Abstract
Many organizations interested in renewable, domestic energy have switched from petroleum diesel to biodiesel blends for use in transportation and heavy-duty equipment. Although considerable evidence exists on the negative health effects of petroleum diesel exhaust exposures in occupational settings, there has been little research examining biodiesel exposures. Working collaboratively with a local municipality, concentrations of particulate matter (PM) and other air toxics were measured at a recycling facility in southwestern New Hampshire while heavy equipment operated first on petroleum diesel and then on a B20 blend (20% soy-based biodiesel/80% petroleum diesel). This pilot study used a combination of established industrial hygiene and environmental air monitoring methods to estimate occupational exposure profiles to PM and air toxics from combustion of petroleum diesel and biodiesel. Results indicate that B20 use dramatically reduces work area respirable particle, PM2.5 (PM ≤2.5 µm in aerodynamic diameter), and formaldehyde levels compared with petroleum diesel. Some volatile organic compound concentrations were higher for petroleum diesel and others were higher for the B20 blend. Overall, this study suggests that biodiesel blends reduce worker exposure to and health risk from petroleum diesel exhaust, but additional exposure research is recommended.
INTRODUCTION
Diesel exhaust emissions are ubiquitous, and their impact on human health is of growing concern. Diesel exhaust itself is a complex mixture of hundreds of components in particulate and gaseous forms. Specific exhaust components vary based on fuel source, engine type, engine load, level of equipment maintenance, and other factors, but many components are known carcinogens or are otherwise toxic. Air toxics from on-road and nonroad diesel engines include benzene, 1,3-butadiene, and formaldehyde. Soot from diesel exhaust is typically less than 2.5 µm in diameter, which is respirable into the deeper parts of the lung. Particulate matter (PM) at this size is associated with numerous negative health effects including but not limited to increased mortality, direct lung injury (i.e., increased inflammation), cardiovascular effects (i.e., increased risk of arrhythmia in people with heart disease), and other organ effects.1,2 Although diesel exhaust has been studied extensively for over 20 yr, the components that are most hazardous to human health and the quantities at which they cause harm are still not fully understood.3,4
Many researchers and regulatory agencies believe that diesel exhaust is a “potential occupational carcinogen” and “likely to be carcinogenic to humans by inhalation” from environmental exposures.5,6 In addition, diesel exhaust has been shown to cause pulmonary inflammation and histopathology, and may contribute to allergic responses and asthma.6–8 The incidence of asthma has more than doubled from 1978 to 1998, affecting over 17 million people and highlighting concerns about possible associations between asthma and diesel exhaust.9 Environmental policy-makers have highlighted the association between diesel and cardiopulmonary health risk at the lower exposure levels typically experienced by the public, but occupational exposure to diesel exhaust may present even greater risk. A detailed review of workplace exposure assessment data for various jobs (such as mine workers, truck drivers, and forklift operators) determined that occupational exposures to diesel particulate matter (DPM) were orders of magnitude higher than ambient exposures, and a cohort study of railroad workers with occupational exposure to diesel exhaust indicated elevated lung cancer mortality.10,11 A more recent survey of particulate exposure in the U.S. trucking industry also found significant differences in exposure intensity of PM2.5 (PM ≤2.5 µm in aerodynamic diameter), elemental carbon, and organic carbon for work locations and jobs relative to background concentrations at trucking terminal sites.12
The only existing U.S. occupational exposure limit for workers exposed to diesel exhaust is for mine workers, as regulated by the Mine Safety and Health Administration (MSHA). MSHA regulates DPM with a permissible exposure limit of 160 µm/m3 (measured as total carbon). Two major technical obstacles to determining what constitutes a harmful above-ground diesel dose are limited measurements of existing human exposures and difficulty developing techniques to identify a unique signature that distinguishes diesel exhaust from background air pollution.3 Research efforts are ongoing, but the quantifiable level of risk posed by diesel exhaust will likely remain unresolved for some time.
Current regulations require enhanced engine and exhaust technology for new (2007 and newer) on-road heavy-duty diesel engines.13 This approach may be slow to change overall total emissions because diesel engines are extremely durable. Many large on-road engines can last 1 million miles before their first rebuild and can be rebuilt multiple times.6 Even if total diesel emissions decrease as a result of enhanced engine and exhaust technology, the effects of new technology and fuels on the chemical and physical characteristics of diesel exhaust are unknown; thus, a reduction of emissions may not mean a reduction in overall health risk.14 Nonroad engines, such as those powering the construction-type equipment in this study, remain significant sources of air pollution. By 2006, the total amount of PM2.5 emitted by all mobile sources had decreased slightly, but the percent contribution of nonroad engines to the total PM2.5 emissions inventory increased to 69%, compared with 64% in 2001.15
Biodiesel is one alternative fuel that is becoming increasingly popular for use in diesel engines. Biodiesel is biodegradable, considerably less toxic to aquatic organisms (in the event of spills) than petroleum diesel, has a high flash point, and is considered sustainable because it can be generated from renewable sources.16 According to the National Biodiesel Board, over 500 fleets in the United States are using the plant-based fuel.17 A 2009 study performed by the U.S. Department of Agriculture determined that soy-based biodiesel yields 4.5 units of fuel product energy for every unit of fossil fuel energy required to produce it.18 By comparison, petroleum diesel yields 0.83 units of fuel product energy per unit of fossil fuel energy consumed.19 Furthermore, the use of soybean-based 100% biodiesel in an urban bus reduced net carbon dioxide emissions by 78%.19 Hill and colleagues20 determined that soy-based biodiesel provides 93% more energy than the fossil fuel energy invested in its production and reduces greenhouse gases by 41% compared with diesel. However, many argue that land-use changes may reduce some of the benefits of biodiesel. Fargione et al.21 assert that land clearing associated with conversion to biofuels (including ethanol and biodiesel) will create a “biofuels carbon debt” by releasing up to 420 times more carbon dioxide than the annual greenhouse gas reductions resulting from displacement of fossil fuels.
Nevertheless, biodiesel is the only alternative fuel that has completed the requirements of the U.S. Environmental Protection Agency (EPA) Clean Air Act Tier I and II testing for health effects.22 As a result of its environmental benefits, many organizations, including Keene State College and a municipal fleet in Keene, NH, have switched to biodiesel blends for on-road and nonroad applications.
The objective of this study was to use a combination of established industrial hygiene and environmental air monitoring methods to evaluate diesel and biodiesel blend exposure profiles in an occupational setting utilizing nonroad diesel equipment. Although numerous studies have evaluated tailpipe emissions from engines fueled by biodiesel blends and operated under controlled conditions, research is needed on biodiesel’s impact on real-world exposures to PM and other air pollutants. The rationale for this study is to better understand if biodiesel emission reductions observed in engine dynamometer studies will translate to reduced exposures in the workplace and near-field environment under real-world operating conditions. The same facility equipment and work activities were monitored during petroleum diesel and biodiesel blend (a 20/80% biodiesel/diesel blend, hereafter “B20”) use at the Keene Recycling Center (KRC) facility in August 2004. Exposures were measured in the equipment cabin and at the perimeter of the work area. Monitoring was performed by a team of researchers from Keene State College, assisted by Keene State College undergraduate students working collaboratively with employees from the municipal facility. The city of Keene has used B20 in other fleet vehicles since 2002 and was particularly interested in quantitative evidence that supports anecdotal employee reports of improved health as a result of biodiesel use.
MATERIALS AND METHODS
Site Description
KRC is a materials recovery facility that serves the city of Keene and surrounding towns in the southwest corner of New Hampshire. Similar to public works facilities in many rural areas, this facility uses forklifts, front loaders, skid steers, and dump trucks to move materials throughout the site. There were four primary pieces of equipment in use at the time of the study: a large front-end loader (2001 John Deere model 624H, 160 hp), a small front-end loader (1994 JCB model 409, 67 hp), a skid steer (2001 New Holland model LS190, 33 hp), and a 1994 TCM propane-powered forklift. This equipment belongs to the city of Keene fleet and as such undergoes regularly scheduled maintenance as part of a documented, comprehensive, preventative maintenance program. The same employees operated the same pieces of equipment during the diesel and B20 sampling days.
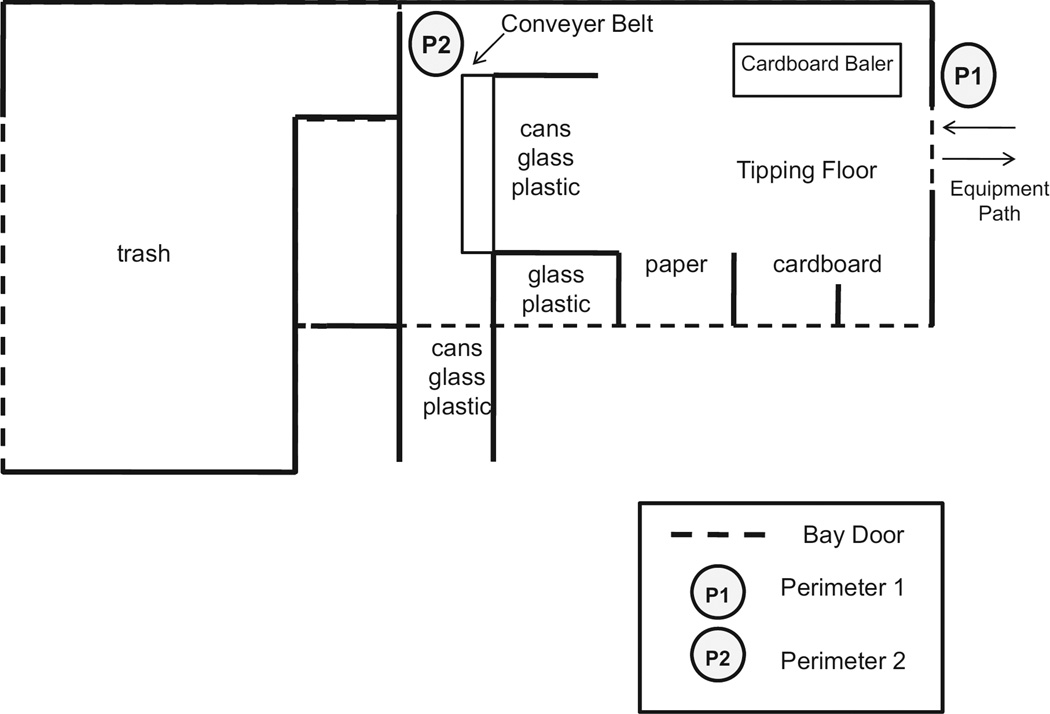
The facility consists of a single large building with one large bay door on the main floor area and five smaller bay doors on the side of the building (Figure 1). This main building has no mechanical ventilation, so airflow occurs by natural ventilation only; the manually operated bay doors were two-thirds open on both sampling days. Air consistently flows across the main floor area from the large bay door to the smaller side doors. Trucks from other towns and local trash hauling companies dump cardboard and paper waste on the main floor. Town residents (mostly in light-duty gasoline-powered vehicles) drop off newspapers, aluminum cans, or plastic containers at the side bays. Employees stand alongside a conveyor belt system inside of the building to separate nonrecyclables from the process stream. The small front loader works on the main floor moving cardboard to a second conveyor belt that leads to a baler located beneath the building. The skid steer moves paper and other materials as needed inside of the building. The large front loader typically works on the metals pile in an outdoor location but also moves paper from the main floor into an open trailer outside.
Figure 1.
Diagram of the municipal facility building layout and stationary sampling locations.
Study Design
The sampling strategy presented here regarded the equipment as pollutant sources, with “in-cabin” measurements assumed to represent “worst-case” employee exposures. Exposures were evaluated inside of the cabins of three pieces of equipment (large front loader, small front loader, and skid steer) for one work shift per fuel type. The small front loader and skid steer cabins were open to the air. Although the large front loader was equipped with air conditioning, the cabin was open to the air during the sampling days. The main sources of PM2.5 exposure at the site were the small front loader, the large front loader, and outside diesel vehicle traffic. Background levels of PM were assumed to be negligible on the basis of the rural character of the site. Air monitoring instrumentation was placed in the same locations for each fuel type—either in the vehicle cabin (sampling media placed within the breathing zone) or within 25 ft of the main tipping floor area. The only exception was perimeter 2 (see Figure 1), located approximately 50 ft from the tipping area in the conveyer belt work area. Perimeter 2 acted as a surrogate for general employee exposure because most employees worked on the conveyor belt or inside vehicle cabins. Sampling occurred during one workday while equipment operated on commercially purchased diesel fuel (500 parts per million by weight [ppmw] maximum allowable sulfur content), and the process was repeated exactly 2 weeks later after the same three pieces of equipment were filled with a B20 blend of soy-based biodiesel (20%) and petroleum (80%) (500 ppmw maximum allowable sulfur content). The biodiesel used in the B20 blend met the American Society for Testing and Materials D 6751 quality standard. However, detailed fuel analysis was not performed on both fuels because of the pilot scale of the study; exact sulfur content and aromatic content are unknown. All monitored employees were nonsmokers in consideration of the potential confounding effects of cigarette smoking found by other researchers.23
Operations and vehicle traffic at the KRC were relatively consistent on a week-to-week basis. However, specific tasks varied day to day, so students recorded activity and vehicle counts for each sampling day. Time-activity logs documented equipment activity, proximity to other equipment, and an assessment of equipment load/duty cycle at 20-min intervals. Weather data were also recorded.
For petroleum diesel and B20 sampling days, researchers and students performed equipment calibrations before and after sampling, positioned the equipment in the same locations, and regularly performed operational checks on all of the equipment in use. Preparation and calibration of the equipment is a precision-based process that was used as an instructional activity for the students, resulting in a reduced (6 vs. 8 hr) sampling period. Despite this shorter sampling time, exposures were still measured over most of the work shift. At the conclusion of field sampling, samples were shipped to an accredited external laboratory for analysis, with the exception of respirable particle samples (defined as a 50% cut point for collection of particles ≤4 µm in aerodynamic diameter). Respirable particle filters were analyzed gravimetrically in the industrial hygiene laboratory at Keene State College.
After the conclusion of petroleum diesel monitoring, the facility’s main fuel supply was drained. The tank was then filled with a B20 biodiesel blend. The large and small front-end loaders were operating on their third tank of B20 fuel mixture by the time biodiesel monitoring occurred 2 weeks later. However, the skid steer was used less frequently and was still operating on its first tank of B20 during biodiesel monitoring.
Similar field preparation and calibration steps were taken on the biodiesel operation day (day 2) as during the diesel operation day (day 1). Work activities at the municipal facility were similar on both sampling days. Important differences include the small front loader being out of service until approximately 10:45 a.m. on day 1 and the work activities conducted during the afternoon of day 1 being performed during the morning of day 2. However, the operators and length of time devoted to the tasks were the same for both days.
Sampling and Analytical Methods
PM2.5 was measured via real-time monitoring, respirable particles via integrated sampling, carbonyl compounds (including acetaldehyde and formaldehyde) via EPA method TO-11A, and volatile organic compounds (VOCs) via EPA method TO-15.
Real-time PM2.5 sampling units (Haz-Dust EPAM-5000) were placed at perimeter 1 and perimeter 2 to measure work area PM levels as they occurred throughout the day. The Haz-Dust EPAM-5000 is a high-sensitivity, real-time, light scattering monitor that measures particles 0.1–100 µm in diameter with a precision of ±0.003 mg/m3 (3 µg/m3) and accuracy of ±10% to National Institute for Occupational Safety and Health (NIOSH) 0600 using Arizona Road Dust. For this study, the EPAM-5000 sampled PM2.5 and took average measurements once every 60 sec.
Respirable particle sampling (integrated time-weighted average) consisted of using an SKC aluminum cyclone (4 µm cut point, as specified by NIOSH 0600) with cassette train (with preweighed polytetrafluoroethylene filter) and appropriately calibrated Gilian personal pumps sampling at a flow rate of 2.5 L/min. Filters were analyzed gravimetrically in a temperature- and humidity-controlled environment. Samples for the EPA TO-11A method for carbonyl compounds (including formaldehyde and acetaldehyde) were collected on SKC sorbent tubes (SKC 226-119) coated with 2,4-dinitrophenylhydrazine. These samples were collected using appropriately calibrated Gilian low-flow air sampling pumps at a flow rate of 200 ml/min. Samples were kept at 4 °C and shipped overnight to an external accredited laboratory where they were analyzed by high-performance liquid chromatography. The EPA TO-15 method for VOCs consisted of using precleaned and evacuated stainless steel Summa canisters with 8-hr orifices (to collect daily integrated average samples) or without orifices (for grab samples). These canisters were sent to an external accredited laboratory for gas chromatography/mass spectrometry analysis for a wide array of VOCs, including air toxics such as benzene and 1,3-butadiene. All of the above samples measured average exposures over the workshift (from ~8:00 a.m. to 3:00 p.m.). Additional “grab” or instantaneous EPA TO-15 samples were collected using Summa canisters (without orifices) during a cold start of the skid steer and in the afternoon near perimeter 2.
RESULTS
Site Data
Activity logs indicated that activity patterns were similar for both days, except as described below. Activity consisted mainly of stop-and-go heavy load cycles to move materials. This type of higher load activity pattern may generate higher levels of PM than the transient test cycle conditions in engine dynamometer studies.
Counts of outside diesel and gasoline vehicles were consistently higher at all site locations during day 2 (B20); total vehicle count for day 2 (B20) was 123 compared with 67 during day 1 (diesel). Temperature was slightly higher on day 1 (66 vs. 62 °F, average) as was wind speed (5.9 vs. 3.6 mph, average). Because the main building where monitoring was performed relies solely on natural ventilation, air exchange rates (and subsequent dilution of emissions) were likely higher during day 1 (diesel).
PM
Haz-Dust EPAM-5000 units measured real-time PM2.5 concentrations at perimeter 1 and perimeter 2 during both sampling days. However, data from the perimeter 2 Haz-Dust EPAM-5000 were lost because of a software malfunction.
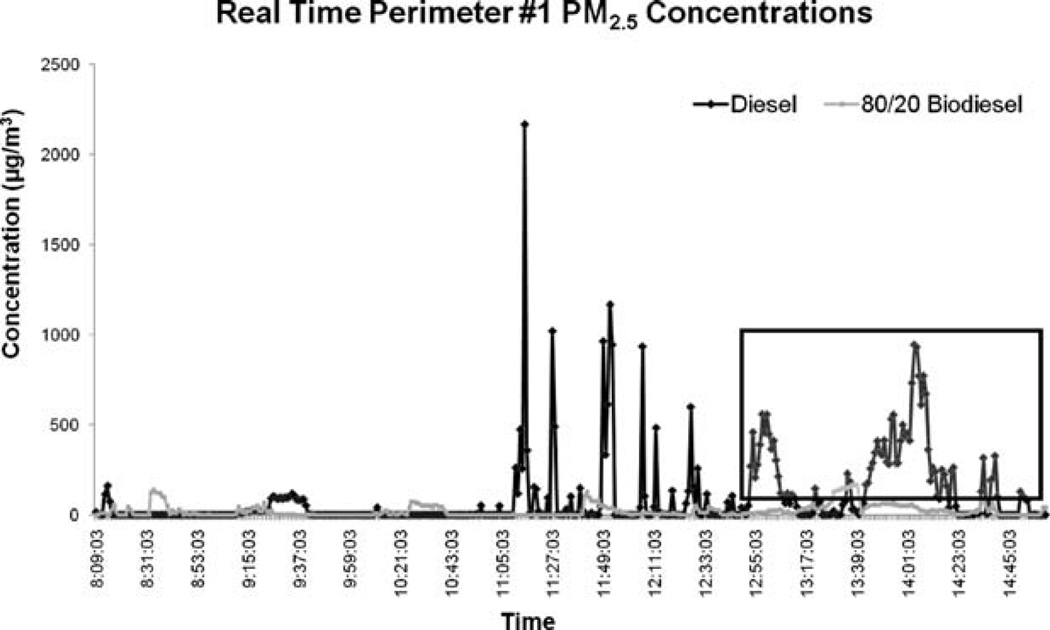
Beginning at approximately 11:00 a.m. on day 1, a series of real-time PM2.5 spikes occurred at perimeter 1 (Figure 2). The series of PM2.5 spikes after 11:00 a.m. were not attributable to any outside traffic; activity from 11:00 a.m. to 12:00 p.m. was associated with idling or movement of the KRC’s small front loader. The highest PM2.5 level during the study (2165 µg/m3) was recorded at this time. The highlighted box in Figure 2 (starting at ~12:50 p.m.) corresponds to a sustained work activity in which the large front loader collected paper from inside of the building and moved it to an open box trailer outside of the building. The perimeter 1 sampling location was effectively isolated from outside truck traffic and other site equipment for 2 hr by this back-and-forth movement of the large front-end loader. The large front loader was thus the main source of diesel emissions at perimeter 1 for this period. Area real-time PM2.5 concentrations measured during this time period reached a high of 943 µg/m3 when the large front loader used petroleum diesel (day 1) compared with a high of 140 µg/m3 during the same 2-hr work activity while using B20 (day 2).
Figure 2.
Real-time plot of PM2.5 concentrations at the lower level entrance (perimeter 1) of the municipal facility.
Real-time PM2.5 concentrations measured during the biodiesel operating day were relatively stable. Although outside vehicle traffic (diesel and gas vehicles) was greater on the biodiesel sampling day, outside traffic did not seem to impact the measurement of area PM2.5 at perimeter 1. When comparing the time-weighted average EPAM-5000 PM2.5 levels, the petroleum diesel (day 1) concentration was 95 µg/m3 over the work shift compared with a B20 (day 2) concentration of 34 µg/m3 (see Table 1).
Table 1.
Integrated particulate mass concentrations for environmental and occupational exposures to diesel and B20.
| Diesel PM2.5 |
B20 PM2.5 |
Diesel Respirable PM |
B20 Respirable PM |
|
|---|---|---|---|---|
| Perimeter 1: environmental exposure | 95 | 34 | 121 | NA |
| Front-end loader: occupational exposure | NA | NA | 5332 | 947 |
Notes: All data reflect time-weighted average concentrations in µg/m3. NA = not applicable.
Results for respirable particle (time-weighted average) samples are summarized in Table 1. These data suggest that exposure to PM2.5 or respirable particle mass in occupationally exposed individuals is approximately 3–5 times higher during petroleum diesel operations than during B20 operations. In addition, occupational exposures were notably higher than environmental exposures. These observations are consistent with results observed from comparisons between occupationally and environmentally exposed individuals in past petroleum diesel assessments.24
Air Toxics
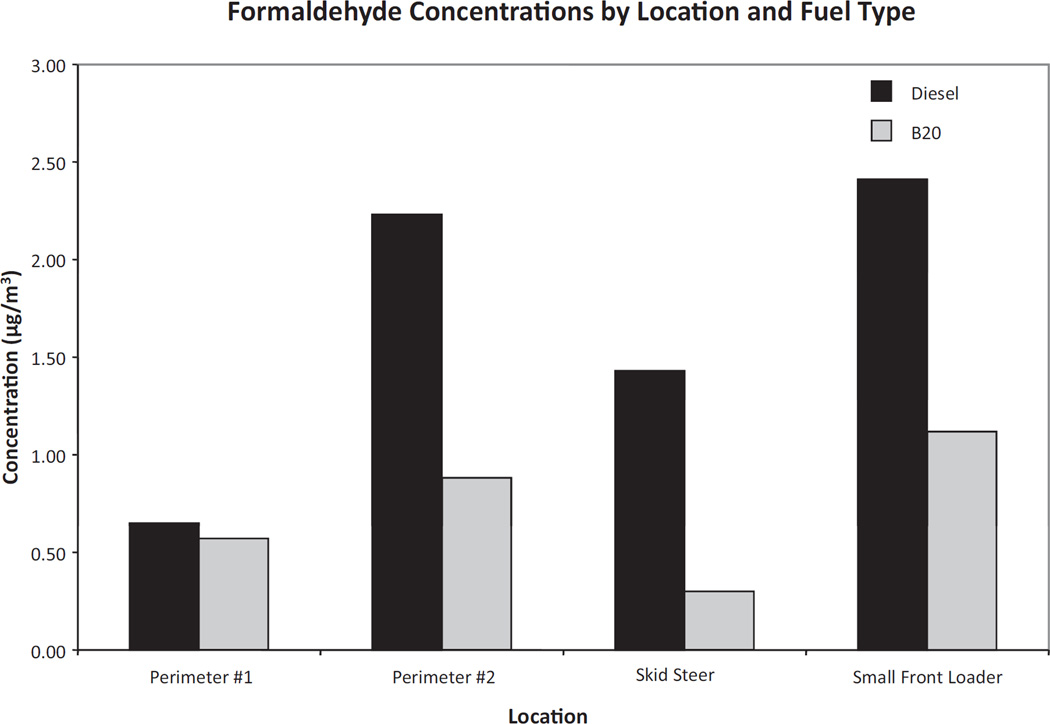
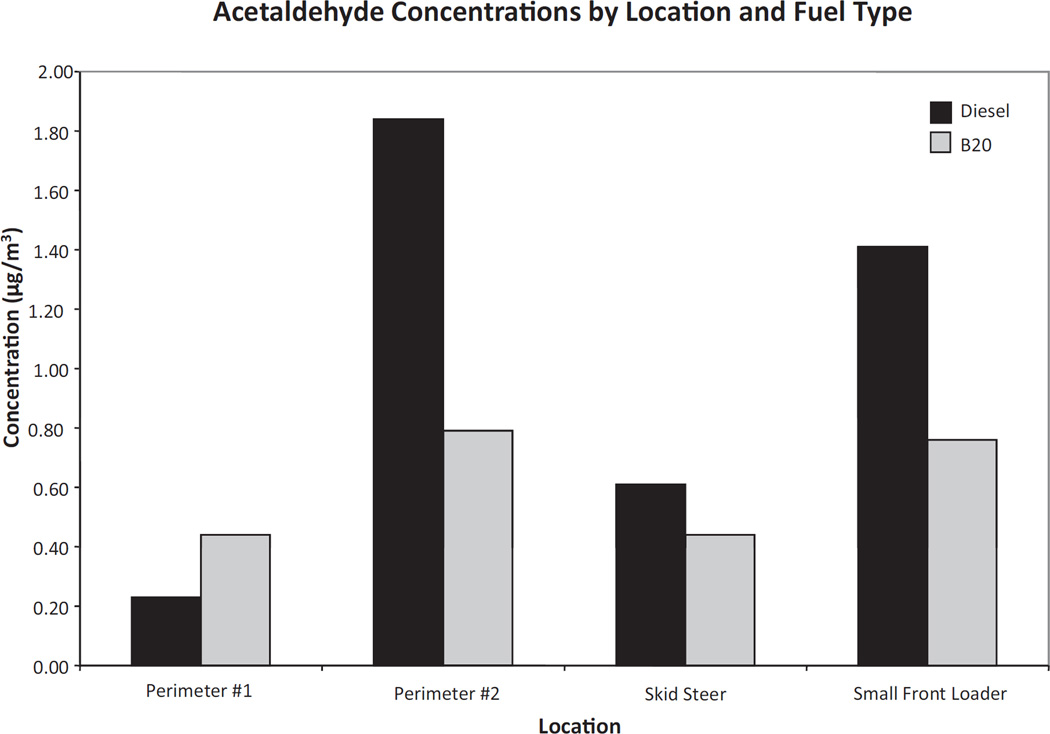
Formaldehyde and acetaldehyde levels were lower inside all of the equipment cabins and at perimeter 2 during B20 operations, although slightly higher acetaldehyde levels were recorded at perimeter 1 on day 2 (Figures 3 and 4). The most notable reduction in air toxics after switching to B20 was measured at perimeter 2, which experienced a 57% reduction (from 1.84 to 0.79 µg/m3) in acetaldehyde concentration and a 61% reduction (from 2.23 to 0.88 µg/m3) in formaldehyde concentration (Figures 3 and 4). Other carbonyl compounds measured during both days were below or very close to the limit of detection and therefore their contribution was considered marginal.
Figure 3.
Formaldehyde concentrations at the municipal facility for day 1 (diesel) and day 2 (B20).
Figure 4.
Acetaldehyde concentrations at the municipal facility for day 1 (diesel) and day 2 (B20).
VOCs measured via the TO-15 method did not reveal any conclusive patterns. The daily time-weighted average concentrations for benzene and toluene at perimeter 2 were less than the analytical detection limits during day 1 (diesel) but were slightly above the limits of detection on day 2 (B20). The values for these contaminants were determined to be 7.2 parts per billion (ppb) for benzene and 8.7 ppb for toluene at perimeter 2 on day 2. At perimeter 1, only acetone was detected on both days, with 79.7 ppb measured on day 1 and 47.6 ppb measured on day 2. A TO-15 grab sample taken in the exhaust plume during the starting of the skid steer resulted in higher day 2 levels of benzene (112 ppb for day 1 vs. 483 ppb for day 2), toluene (53.5 ppb for day 1 vs. 189 ppb for day 2), and 1,3-butadiene (75.4 ppb for day 1 vs. 137 ppb for day 2). A TO-15 grab sample of ambient air taken at perimeter 2 on both afternoons revealed higher benzene and toluene concentrations on day 1 (6.2 and 8.5 ppb for benzene and toluene, respectively) than on day 2 when they were not detected. All of these ambient grab sample concentrations are at or near the limit of detection for this analytical method.
DISCUSSION
Unlike transient work areas such as construction sites, the KRC is a stable and permanent source of diesel emissions in the local environment of southwest New Hampshire. As such, it has the potential to produce long-term occupational exposure and prolonged environmental impact, making it an interesting case study for examining the relationship between occupational and environmental risk. Diesel exhaust occupational exposure levels are typically much higher than public exposure levels, as indicated by exposure assessment data collected for the trucking, railroad, and mining industries.10 Although employees working in close proximity to diesel emissions are expected to experience higher exposure levels, it is important to remember that these same sources also contribute to local ambient air pollution outside of the workplace. Cleaner burning fuels that can be implemented today—without additional engine modification or regulation—may offer more immediate reductions in the environmental and occupational health risks associated with exposure to diesel exhaust.
Several studies have shown that burning biodiesel in heavy-duty diesel engines results in reduced tailpipe emissions of PM and total hydrocarbons.25–29 It was investigated whether these tailpipe emission reductions would translate to reduced exposures in the workplace. In this comparison of diesel and biodiesel exposures, the difference in real-time PM2.5 and in-cabin integrated respirable particles after switching fuels was dramatic.
Examination of the highlighted box in the PM2.5 data plot in Figure 2 and the activity logs serves as a focal point for this comparison. On the afternoon of day 1, the large front loader worked consistently near perimeter 1 for almost 2 hr. This work activity required entering and exiting the main floor of the building and loading paper into a box trailer for off-site processing. This same activity was repeated the morning of day 2 for a similar time period. When the large front loader was fueled by petroleum diesel on day 1, the perimeter 1 area sample for PM2.5 reached a high of 943 µg/m3. In contrast, when the same activity was performed by the large front loader fueled by a B20 blend on day 2, the peak PM2.5 level measured was 140 µg/m3. Although immediate background PM2.5 levels were not measured for this study, regional ambient levels of PM2.5 were examined for comparative purposes. Available PM2.5 measurements from the New Hampshire state air monitoring system were reported as 21.76 µg/m3 in Manchester, NH (40 mi away) on day 1 and 5.87 µg/m3 in downtown Keene, NH (7 mi away) the day after day 2. There were no known significant PM2.5 local or regional events (i.e., nearby boiler operations and/or forest fires) on or near the municipal facility on either sampling day. Wind speeds were comparable for both sampling days. Although it appears reasonable to conclude that background PM2.5 levels were not a significant contributor to the site levels observed in this study, in the future direct background measurements of PM2.5 are recommended.
Integrating real-time data for this 2-hr work activity results in a diesel PM2.5 concentration of 131 µg/m3 compared with a B20 (Day 2) concentration of 19 µg/m3. Therefore, during a similar work activity with the same equipment, engine load, operator, and duty cycle, real-time work area PM2.5 levels were reduced by up to 85% during B20 use. Integrating the EPAM data over the entire work shift results in a diesel PM2.5 concentration of 95 µg/m3 (day 1) compared with a B20 (day 2) concentration of 34 µg/m3 (see Table 1)—a 61% reduction. This finding is notable because tailpipe emissions studies only predict an average 10% reduction in PM from B20 use.25 This prediction is based on a review of biodiesel use in on-road engines; EPA notes that nonroad biodiesel data are especially lacking.25
Finally, although limited respirable particle (time-weighted average) samples were collected in this study, respirable particles were measured in the large front loader cabin during the work shift on day 1 and day 2. The integrated respirable particle average on day 2 (947 µg/m3) was 82% lower than the result for day 1 (5332 µg/m3), consistent with the dramatic reduction noted in real-time PM2.5 area levels after switching to B20. Further examination of PM reduction trends is recommended and should include on-site meteorological assessment and direct comparison with local background levels of PM2.5 and respirable particles.
Fuel chemistry, especially combustion in nonroad engines, may be an important explanatory factor behind the PM reductions documented in this study. Heavier engine loads or more fuel-rich conditions typically lead to increased PM emissions. Unlike petroleum diesel, biodiesel does not contain aromatics or sulfur but is composed of methyl esters, which have oxygen embedded within the hydrocarbon chain. This increased oxygen content may enhance combustion, thereby reducing soot formation, which when combined with the lack of sulfur and aromatics may result in fewer particulates overall.27 Other researchers have concluded that the higher oxygen content available from oxygenates such as biodiesel may also lead to obvious reductions in smoke and PM emissions during nonroad engine, heavy-load operations when more fuel is burned in the diffusion mode.30 In their study of the effects of oxygenates on soot formation processes in diesel engines, Mueller et al.31 determined that selection of an appropriate oxygenate (with an optimal molecular structure for reducing soot precursors) can be especially effective at reducing in-cylinder soot formation. Therefore, the increased oxygen content in biodiesel may have effectively increased air/fuel ratios in the fuel-rich zones during heavy engine load stop-and-go conditions such as those seen during this study, leading to reduced PM emissions and exposures.
Sulfur content differences between the fuels may also have influenced the PM results presented here. Although the petroleum diesel fuel and the diesel fraction of the B20 blend were commercially purchased as 500 ppmw maximum allowable sulfur content, exact sulfur levels are unknown. Variation in sulfur content between fuels could have contributed to the reduction in PM, complicating interpretation of the impact of the biodiesel fraction itself. Future research should determine specific fuel properties.
Reviewing the historical data from the New Hampshire PM2.5 ambient air monitoring network for Manchester and Keene, NH, the authors are reasonably confident that local equipment is the primary source of the high PM2.5 levels measured in this pilot study. The EPA National Ambient Air Quality Standard for PM2.5 is 35 µg/m3 (over a 24-hr period). In its Health Assessment Document for Diesel Engine Exhaust, EPA established a nonbinding exposure standard or reference concentration of 5 µg/m3 for DPM.6 The American Conference of Governmental Industrial Hygienists (ACGIH) proposed (although later removed) a threshold limit value exposure of 150 µg/m3 for DPM.32 MSHA has implemented a DPM limit of 160 µg/m3. Because this study was not designed to assess compliance, it cannot be concluded that exposure standard exceedances occurred at the site. However, the levels of PM2.5 measured during the diesel operations on day 1 were orders of magnitude higher than the reference concentration for DPM, even after applying a conservative assumption that DPM contributes between 6 and 36% of the measured PM2.5 for an area.6,25 These data indicate that petroleum diesel operations at the municipal facility could be a relatively important source of DPM exposure for workers and the local environment. Although more data are needed, this study also indicates that B20 may be effective at reducing PM exposure from diesel engine operations.
Biodiesel may also have potential for reducing carbonyl concentrations. Many carbonyl compounds are pronounced respiratory irritants, and some are believed to be carcinogenic. Acetaldehyde and formaldehyde levels are expected to decrease with increasing biodiesel concentrations.25 The pilot results presented here support this trend. Individual inhalation exposure to an average formaldehyde concentration of 0.08 µg/m3 over a lifetime represents a 1 in 1,000,000 excess cancer risk.33 Levels of formaldehyde measured during the study exceeded this threshold for day 1 and day 2, although biodiesel use did result in an overall reduction in total concentration. Additional data are necessary to clarify whether biodiesel presents significant carcinogenic risk reduction benefit when compared with petroleum diesel.
The variation in targeted VOCs is more puzzling. Measurements inside of the building at perimeter 2 indicated no detectable VOC during day 1 (diesel) and slight levels of benzene and toluene during day 2 (B20). A grab sample taken during the afternoon at perimeter 2 indicated low levels of benzene and toluene during day 1 and nondetectable levels during day 2. The skid steer exhaust grab sample showed the most dramatic results, with higher levels of VOCs detected during biodiesel operations. However, it is difficult to draw firm conclusions with such limited data. EPA biodiesel emissions data on the air toxics benzene, toluene, and 1,3-butadiene have also been inconclusive in trend and magnitude.25 Further study is necessary to determine the impact of diesel and biodiesel on targeted VOCs.
CONCLUSIONS
This study indicates that the use of biodiesel in some settings may provide more substantial reductions of occupational and environmental exposures to PM than predicted on the basis of engine dynamometer testing. In addition, the study highlights the need to concurrently examine the impact of diesel nonroad equipment sources on exposures in the workplace and the near-field environment because these sources can contribute to occupational and public health risks. However, although biodiesel may hold promise for reducing exposure to PM and carbonyls, more comprehensive biodiesel exposure data are needed to determine if these reductions are replicable and statistically significant. VOC results were inconclusive on the basis of the data collected and more exposure data are recommended. In addition, emissions of nitrogen oxides from biodiesel remain a concern, especially in ozone nonattainment areas, and deserve scrutiny in future work.25
Since 2007, new on-road engines with cleaner emissions profiles have begun to replace older engines, but cleaner nonroad engines will not be required by law until 2014. Diesel engine durability means that the full replacement of existing engines with cleaner models could take decades. Finally, although health hazards associated with diesel exhaust have been widely examined, the fate of diesel emissions in the atmosphere, the effect of different fuel formulations on emissions, and the impact of diesel on noncancer health effects (e.g., asthma exacerbation) are still relatively unknown.4,34 Biodiesel may offer immediate, nationwide risk reduction opportunities, even as the debate regarding the level of health risk posed by diesel continues.
IMPLICATIONS.
Increasing public health concerns and limited regulatory initiatives for controlling occupational exposures to petroleum diesel emissions have led to increased use of alternative fuels like biodiesel. Although considerable evidence exists on the negative health effects of petroleum diesel exhaust exposures in occupational settings, there has been little research examining the effects of biodiesel exhaust on worker exposure and the local environment. This pilot study provides preliminary characterization of the occupational and environmental exposures resulting from switching to biodiesel, but more research is still needed.
Acknowledgments
The authors thank Steve Russell; Duncan Watson; the city of Keene, NH; and the Keene State College Safety Studies students who directly supported this work. This work was supported through funding provided by the National Institutes of Health (NIH) Centers of Biomedical Research Excellence grant P42 ES07373 and the EPA Science to Achieve Results fellowship grant FP916576. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, NIH, or EPA.
Contributor Information
Nora Traviss, Department of Environmental Studies, Keene State College, Keene, NH.
Brett Amy Thelen, Department of Environmental Studies, Keene State College, Keene, NH.
Jaime Kathryn Ingalls, Department of Safety Studies, Keene State College, Keene, NH.
Melinda Dawn Treadwell, Department of Professional and Graduate Studies, Keene State College, Keene, NH.
REFERENCES
- 1.Lippmann M, Frampton M, Schwartz J, Dockery D, Schlesinger R, Koutrakis P, Froines J, Nel A, Finkelstein J, Godleski J. The U.S. Environmental Protection Agency Particulate Matter Health Effects Research Center Program: a Midcourse Report of Status, Progress, and Plans. Environ. Health Perspect. 2003;111:1074–1092. doi: 10.1289/ehp.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Environmental Protection Agency; [accessed February 2008]. Fact Sheet: Final Revisions to the National Ambient Air Quality Standards for Particle Pollution (Particulate Matter) available at http://www.epa.gov/particles/pdfs/20060921_factsheet.pdf. [Google Scholar]
- 3.Research Directions to Improve Estimates of Human Exposure and Risk from Diesel Exhaust: Report of the Diesel Epidemiology Working Group. Boston, MA: Health Effects Institute; 2002. [Google Scholar]
- 4.Mauderly J. Diesel Emissions: Is More Health Research Needed? Toxicol. Sci. 2001;62:6–9. doi: 10.1093/toxsci/62.1.6. [DOI] [PubMed] [Google Scholar]
- 5.NIOSH Current Intelligence Bulletin 50. Atlanta, GA: Department of Health and Human Services; National Institute of Occupational Safety and Health; 1988. Carcinogenic Effects of Exposure to Diesel Exhaust. Publication No. 88–116. [Google Scholar]
- 6.Health Assessment Document for Diesel Engine Exhaust. Washington, DC: U.S. Environmental Protection Agency; 2002. EPA/600/8-90/057F. [Google Scholar]
- 7.Wade J, Newman L. Diesel Asthma: Reactive Airways Disease following Overexposure to Locomotive Exhaust. J. Occup. Med. 1993;35:149–154. [PubMed] [Google Scholar]
- 8.Mauderly J. Diesel Exhaust. In: Littman M, editor. Environmental Toxicants. 2nd. New York: John Wiley and Sons; 2000. [Google Scholar]
- 9.Asthma Research Strategy. Washington, DC: U.S. Environmental Protection Agency; 2002. EPA 600/R-01/061. [Google Scholar]
- 10.Cantrell BK, Watts WF. Diesel Exhaust Aerosol: Review of Occupational Exposure. Appl. Occup. Environ. Hyg. 1997;12:1019–1027. [Google Scholar]
- 11.Garshick E, Laden F, Hart JE, Rosner B, Smith TJ, Dockery DW, Speizer FE. Lung Cancer in Railroad Workers Exposed to Diesel Exhaust. Environ. Health Perspect. 2004;112:1539–1543. doi: 10.1289/ehp.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TJ, Davis ME, Reaser P, Natkin J, Hart JE, Laden F, Heff A, Garshick E. Overview of Particulate Exposures in the U.S. Trucking Industry. J. Environ. Monit. 2006;8:711–720. doi: 10.1039/b601809b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heavy Duty Engine and Vehicle Standards and Highway Diesel Fuel Sulfur Control Requirements (Final Rule) Fed. Regist. 2001;66(12):5135–5193. [Google Scholar]
- 14.Diesel Exhaust: a Critical Analysis of Emissions, Exposure, and Health Effects: a Special Report of the Institute’s Diesel Working Group. Cambridge, MA: Health Effects Institute; 1995. [Google Scholar]
- 15.U.S. Environmental Protection Agency; [accessed January 2008]. National Emissions Inventory: 1970–2006. available at http://www.epa.gov/ttn/chief/trends/index.html. [Google Scholar]
- 16.Khan N, Warith MA, Luk G. A Comparison of Acute Toxicity of Biodiesel, Biodiesel Blends, and Diesel on Aquatic Organisms. J. Air & Waste Manage. Assoc. 2007;57:286–296. doi: 10.1080/10473289.2007.10465333. [DOI] [PubMed] [Google Scholar]
- 17.More Truckers Hit the Road with American-Made Biodiesel National Biodiesel Board. [accessed November 2005]; available at http://www.nbb.org/resources/pressreleases/fle/20050825_biotrucker.pdf. [Google Scholar]
- 18.Sheehan J, Camobreco V, Duffield J, Graboski M, Shapouri H. Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus. Golden, CO: National Renewable Energy Laboratory; 1998. Final Report; NREL/SR-580-2-4089. [Google Scholar]
- 19.Pradhan A, Shrestha D, McAloon A, Yee W, Haas M, Duffield J, Shapouri H. Energy Life-Cycle Assessment of Soybean Biodiesel. Washington, DC: U.S. Department of Agriculture; 2009. [Google Scholar]
- 20.Hill J, Nelson E, Tilman D, Polasky S, Tiffany D. Environmental, Economic, and Energetic Costs and Benefits of Biodiesel and Ethanol Biofuels. Proc. Natl. Acad. Sci. 2006;103:11206–11210. doi: 10.1073/pnas.0604600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fargione J, Hill J, Tilman D, Polasky S, Hawthorne P. Land Clearing and the Biofuel Carbon Debt. Science. 2008;319:1235–1238. doi: 10.1126/science.1152747. [DOI] [PubMed] [Google Scholar]
- 22.Health Effects Testing; National Biodiesel Board. [accessed February 2010]; http://www.biodiesel.org/pdf_files/fuelfactsheets/HealthEffectsTesting.pdf. [Google Scholar]
- 23.Zaebst DD, Clapp DE, Blade LM, Marlow DA, Steenland K, Hornung RW, Scheutzle D, Butler J. Quantitative Determination of Trucking Industry Workers’ Exposure to Diesel Exhaust Particles. Am. Ind. Hyg. Assoc. J. 1991;52:529–541. doi: 10.1080/15298669191365162. [DOI] [PubMed] [Google Scholar]
- 24.Treadwell MD, Woskie S, Youngs F. Evaluating the Occupational and Environmental Impact of Nonroad Diesel Equipment in the Northeast: Northeast States for Coordinated Air Use Management. [accessed June 2007]; available at http://www.ct.gov/dep/lib/dep/air/diesel/docs/nescaumnonroad.pdf.
- 25.A Comprehensive Analysis of Biodiesel Impacts on Exhaust Emissions. Washington, DC: U.S. Environmental Protection Agency; 2002. EPA-420-P-02-001. [Google Scholar]
- 26.McCormick RL, Williams A, Ireland J, Brimhall M, Hayes RR. Effects of Biodiesel Blends on Vehicle Emissions. Golden, CO: National Renewable Energy Laboratory; 2006. NREL/MP-540-4-0554. [Google Scholar]
- 27.Lapuerta M, Armas O, Rodriguez-Fernandez J. Effect of Biodiesel Fuels on Diesel Engine Emissions. Prog. Energy Combust. Sci. 2007;34:198–223. [Google Scholar]
- 28.Yanowitz J, McCormick R. Effect of Biodiesel Blends on North American Heavy-Duty Diesel Engine Emissions. Eur. J. Lipid Sci. Technol. 2009;111:763–772. [Google Scholar]
- 29.Frey HC, Kim K, Pang S, Rasdorf WJ, Lewis P. Characterization of Real-World Activity, Fuel Use, and Emissions for Selected Motor Graders Fueled with Petroleum Diesel and B20 Biodiesel. J. Air & Waste Manage. Assoc. 2008;58:1274–1287. [PubMed] [Google Scholar]
- 30.Di Y, Cheung CS, Huang Z. Comparison of the Effect of Biodiesel-Diesel and Ethanol-Diesel on the Particulate Emissions of a Direct Injection Diesel Engine. Aerosol Sci. Technol. 2009;43:455–465. [Google Scholar]
- 31.Mueller CJ, Pitz WJ, Pickett LM, Martin GC, Siebers DL, Westbrook CK. Warrendale, PA: SAE; 2003. Effects of Oxygenates on Soot Processes in DI Diesel Engines: Experiments and Numerical Simulations. Society of Automotive Engineers (SAE) paper 2003-01-1791. [Google Scholar]
- 32.Threshold Limit Values for Chemical Substances and Physical Agents. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 1995. [Google Scholar]
- 33.U.S. Environmental Protection Agency; [accessed February 2010]. Integrated Risk Information System (IRIS) Database Listing on Formaldehyde. available at http://www.epa.gov/ncea/iris/subst/0419.htm. [Google Scholar]
- 34.Arey J. A Tale of Two Diesels. Environ. Health Perspect. 2004;112:812–813. doi: 10.1289/ehp.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]