6J background were obtained from Taconic, (Hudson, NY). B6.MRLTnfrsf6lpr (B6.lpr) female mice age 7–8 months were purchased from The Jackson Laboratory (Bar Harbor, ME) and used to isolate IgG from serum. Tcrd−/− and Rorc−/− mice on B6.129 background were backcrossed on to a C57BL/6 background for at least 12 and 6 generations respectively. Mice underwent at least 7 days of acclimatization before experimentation. All mice used in this study were 8–12 week old males, except the bone marrow chimeras that were 18–22 weeks old, and were maintained in pathogen-free conditions in the animal research facility at the Beth Israel Deaconess Medical Center, Boston, MA. All experiments were performed in accordance with the guidelines and approval of the Harvard University Institutional Animal Care and Use Committee.
Ischemia-Reperfusion (IR) Injury
Mice were randomly assigned to sham or IR groups. Mice were anesthetized by intraperitoneal injection of 72 mg/kg pentobarbital or 250 mg/kg Avertin and anesthesia was maintained by subcutaneous 36 mg/kg pentobarbital injected or 125 mg/kg Avertin. A midline laparotomy incision was made and the superior mesenteric artery (SMA) was identified, isolated and clamped for 30 min using a microvascular clip (Roboz Surgical Instruments, Rockville MD) delivering ~8.5 g of pressure. The clip was removed after 30 minutes of ischemia and the intestines were re-perfused for 2 hours. The laparotomy incision was sutured closed using 4.0 prolene suture and the mice were resuscitated with 1 ml warm PBS injected subcutaneously. The mice were monitored throughout the experiment. Body temperature was maintained at 37°C throughout the experiment on a temperature controlled heating pad. Sham-operated mice underwent identical abdominal manipulations (laparotomy, intestinal retraction, and positioning) as mice subjected to SMA clamping. Intestines were collected 2 h after sham operation or intestinal IR injury unless otherwise noted.
Histological analysis of intestinal IR injury
Small intestine (jejunum and ileum) was washed in ice-cold PBS and fixed overnight in 10% formalin. After automated dehydration through a graded alcohol series, tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin (H&E). Intestinal H&E sections were graded for intestinal IR-induced mucosal injury. Villi were scored using a published algorithm (3) to measure intestinal damage in a blinded manner by one of us (MGT).
Immunohistochemistry
Formalin-fixed paraffin sections of small intestine were subjected to rehydration, and endogenous peroxidase activity was quenched with 3% H2O2. Then antigen retrieval was performed using Retrievagen A (BD Pharmingen, San Jose, CA) according to the manufacturer’s directions. The sections were blocked with 10% BSA/PBS containing the serum from host species of secondary antibody. The primary antibody rat-anti-mouse Ly-6B.2 (Gr1) clone 7/4 at 1/250 dilution (Bio-Rad, Herculese, CA) was used to stain for neutrophils. Primary antibody or isotype control antibody prepared in 10% BSA/PBS were applied overnight at 4°C. The slides were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 60 min at room temperature, developed with NovaRED (Vector Laboratories, Burlingame, CA), counterstained with hematoxylin, and dehydrated. The sections were mounted in mounting medium (Thermo Scientific, Waltham, MA) and evaluated with Nikon eclipse 80i microscope. Images were analyzed using Nikon NIS-Elements software (Nikon, Melville, NY). For neutrophil infiltration positive staining cells in the area of injury were counted in 20 high power fields (HPF) at 200× and the average was calculated and expressed as number of neutrophils per HPF.
Neutralization of endogenous IL-17A and IL-23
To neutralize IL-17A, 200 μg of rat-anti-mouse anti-IL-17A Ab (R&D Systems) were given in 3 doses by intraperitoneal (i.p.) injection 96, 48 and 24 hours prior to IR as described (47). Control animals were given 200 μg rat IgG isotype control antibody (R&D systems) also in 3 doses 96, 48 and 24 hours prior to IR. To neutralize IL-23, 10 μg of goat-anti-mouse anti-IL-23 Ab (R&D Systems) were given in 3 doses by i.p. injection 96, 48 and 24 hours prior to IR as described by (48). Control animals were given 20 μg goat IgG isotype control antibody (R&D systems) also in 3 doses 96, 48 and 24 hours prior to IR.
Reconstitution of Rag2−/− and Rag2−/−γc−/− mice with IgG from B6.lpr mice
Serum from B6.lpr mice was obtained by cardiac puncture. The MelonTM Gel IgG Purification Kit (Thermo Scientific) was used to Isolate and purify IgG. A buffer exchange to PBS was performed on the eluted material. 200 μg of IgG was injected intravenously in Rag2−/− or Rag2−/−γc−/− mice 30 minutes prior to ischemia or sham operation as previously described (49).
Generation of Bone Marrow (BM) Radiation Chimeras
Eight-week-old recipient CD45.2+ WT and Il17a−/− mice were lethally irradiated (1,100 rads delivered in 2 doses of 550 rads each at 3 hrs. intervals), and injected i.v. with 5×106 BM cells obtained from congenic CD45.1+ WT mice and vice versa. Chimerism was assessed by measuring the percentages of CD45+ donor and recipient cells in the chimeric mice 8 weeks after BM reconstitution using FACS analysis for the CD45.1 and CD45.2 markers on blood after red blood cell lysis.
qRT-PCR analysis
Intestines were harvested and placed in RNAlater (Thermo Fisher Scientific). Total RNA was isolated using the RNeasy Mini kit (Qiagen). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to manufacturer protocol. Quantitative real-time PCR were performed (Light Cycler 480; Roche) for Il17a, Il23, Cxcl1, Cxcl2, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Cyclophilin A (CycloA) with 40 cycles at 94 °C for 12 s and 60 °C for 60 s using appropriate murine TaqMan assays for Il17a, Il23, Cxcl1, Cxcl2 and GAPDH (Applied Biosystems) or using SYBR Green I Brilliant Mastermix (Stratagene, La Jolla, CA) with appropriate primers for Il17a, Il23 and CycloA Ct values were determined by using Mx3000P software. All PCR reactions were run in triplicates. The averaged cycle threshold values for each target gene were normalized for GAPDH or CycloA mRNA, and relative expression of the target gene mRNA was calculated with the ΔΔCt relative quantification method.
Statistical analysis
Numeric data are presented as mean ± SD or mean ± SEM. Statistical analyses were performed using GraphPad Prisim 6.0 (GraphPad Software, San Diego, CA). The ordinal values of the injury scores and PMN/HPF were analyzed by the non-parametric Mann-Whitney U. The two-tailed Student’s t test for unpaired samples was used in the comparison of means of two groups. A value of P < 0.05 was considered statistically significant.
RESULTS
IL-17A is important for mesenteric IR injury
We confirmed the critical role of IL-17A in our model of mesenteric IR injury in which mice are subjected to 30 minutes of SMA occlusion followed by 2 hours of reperfusion. Il17a−/− mice developed markedly less intestinal damage, with lower injury scores, compared to WT mice (Fig. 1A & B). Il17a−/− mice subjected to mesenteric IR injury had significantly less neutrophil infiltration in the intestine than WT controls as determined by examination of intestinal sections stained immunohistochemically for Gr1 (Fig. 1C & D).
Figure 1. IR injury is attenuated in Il17a−/− mice.
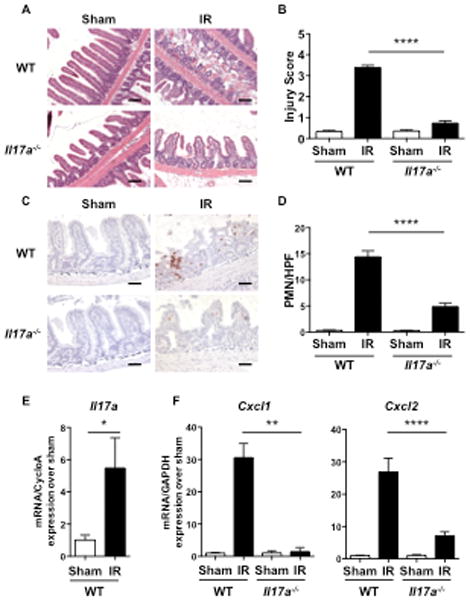
A–E. Representative H&E sections (A) Injury scores (B), representative immunohistochemical staining for Gr1 (C) quantitation of infiltrating Gr1+ cells/HPF (D), and qRT-PCR analysis of Il17a (E), Cxcl1 and Cxl2 expression (F) in Il17a−/− mice and WT controls subjected to intestinal IR injury or sham operated. Results are derived from three independent experiments each with 3–4 mice per group. Columns and bars represent the mean+SEM. *p< 0.05, ** p< 0.01, *** p<0.001. Photomicrographs in A 100× magnification, scale bars represent 100 μM. Photomicrographs in C 200× magnification, scale bars represent 50 μM.
Mesenteric IR injury in WT mice was associated with a significant increase in Il17a mRNA levels in the small intestine when compared to sham operated mice, as determined by qRT-PCR (Fig. 1E). IL-17A is known to drive neutrophil infiltration into tissues by inducing resident cells to express neutrophil attracting chemokines, including Cxcl1 and Cxcl2,(50). Cxcl1 and Cxcl2 mRNA levels were significantly higher in the small intestine of WT mice subjected mesenteric IR injury compared to sham operated mice. In contrast, there was no significant increase in Cxcl1 and Cxcl2 mRNA levels in the small intestine of Il17a−/− mice subjected to mesenteric IR injury when compared to WT controls (Fig. 1F).
Natural antibodies (7) that include self-reacting autoantibodies (49, 51) are raised in response to the bacterial flora and play a critical role in intestinal IR injury by cross reacting with neoantigens expressed on damaged intestinal cells and triggering complement activation. Given the interaction between IL-17A and the microbiome, we considered the possibility that the failure of Il17a−/− mice to develop IR injury may be due to defective production of natural antibodies. Administration of serum IgG from B6.lpr mice, which contains autoantibodies known to restore IR injury in Rag deficient recipients (49, 52–55), failed to restore IR injury in Il17a−/− mice (Fig S1). These observations conclusively place IL-17A downstream from the recognition of damaged cells in ischemic intestinal tissue by autoantibodies and subsequent fixation and activation of complement.
Lack of IL17-A could avert IR injury by exerting effects during development. Furthermore, IL-17A plays an important role in the maintenance and composition of the gut normal microbiome (56). Alteration of the intestinal microbiota, in the absence of IL-17A, may affect the profile of natural antibodies, which play an essential role in intestinal IR injury (49, 52–55). To circumvent these limitations, we examined the effect of administration of neutralizing IL-17A on intestinal IR injury in WT mice. WT mice were treated with a neutralizing IgG antibody to IL-17A, or IgG isotype control antibody, on days -4, -2 and -1 prior to IR injury. WT mice treated with neutralizing anti-IL-17A antibody developed significantly lower intestinal injury scores after IR (Fig. S2 A) and significantly diminished neutrophil infiltration in the lamina propria (Fig. S2 B) compared to mice treated with IgG isotype control. Collectively, these results confirm that that IL-17A plays a critical role in intestinal IR injury.
The transcription factor RORγt is essential for intestinal IR injury
The transcription factor RORγt encoded by Rorc plays an important role in driving Il17a gene expression(57). We used Rorc−/− mice to examine whether RORγt is essential for IR injury. Rorc−/− mice developed significantly less intestinal IR injury compared to WT controls, as assessed by injury scores and numbers of infiltrating neutrophils in the small intestine (Fig. 2A & B). These results indicate that RORγt plays an essential role in intestinal IR injury.
Figure 2. The transcription factor RORγt is essential for intestinal IR injury.
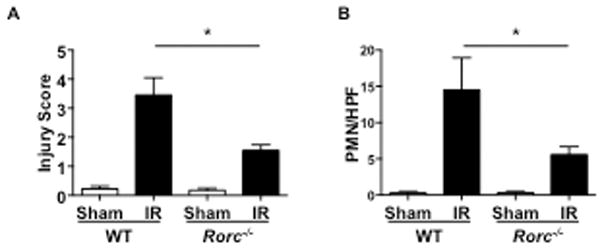
A–B. Quantitative Injury scores (A) and quantitation of infiltrating Gr1+ cells (B), in Rorc−/− mice and WT controls subjected to intestinal IR injury or sham operated (n = 4 mice per group). Columns and bars represent the mean+SD statistical analysis was performed by the Mann-Whitney U test. *p< 0.05
IL-23 plays an important role in intestinal IR injury
IL-23 plays an important role in IL-17A production and is primarily expressed by epithelial cells and dendritic cells (50, 58). Intestinal IR injury did not cause a significant increase in Il23 mRNA levels in the small intestine of WT mice or Il17a−/− mice (Fig S3). Nevertheless, intestinal damage, neutrophil infiltration and expression of Cxcl1 and Cxcl2 mRNA after intestinal IR injury were significantly, albeit partially, attenuated in Il23r−/− mice compared to WT controls (Fig. 3A–E).
Figure 3. IR injury is attenuated in Il23r−/− mice.
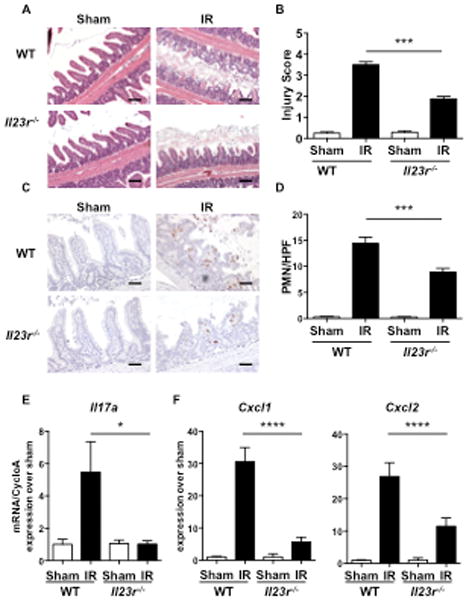
A–E. Representative H&E sections (A) quantitative Injury scores (B), representative immunohistochemical staining for Gr1 (C) quantitation of infiltrating GR1+ cells (D), and qRT-PCR analysis of Il17a (E), Cxcl1 and Cxl2 expression (F) in Il23r−/− mice and WT controls subjected to intestinal IR injury or sham operated. Results are derived from three independent experiments each with 3–4 mice per group. Columns and bars represent the mean+SEM. *p< 0.05, ** p< 0.01, *** p<0.001. Photomicrographs in A 100× magnification, scale bars represent 100 μM. Photomicrographs in C 200× magnification, scale bars represent 50 μM.
To confirm the importance of IL-23 in IR injury, WT mice were treated with neutralizing IgG antibody to IL-23, or IgG isotype control, on days -4, -2 and -1 prior to IR. WT mice treated with neutralizing IL-23 antibody developed significantly less small intestinal damage after IR injury than mice treated with IgG isotype control, as evidenced by significantly lower injury scores (Fig. S4 A), and significantly lower numbers of infiltrating neutrophils (Fig. S4 B). These results suggest that IL-23 constitutively expressed in the small intestine plays an important role in intestinal IR injury.
IL-17A produced by hematopoietic cells is essential for intestinal IR injury
It has been reported that Paneth cells are the major IL-17A-containing intestinal cells in IR injury (33) but it is not clear whether Paneth cells produce IL-17A or simply store and release IL-17A made by other cells. We used BM chimeras to ascertain whether the source of IL-17A in IR injury is of hematopoietic or non-hematopoietic cell origin. To determine the contribution of IL-17A derived from hematopoietic cells BM from CD45.1+ WT donors was used to reconstitute lethally irradiated CD45.2+ Il17a−/− or WT recipients. To assess the contribution of IL-17A derived from non-hematopoietic cells BM from CD45.2+ WT Il17a−/− donors were used to reconstitute lethally irradiated CD45.1+ WT recipients or CD45.2+ Il17a−/− recipients. FACS analysis eight weeks after BM reconstitution revealed that >91% of the blood cells in the WT->Il17a−/−, WT->WT and Il17a−/− ->WT BM chimeras were donor-derived (Fig. 4A–C). We could not assess the percentage of donor cells in Il17a−/− ->Il17a−/− BM chimeras, as both donors and recipients were on the CD45.2 background; however Il17a−/− ->Il17a−/− BM chimeras had numbers of leukocytes in blood and spleen that were comparable to those in the other three chimeras (data not shown). Intestinal IR injury was comparable between WT->Il17a−/− and WT->WT BM chimeras as assessed by injury scores and numbers of infiltrating neutrophils in the small intestine (Fig. 4D & E). In contrast, minimal intestinal injury after IR was observed in Il17a−/− ->WT and Il17a−/− ->Il17a−/− BM chimeras (Fig. 4D & E). These results indicate that the major source of IL-17A important for intestinal damage after intestinal IR is a cell of hematopoietic origin.
Figure 4. IL-17A derived from hematopoietic cells is essential for intestinal IR injury.
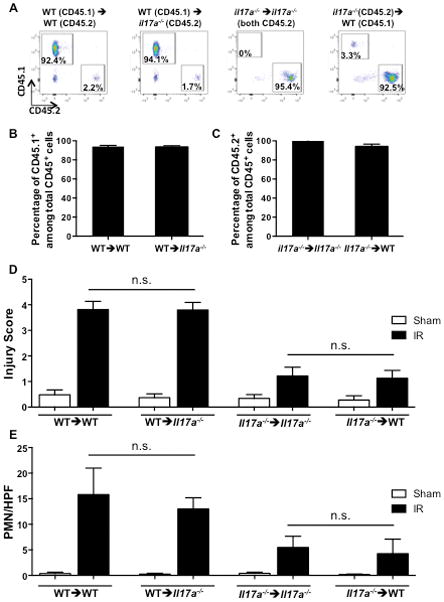
A–C. Representative FACS analysis of CD45.1 and CD45.2 expression (A) and quantitative analysis of the percentages of CD45.1+ and CD45.2+ cells (B & C) in blood leukocytes from WT->WT, WT ->Il17a−/−, Il17a−/−->WT Il17a−/−-> Il17a−/− bone marrow radiation chimeras. Gating was on live cells following red cell lysis. D, E. Injury scores (D), and quantitation of infiltrating GR1+ cells (E) in the four bone marrow radiation chimeras (n = 4–6 mice per group). Columns and bars represent the mean+SD. n.s.: not significant.
TCRγδ cells do not contribute significantly to intestinal IR injury
The intestine is rich in TCRγδ cells and type 3 ILCs, both of which can rapidly release IL-17A in response to stimulation with IL-23 (59). We used Tcrd−/− mice to examine the role of TCRγδ cells in IRI. Intestinal damage and neutrophil infiltration was not significantly different Tcrd−/− mice compared to WT controls (Fig. 5A & B). These results indicate that TCRγδ cells do not contribute significantly to intestinal IR injury.
Figure 5. TCRγδ T cells do not contribute to intestinal IR injury.
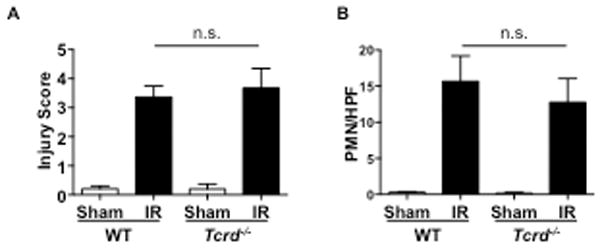
A–B. Quantitative Injury scores (A) and quantitation of infiltrating GR1+ cells (B), in Tcrd−/− mice and WT controls subjected to small intestinal injury or sham operated (n = 4 mice per group). Columns and bars represent the mean+SD. n.s.: not significant.
Innate lymphoid cells (ILCs) play an important role in intestinal IR injury that depends on IL-17A
It has been previously demonstrated that intestinal IR injury depends on the presence of natural antibodies or autoantibodies (49). Furthermore it has been shown that Rag2−/− mice, which lack mature T and B cells, are resistant to intestinal IR injury and that administration of IgM directed against intestinal neoantigens, or of IgG from autoimmune-prone B6.lpr mice, restores intestinal IR injury in these mice (6, 7). To investigate the potential role of ILCs in intestinal IR injury, we assessed the ability of IgG from autoimmune-prone B6.lpr mice to restore intestinal IR injury in Rag2−/−γc−/− mice, which lack ILCs in addition to lacking mature T and B cells.
We first verified that the batch of IgG we used restores IR injury in Rag2−/− mice. As previously reported (13, 51, 55), Rag2−/− mice had minimal intestinal IR injury compared to WT controls and neutrophil infiltration (Fig. 6A & B). Administration of IgG from B6.lpr mice restored IR injury in Rag2−/− mice, albeit to a level lower than that in WT controls (Fig. 6A & B). Restoration of intestinal IR injury in Rag2−/− mice was associated with the induction of Il17a, Cxcl1 and Cxcl2 expression in the small intestine (Fig. 6C). Importantly, co-administration of anti-IL-17A neutralizing antibody prevented the restoration of intestinal IR injury in Rag2−/− mice treated with IgG from B6.lpr mice (Fig. 6A & B), demonstrating that this restoration depended on IL-17A.
Figure 6. Reconstitution of injury in Rag2−/− is IL-17A dependent.
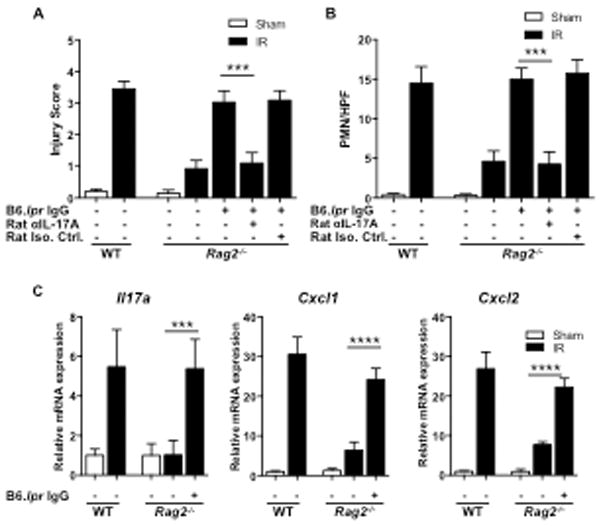
A, B. Quantitative injury scores (A) and quantitation of small intestinal infiltrating GR1+ cells (B) in Rag2−/−, WT and in Rag2−/− mice administered IgG from B6.lpr mice alone, or with rat anti-IL-17A IgG neutralizing antibody or with rat IgG isotype control subjected to intestinal IR injury or sham operated (n=3–5 mice per group). C. Il17a, Cxcl1 and Cxcl2 expression in the small intestine in WT, Rag2−/− and Rag2−/− mice administered IgG from B6.lpr mice (n=3–5 mice per group) subject to small intestinal IR injury or sham operated. Columns and bars represent the mean+SD. *** p<0.001.
Like Rag2−/− mice, Rag2−/−γc−/− mice developed minimal intestinal IR injury (Fig. 7A). However, in contrast to Rag2−/− mice, administration of IgG from B6.lpr mice failed to restore intestinal IR injury in Rag2−/−γc−/− mice (Fig. 7A & B). It also failed to induce Il17a, Cxcl1 and Cxcl2 expression in the small intestine (Fig. 7C). These results indicate that ILCs play an essential role in intestinal IR injury, likely by producing IL-17A.
Figure 7. Innate lymphoid cells are important in intestinal IR injury by producing IL-17A.
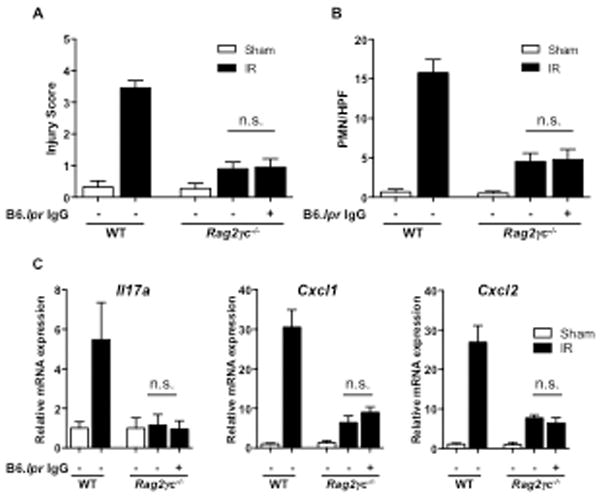
A–C. Injury scores (A) quantitation of small intestinal infiltrating GR1+ cells (B) and Il17a, Cacl1 and Cxcl2 expression (C) in Rag2−/−γc−/− mice administered IgG from B6.lpr mice subject to small intestinal IR injury compared to Rag2−/−γc−/− and WT (n= 3–6 mice per group) subject to small intestinal IR injury. Columns and bars represent the mean+SD. n.s.: not significant.
DISCUSSION
We demonstrate a critical role for IL-17A in the development of intestinal epithelial damage and neutrophil-dominated inflammation during intestinal reperfusion after mesenteric IR injury. We also show that IL-23 is important for intestinal IR injury. In addition, we demonstrate for the first time that the major source of IL-17A in this model are ILCs, which are known to rapidly release IL-17A in response to IL23.
Intestinal IR injury was associated with a significant increase in Il17a expression in the small intestine, and was markedly attenuated in Il17a−/− mice, evidenced by a significant decrease in injury scores and neutrophil infiltration in the lamina propria. Similar findings were observed in WT mice treated with anti-IL-17A neutralizing antibody, ruling out a role for secondary effects of lack of IL-17A on the microbiome and on the development of natural antibodies, essential for intestinal IR injury, in Il17a−/− mice. These observations conclusively place IL-17A downstream from the recognition of damaged cells in ischemic tissue by natural antibodies and subsequent fixation and activation of complement. The role of IL-17A in intestinal IR injury was further supported by the novel observation that intestinal IR injury was significantly attenuated in Rorc−/− mice, which lack the transcription factor RORγt essential for Il17a gene expression. The mechanism of villous damage in intestinal IR injury is largely dependent on neutrophils (60). Consistent with the role of IL-17A in driving expression of neutrophil chemoattractants, the expression of Cxcl1 and Cxcl2 was significantly upregulated following IR injury in WT mice, but not in Il17a−/− mice. The residual neutrophil infiltration observed in Il17a−/− mice could be due to cytokines other than IL-17A that cause neutrophil recruitment to injured tissues. These may include IL-1 and IL-6, which are known to be upregulated in intestines subjected to IR injury (61, 62)
We demonstrate for the first time that intestinal IR injury was significantly attenuated in Il23r−/− mice, as evidenced by decreased injury scores as well as by decreased neutrophil infiltration in the lamina propria. This is consistent with our previous report that intestinal IR injury is attenuated in IL-23 deficient p19−/− mice (13). Furthermore, administration of anti-IL-23 neutralizing antibody to WT mice attenuated intestinal IR injury, indicating that IL-23-IL-23R signaling is important for IR injury independent of potential alterations in the microbiota in p19−/− and Il23r−/− mice. We did not detect a significant increase in intestinal Il23 mRNA levels in WT mice following IR injury, suggesting that preformed IL-23 is the culprit. Notably IL-23 is constitutively expressed in the intestines by endothelial cells, and to a lesser extent by epithelial cells (63). Upregulation of Il7a expression in the intestine following IR was abrogated in Il23r−/− mice, indicating that this upregulation is strictly dependent on IL-23. Upregulation of Cxcl1 and Cxcl2 expression was significantly diminished, but not abolished, in Il23r−/− mice. Since upregulation of Cxcl1 and Cxcl2 expression was abolished in Il17a−/− mice, these findings together suggest that the release of preformed IL-17A contributes to the intestinal upregulation of Cxcl1 and Cxcl2 expression caused by IR injury. This may explain why intestinal IR injury was attenuated less in Il23r−/− mice compared to Il17a−/− mice and in WT mice treated with anti-IL-23 neutralizing antibody compared to WT mice treated with anti-IL-17A neutralizing antibody. In addition, pathways independent of IL-23-IL-23R signaling pathway may also be involved in driving intestinal IR injury.
We definitively demonstrate, using BM chimeras, that the source of IL-17A important for intestinal IR injury is a cell of hematopoietic origin. WT->Il7a−/− chimeras exhibited intestinal injury that was comparable to that observed in WT->WT chimeras. In contrast, Il7a−/−->WT chimeras developed minimal intestinal IR injury. Paneth cells have been demonstrated to store IL-17A and to be important for IR injury (33). However, gene expression array analyses do not reveal detectable expression of Il17a mRNA in Paneth cells. This suggests that Paneth cells store IL-17A normally produced by a cell of hematopoietic origin, and thereby contribute to intestinal IR injury. The 8–9 week interval after irradiation of WT mice and their reconstitution with Il7a−/− BM likely resulted in the depletion of IL-17A from the Paneth cells of Il7a−/−->WT chimeras explaining their minimal intestinal IR injury, that was no different from that of Il7a−/−-> Il7a−/− control chimeras.
TCRγδT cells rapidly express Il17a and secrete IL-17A in response to IL-23 released by tissue injury (64). However, there was no reduction in the severity of intestinal IR injury in TCRγδ deficient Tcrd−/− mice suggesting that TCRγδ T cells are not the important source of IL-17A in our model. In addition to releasing preformed IL-17A in response to IL-23 stimulation, ILCs, like TCRγδ T cells, rapidly upregulate Il17a mRNA expression in response to IL-23 stimulation. In contrast to Rag2−/− mice, Rag2−/−γc−/− mice reconstituted with IgG from B6.lpr mice developed minimal intestinal IR injury. Furthermore, intestinal IR in Rag2−/− mice, but not Rag2−/−γc−/− mice, reconstituted with B6.lpr IgG was associated with robust expression of Il17a, Cxcl1 and Cxcl2 in the intestine. As previously mentioned, intestinal IR injury in the reconstituted mice depends on IL-17A, because it was abrogated by administration of anti-IL-17A neutralizing antibody. Taken together with the observation that intestinal IR injury depends on RORγt, these findings strongly suggest that RORγt expressing, IL-17A producing ILC3s are essential for the development of intestinal IR injury
Based on our current and previous data (65, 66) we propose a mechanism for intestinal IR injury in which ischemia causes the expression of damage-associated neoantigens on intestinal cells. Binding of natural antibodies that crossreact with intestinal neoantigens results in fixation and activation of complement (7). Complement binding to receptors, such as C3aR and C5aR, on intestinal cells releases IL-23, which drives rapid IL-17A production and release by ILC3s. The locally released IL-17A acts on its receptors on epithelial and other stromal cells to induce the expression and release of chemokines that attract neutrophils. The release of granular contents from neutrophils causes tissue damage, resulting in intestinal IR injury. Accordingly, blockade of the IL-23/IL-17A axis may provide a powerful therapeutic strategy to attenuate intestinal IR injury.
Supplementary Material
Acknowledgments
We thank Stella Kourembanas, MD, Division of Newborn Medicine, Children’s Hospital, Boston, MA and Paul H. Lerou, MD, Division of Newborn Medicine, Massachusetts General Hospital, Boston, MA, for support, and Drs. Rene de Waal Malefyt and J. Kolls for providing il23r−/− and Il7a−/− mice.
Abbreviations used in this manuscript
- IR
ischemia-reperfusion
- ILC
innate lymphoid cells
- B6
C57BL/6J
- WT
wild type
- B6.lpr
B6.MRLTnfrsf6lpr
- SMA
superior mesenteric artery
- HPF
high power field
- qRT-PCR
quantitative RT-PCR
- CycloA
Cyclophilin A
- BM
bone marrow
Footnotes
This work was supported by U.S. Department of the Army Medical Research and Material Command Grant W81XWH-12-1-0526 and by NIH T32 HD-007466 (SK).
References
- 1.Kong SE, Blennerhassett LR, Heel KA, McCauley RD, Hall JC. Ischaemia-reperfusion injury to the intestine. Aust N Z J Surg. 1998;68:554–561. doi: 10.1111/j.1445-2197.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 2.Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery. 1993;114:489–490. [PubMed] [Google Scholar]
- 3.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 4.Shandall AA, Williams GT, Hallett MB, Young HL. Colonic healing: a role for polymorphonuclear leucocytes and oxygen radical production. Br J Surg. 1986;73:225–228. doi: 10.1002/bjs.1800730325. [DOI] [PubMed] [Google Scholar]
- 5.Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009;249:889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 8.Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978;61:661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- 10.Lapchak PH, Kannan L, Ioannou A, Rani P, Karian P, Dalle Lucca JJ, Tsokos GC. Platelets orchestrate remote tissue damage after mesenteric ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2012;302:G888–897. doi: 10.1152/ajpgi.00499.2011. [DOI] [PubMed] [Google Scholar]
- 11.Pamuk ON, Lapchak PH, Rani P, Pine P, Dalle Lucca JJ, Tsokos GC. Spleen tyrosine kinase inhibition prevents tissue damage after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2010;299:G391–399. doi: 10.1152/ajpgi.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Crispin JC, Tedder TF, Dalle Lucca J, Tsokos GC. B cells contribute to ischemia/reperfusion-mediated tissue injury. Journal of autoimmunity. 2009;32:195–200. doi: 10.1016/j.jaut.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgerton C, Crispin JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang YT, Tsokos GC. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeremenko N, Paramarta JE, Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol. 2014;26:361–370. doi: 10.1097/BOR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends in immunology. 2013;34:151–154. doi: 10.1016/j.it.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nature immunology. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 19.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. The Journal of experimental medicine. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 21.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM, Collins M, Dunussi-Joannopoulos K, Chatterjee-Kishore M, Carreno BM. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 22.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44:183–193. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 24.Halwani R, Al-Muhsen S, Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest. 2013;143:494–501. doi: 10.1378/chest.12-0598. [DOI] [PubMed] [Google Scholar]
- 25.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25:736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 28.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunological reviews. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 31.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 32.Du J, Shen X, Zhao Y, Hu X, Sun B, Guan W, Li S, Zhao Y. Wip1-deficient neutrophils significantly promote intestinal ischemia/reperfusion injury in mice. Current molecular medicine. 2015;15:100–108. doi: 10.2174/1566524015666150114122929. [DOI] [PubMed] [Google Scholar]
- 33.Lee HT, Kim M, Kim JY, Brown KM, Ham A, D’Agati VD, Mori-Akiyama Y. Critical role of interleukin-17A in murine intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G12–25. doi: 10.1152/ajpgi.00201.2012. [DOI] [PubMed] [Google Scholar]
- 34.Barry SP, Ounzain S, McCormick J, Scarabelli TM, Chen-Scarabelli C, Saravolatz LI, Faggian G, Mazzucco A, Suzuki H, Thiemermann C, Knight RA, Latchman DS, Stephanou A. Enhanced IL-17 signalling following myocardial ischaemia/reperfusion injury. Int J Cardiol. 2013;163:326–334. doi: 10.1016/j.ijcard.2011.08.849. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, Yuan J, Jevallee H, Wei F, Shi GP, Cheng X. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–429. doi: 10.1016/j.jacc.2011.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng M, Li G, Qian X, Fan Y, Huang X, Zhang F, Lu L. IL-17A-producing NK cells were implicated in liver injury induced by ischemia and reperfusion. Int Immunopharmacol. 2012;13:135–140. doi: 10.1016/j.intimp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Kono H, Fujii H, Ogiku M, Hosomura N, Amemiya H, Tsuchiya M, Hara M. Role of IL-17A in neutrophil recruitment and hepatic injury after warm ischemia-reperfusion mice. J Immunol. 2011;187:4818–4825. doi: 10.4049/jimmunol.1100490. [DOI] [PubMed] [Google Scholar]
- 38.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue L, Xie K, Han X, Yang Z, Qiu J, Zhao Z, Bao T. Detrimental functions of IL-17A in renal ischemia-reperfusion injury in mice. J Surg Res. 2011;171:266–274. doi: 10.1016/j.jss.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 42.Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Holscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, Magnus T. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120:3793–3802. doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- 43.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science (New York, NY) 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Proteomics. Tissue-based map of the human proteome. Science (New York, NY) 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 45.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 46.Hoeve MA, Savage ND, de Boer T, Langenberg DM, de Waal Malefyt R, Ottenhoff TH, Verreck FA. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol. 2006;36:661–670. doi: 10.1002/eji.200535239. [DOI] [PubMed] [Google Scholar]
- 47.He R, Kim HY, Yoon J, Oyoshi MK, MacGinnitie A, Goya S, Freyschmidt EJ, Bryce P, McKenzie AN, Umetsu DT, Oettgen HC, Geha RS. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. The Journal of allergy and clinical immunology. 2009;124:761–770.e761. doi: 10.1016/j.jaci.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1640–1651. doi: 10.1096/fj.11-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleming SD, Monestier M, Tsokos GC. Accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. J Immunol. 2004;173:4230–4235. doi: 10.4049/jimmunol.173.6.4230. [DOI] [PubMed] [Google Scholar]
- 50.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 52.Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmunity reviews. 2006;5:89–92. doi: 10.1016/j.autrev.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshiya K, Lapchak PH, Thai TH, Kannan L, Rani P, Dalle Lucca JJ, Tsokos GC. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1020–1030. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Molecular immunology. 2008;45:4036–4039. doi: 10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142:24–31. doi: 10.1111/imm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buonocore S, Ahern PP, Uhlig Ivanov HH, II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology. 1992;103:807–812. doi: 10.1016/0016-5085(92)90010-v. [DOI] [PubMed] [Google Scholar]
- 61.Kannan L, Kis-Toth K, Yoshiya K, Thai TH, Sehrawat S, Mayadas TN, Dalle Lucca JJ, Tsokos GC. R-spondin3 prevents mesenteric ischemia/reperfusion-induced tissue damage by tightening endothelium and preventing vascular leakage. Proc Natl Acad Sci U S A. 2013;110:14348–14353. doi: 10.1073/pnas.1309393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of interleukin-1alpha administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg. 2003;237:101–109. doi: 10.1097/00000658-200301000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal immunology. 2008;1:339–349. doi: 10.1038/mi.2008.28. [DOI] [PubMed] [Google Scholar]
- 64.Gelderblom M, Arunachalam P, Magnus T. gammadelta T cells as early sensors of tissue damage and mediators of secondary neurodegeneration. Frontiers in cellular neuroscience. 2014;8:368. doi: 10.3389/fncel.2014.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Ioannou A, Kannan L, Tsokos GC. Platelets, complement and tissue inflammation. Autoimmunity. 2013;46:1–5. doi: 10.3109/08916934.2012.722144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


