Abstract
Acetaminophen (APAP) overdose induces acute liver damage and failure via reactive oxygen species production and glutathione (GSH) depletion. Methionine sulfoxide reductase B1 (MsrB1) is an antioxidant selenoenzyme that specifically catalyzes the reduction of methionine R-sulfoxide residues. In this study, we used MsrB1 gene-knockout mice and primary hepatocytes to investigate the effect of MsrB1 on APAP-induced hepatotoxicity. Analyses of histological alterations and serum indicators of liver damage showed that MsrB1−/− mice were more susceptible to APAP-induced acute liver injury than wild-type (MsrB1+/+) mice. Consistent with the in vivo results, primary MsrB1−/− hepatocytes displayed higher susceptibility to APAP-induced cytotoxicity than MsrB1+/+ cells. MsrB1 deficiency increased hepatic oxidative stress after APAP challenge such as hydrogen peroxide production, lipid peroxidation, and protein oxidation levels. Additionally, basal and APAP-induced ratios of reduced-to-oxidized GSH (GSH/GSSG) were significantly lower in MsrB1−/− than in MsrB1+/+ livers. Nrf2 nuclear accumulation and heme oxygenase-1 expression levels after APAP challenge were lower in MsrB1−/− than in MsrB1+/+ livers, suggesting that MsrB1 deficiency attenuates the APAP-induced activation of Nrf2. Collectively, the results of this study suggest that selenoprotein MsrB1 plays a protective role against APAP-induced hepatotoxicity via its antioxidative function.
Keywords: Methionine sulfoxide, Selenoenzyme MsrB1, Acetaminophen, Hepatic damage, Oxidative stress
Introduction
Oxidation of methionine occurs mostly in non-enzymatic reactions mediated by reactive oxygen species (ROS), whereas its reduction requires an enzymatic reaction led by methionine sulfoxide reductase (Msr) [1,2]. The cyclic oxidation and reduction of methionine is a defense mechanism that protects cells from oxidative stress [3]. Therefore, Msr is considered as an important antioxidant enzyme, capable of eliminating cellular ROS [4,5]. There are two Msr families, displaying different substrate stereospecificities. The conjunct action of these two types of Msr allows for the complete reduction of any mixture of methionine-(R,S)-sulfoxide residues in proteins [6,7]. MsrA is specific to the S-form of methionine sulfoxide, whereas MsrB only processes the R-form.
Acetaminophen (N-acetyl-p-aminophenol, APAP) is used worldwide as an analgesic and antipyretic drug. However, APAP overdose can cause severe acute liver damage, including total failure and death [8], and is the most common cause of drug-induced liver injury in many countries, including the United States and the United Kingdom [9]. The reactive metabolite N-acetyl-p-benzoquinone imine, which is generated through the biotransformation of APAP in the liver, depletes glutathione (GSH), covalently binds to thiol groups of proteins, and induces ROS production, thereby, ultimately, generating hepatotoxicity [10]. An effective therapy for APAP overdose patients is treatment with GSH (or N-acetylcysteine) to enhance hepatic GSH levels and thereby, reduce ROS levels [10,11].
Mammalian cells possess three MsrB proteins (MsrB1–MsrB3), located within different cellular compartments [12]. Among these three MsrB enzymes, MsrB1 is a cytosolic and nuclear selenoprotein that contains selenocysteine within its active site [12,13,14]. MsrB1 is highly expressed in liver, representing the main hepatic MsrB enzyme [15,16]. MsrB1 knockout mice exhibit increased protein oxidation, lipid peroxidation, and oxidized GSH levels in liver and kidney [16], suggesting that MsrB1 plays an antioxidative role within these detoxifying organs. Additionally, MsrB1 knockdown in mammalian cells increases ROS production and oxidative stress-induced cell death [17], whereas its overexpression enhances cell survival under oxidative stress conditions [18].
We have previously shown that MsrA knockout mice are more susceptible to APAPinduced acute liver damage than wild-type mice, suggesting that MsrA plays a protective role against APAP-induced hepatotoxicity [19]. Since MsrB is the counterpart of MsrA, it would be of interest to establish whether MsrB produces a similar effect against APAP-induced hepatic damage. In the present study, we used MsrB1-deficient mice and primary hepatocytes to investigate the role of MsrB1, the major hepatic MsrB enzyme, in APAP-induced hepatotoxicity.
Material and Methods
Animal preparation
The generation of MsrB1 knockout (MsrB1−/−) mice is described elsewhere [16]. The breeding lines for MsrB1−/− mice were obtained by backcrossing into the C57BL/6N genetic background for >8 generations, as previously described [20]. MsrB1−/− and wild-type MsrB1+/+ mice at 8–10 weeks of age were used for all the experiments, which were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Yeungnam University.
APAP challenge in mice
Age-matched male MsrB1−/− and MsrB1+/+ mice (~10 weeks old) were used to carry out the APAP challenge. After overnight starvation, mice were intraperitoneally administered APAP (300 mg/kg body weight; Sigma–Aldrich) dissolved in warm saline solution or sterile saline control (10 µL/g body weight). Food was immediately returned to the mice following administration. Mice were sacrificed 6 h after APAP treatment to obtain liver tissues and blood from the heart.
Histology
A slice of liver was fixed in a freshly prepared 4 % paraformaldehyde solution, embedded in paraffin, and then cut into 5 µm thick sections. After deparaffinization and dehydration, liver sections were stained with hematoxylin and eosin (H&E) for microscopic examinations.
Isolation of primary hepatocytes
Primary hepatocytes were isolated from the livers of male MsrB1+/+ and MsrB1−/− mice (8–10 weeks old) using a two-step collagenase perfusion method, as previously described [21,22]. Isolated cells were seeded onto culture plates in DMEM supplemented with 10 % fetal bovine serum, 100 U penicillin/streptomycin, and 3.2 mg/L insulin. Cells were allowed to attach for 5 h at 37 °C in a 5% CO2 incubator, after which the medium was changed to remove unattached and dead cells.
Cell viability assay
Primary hepatocytes were seeded into 24-well plates at a density of 5×104 cells/well, and subsequently treated with or without APAP for 24 h. Cell viability was evaluated using an established colorimetric 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT)-based assay.
Measurements of ALT, AST, and LDH levels
Alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH) levels were measured in serum or culture medium using assay kits (IVD Lab), according to the manufacturer’s instructions, using a Shimadzu UV visible spectrophotometer.
Western blot analysis
Antibodies against MsrB1, Nrf2, heme oxygenase-1 (HO-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as previously described [23,24]. Antibodies against histone 1 and HSP90 were purchased from Santa Cruz Biotechnology and Cell Signaling Technology, respectively. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) using a NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred onto a polyvinylidene difluoride membrane. Membranes were subsequently probed with primary antibodies, followed by the addition of horseradish peroxidase-conjugated secondary antibodies. GAPDH expression was used as the protein loading control. Quantitative analysis of blot signals was performed using the LAS-4000 imaging system (GE Healthcare Life Sciences) and ImageJ software (open source).
Measurements of hydrogen peroxide, 4-hydroxynonenal, and protein-carbonyl levels
H2O2 levels in liver samples were determined using the ferric-sensitive dye, xylenol orange (Sigma–Aldrich), as previously described [25]. 4-Hydroxynonenal (HNE) levels were determined by measuring HNE-protein adducts using anti-HNE antibodies, as previously described [19]. Protein-carbonyl levels were measured using an OxyBlot protein oxidation detection kit (Millipore), according to the manufacturer’s recommendations. The blot signals were quantitatively analyzed using the ImageJ software.
Measurement of GSH contents
Free and total GSH contents were measured using a GSH fluorescent detection kit (Arbor Assays), according to the manufacturer’s instructions. Briefly, liver homogenates were prepared in 5 % sulfosalicylic acid to remove proteins. The oxidized GSH (GSSG) contents were calculated by subtracting the measured free GSH from the measured total GSH. The GSH contents were expressed as µmol/g of total protein.
Nuclear protein fractionation
Nuclear and cytoplasmic proteins from livers were fractionated using an NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Scientific), according to the manufacturer’s instructions. Histone 1 and HSP90 were used as nuclear and cytoplasmic marker proteins, respectively.
Reverse transcription-PCR (RT-PCR)
Total RNA was extracted from livers with the TRI-Solution (Bioscience), and then reverse-transcribed to cDNA using a Reverse Transcription Master Premix kit (ELPIS Biotech). The primers used for RT-PCR were: 5´-GCCAGGTGTCTACGTGTGTG-3´ and 5´-TTCTTTGCCTTTAGGGACGA-3´ for MsrB1; 5´-CTATTCAGCAGAGCGGTTCC-3´ and 5´-ATTTTCGTTGGGACGAACTG-3´ for thioredoxin reductase 1; 5´-GGAGCCAAAAGGGTCATCAT-3´ and 5´-GTGATGGCATGGACTGTGGT-3´ for GAPDH. GAPDH mRNA expression was used as the internal control.
Statistical analysis
Statistical analysis was conducted with the Prism 5 software (GraphPad) using two-way ANOVA followed by Bonferroni post-tests. In the case of the data with added samples, p values were adjusted using the alpha spending function. Differences were considered significant at a p value < 0.05.
Results
MsrB1 knockout mice exhibit higher susceptibility to APAP-induced liver injury
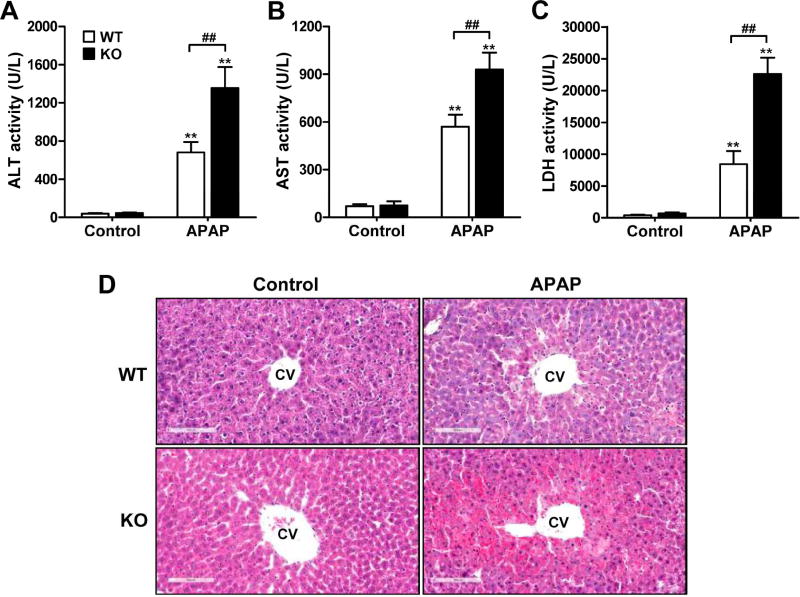
MsrB1−/− and MsrB1+/+ mice were challenged with APAP (300 mg/kg body weight) for 6 h, after which liver injury was analyzed, based on levels of liver damage indicators and histological alterations. The serum ALT and AST levels were significantly higher in APAP-treated MsrB1−/− than in APAP-treated MsrB1+/+ mice (Fig. 1A and B). Additionally, MsrB1−/− mice challenged with APAP displayed significantly higher serum LDH levels than did MsrB1+/+ mice challenged with APAP (Fig. 1C). Notably, there were statistically significant interactions between drug and genotype factors from all the serum data. The histological analysis also evidenced an increased susceptibility to APAP-induced liver damage in MsrB1−/− mice. While livers from control MsrB1−/− mice were histologically normal, similar to those obtained from control MsrB1+/+ mice, APAP challenge induced stronger liver damage in MsrB1−/− than in MsrB1+/+ mice (Fig. 1D). Indeed, the central lobule area of the MsrB1−/− liver displayed more necrotic lesions. Taken together, these results suggest that MsrB1 plays a protective role against APAP-induced liver injury.
Fig. 1. Increased susceptibility to APAP-induced acute liver injury in MsrB1-deficient mice.
Male MsrB1+/+ (WT) and MsrB1−/− (KO) mice were intraperitoneally challenged with APAP (300 mg/kg) or saline control for 6 h. (A–C) Serum ALT (A), AST (B), and LDH (C) levels. Representative data from two independent experiments are shown as mean ± SE (n = 6 for each group). **p < 0.01 vs. WT or KO controls; ##p < 0.01, based on comparisons with WT mice. (D) Histological analysis. Liver sections were stained with H&E, and representative pictures are shown (magnitude 200×). Livers from control MsrB1−/− mice were histologically normal. APAP challenge caused centrilobular hepatotoxicity and central lobular regions contained more necrotic lesions in MsrB1−/− mice. CV, central vein.
MsrB1-deficient hepatocytes are more susceptible to APAP-induced toxicity
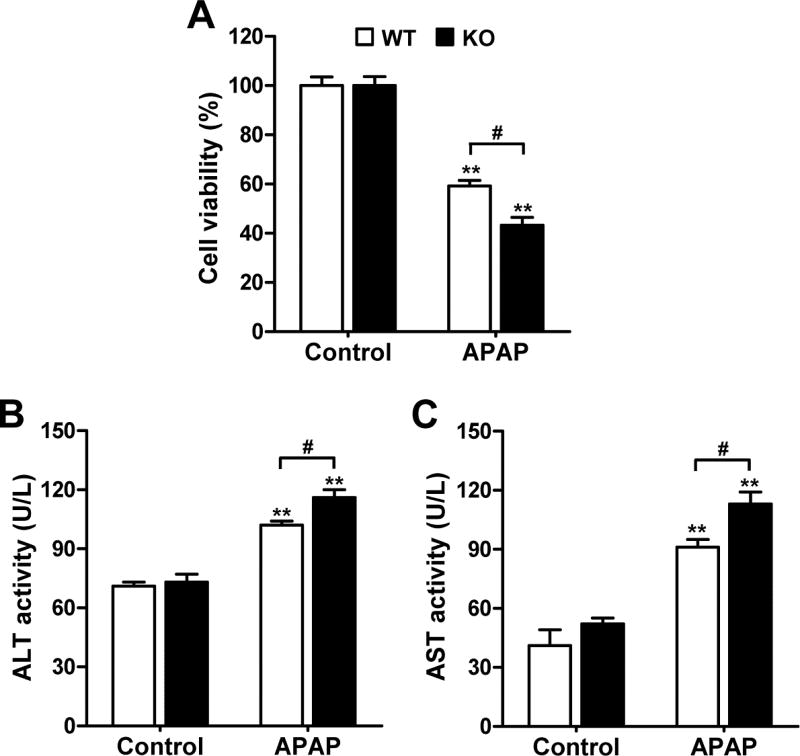
We further investigated the protective role of MsrB1 against APAP-induced hepatotoxicity using primary hepatocytes isolated from livers of MsrB1+/+ and MsrB1−/− mice. Primary MsrB1+/+ and MsrB1−/− hepatocytes were treated for 24 h with various concentrations of APAP (0, 1, 5, and 10 mM). While APAP treatment reduced viability in a dose-dependent manner for both types of cells, MsrB1 deficiency significantly increased APAP-induced cell mortality at 5 and 10 mM concentrations (Supplementary Fig. S1). We chose the 10 mM concentration of APAP to analyze the levels of ALT and AST in the culture media after 24 h. The increased APAP-induced cell mortality was evident in MsrB1−/− cells (Fig. 2A). The basal activities of ALT and AST were similar in both media (Fig. 2B and C). The levels of ALT and AST increased in response to APAP in both MsrB1−/− and MsrB1+/+ hepatocytes; however, the culture medium from APAP-treated MsrB1−/− cells displayed significantly higher levels of both these hepatocellular damage indicators (Fig. 2B and C). Taken together, these results demonstrate a higher susceptibility to APAP-induced cytotoxicity in MsrB1-deficient hepatocytes, which is consistent with the in vivo results.
Fig. 2. Increased susceptibility of MsrB1-deficient hepatocytes to APAP-induced toxicity.
Primary hepatocytes isolated from livers of male MsrB1+/+ (WT) and MsrB1−/− (KO) mice were treated with 10 mM APAP for 24 h. (A) Cell viability. (B, C) Activities of ALT (B) and AST (C) detected in the culture media. Data are representative of at least two independent experiments and expressed as mean ± SE (n = 3 for each group). **p < 0.01 vs. WT or KO controls; #p < 0.05, based on comparisons with WT cells.
MsrB1 depletion increases hepatic oxidative stress
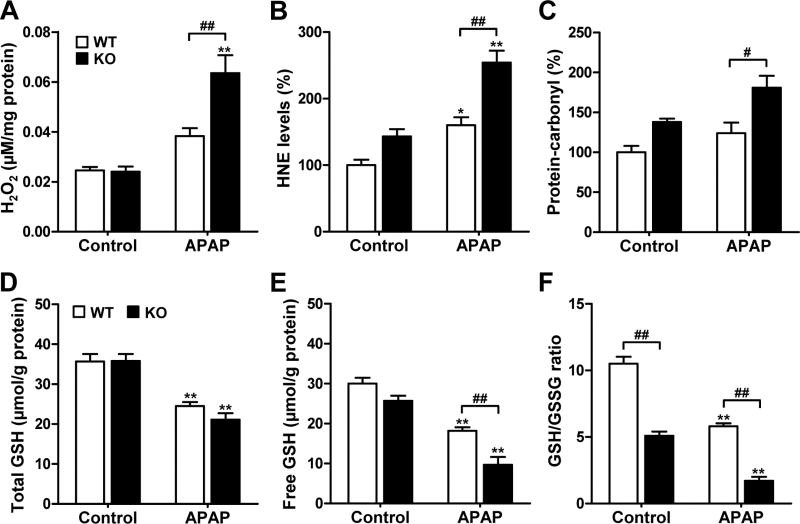
We examined the oxidative stress status of livers upon APAP challenge by measuring the hydrogen peroxide, HNE, and protein-carbonyl levels (Fig. 3A–C). Basal H2O2 levels were similar in MsrB1−/− and MsrB1+/+ livers. The APAP challenge significantly increased the H2O2 levels in MsrB1−/− livers, while a marginal increase in H2O2 level was observed in MsrB1+/+ livers (Fig. 3A). Basal HNE levels were similar in MsrB1−/− and MsrB1+/+ livers. Although the HNE levels significantly increased in both livers after APAP challenge, this increase was significantly higher in MsrB1−/− mice (Fig. 3B), suggesting that MsrB1 plays an antioxidative function against lipid peroxidation. Basal protein-carbonyl levels were similar in MsrB1−/− and MsrB1+/+ livers. Although APAP treatment slightly increased the protein-carbonyl levels in both livers, significantly higher levels were observed in MsrB1−/− livers (Fig. 3C). Taken together, these results suggest that MsrB1 deficiency increases hepatic oxidative stress after APAP exposure.
Fig. 3. Increased oxidative stress and free GSH depletion in MsrB1-deficient liver upon APAP challenge.
Male MsrB1+/+ (WT) and MsrB1−/− (KO) mice were intraperitoneally challenged with APAP (300 mg/kg) or saline control for 6 h. (A) H2O2 contents. (B) 4-Hydroxynonenal (HNE) levels. (C) Protein-carbonyl levels. HNE and protein-carbonyl were used as lipid peroxidation and protein oxidation markers, respectively. Data are representative of two independent experiments. (D) Total GSH contents. (E) Free GSH (reduced form) contents. (F) GSH (reduced) to GSSG (oxidized) ratios. All data are expressed as mean ± SE (n = 6 for each group in H2O2 data; in other data, n = 3 for control groups and n = 4 for APAP-treated groups). *p < 0.05 and **p < 0.01 vs. WT or KO controls; #p < 0.05 and ##p < 0.01, based on comparisons with WT mice.
MsrB1 deficiency decreases the ratio of free to oxidized GSH
Since GSH depletion is a well-known mechanism in APAP-induced hepatotoxicity [11], we measured the total hepatic GSH contents in MsrB1+/+ and MsrB1−/− mice (Fig. 3D). Basal GSH levels were similar among both livers. Although APAP challenge significantly depleted GSH in both livers, the reduction in GSH levels was not significantly different in MsrB1−/− livers. We also measured free (reduced) GSH contents and the ratios of free to oxidized GSH (GSH/GSSG). Basal free GSH levels was not significantly different between MsrB1−/− and MsrB1+/+ livers (Fig. 3E). The free GSH levels significantly decreased in both livers after APAP challenge; however, a significantly higher decrease was observed in the MsrB1−/− livers (Fig. 3E). The basal ratio of GSH/GSSG was significantly lower (2-fold) in MsrB1−/− than in MsrB1+/+ livers (Fig. 3F). APAP exposure significantly decreased the GSH/GSSG ratios in both livers; however, this decrease was significantly larger in MsrB1−/− livers (Fig. 3F). These results suggest that MsrB1 deficiency induces a decrease in GSH/GSSG ratios after APAP exposure, as well as at a basal state, through increasing oxidative stress.
MsrB1 deficiency attenuates APAP-induced Nrf2 activation
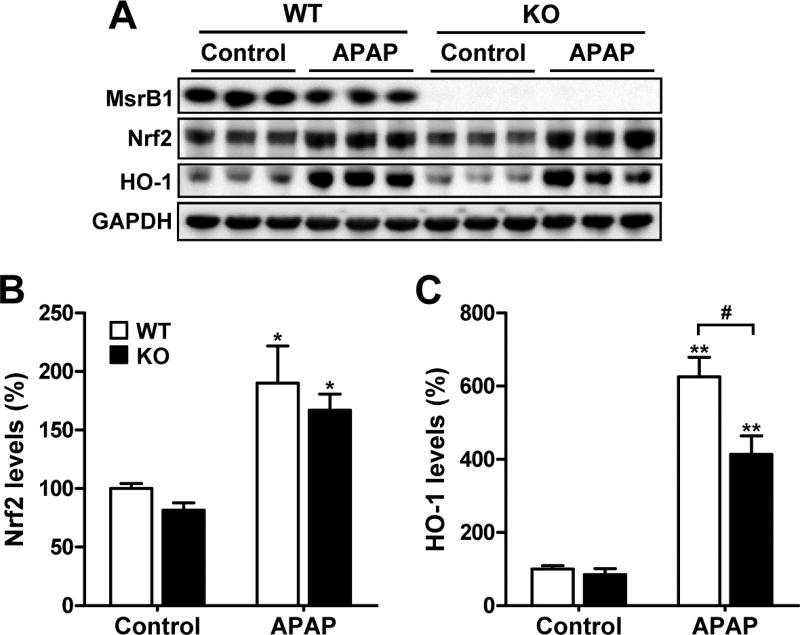
Since APAP challenge activates hepatic Nrf2 [26,27], which is a key regulator of the response to oxidative stress, we investigated whether MsrB1 affects the activation of the Nrf2 pathway. The basal Nrf2 protein levels were lower in MsrB1−/− than in MsrB1+/+ livers (Fig. 4A and B). Nrf2 levels significantly increased in response to APAP in both livers. However, the APAP-induced Nrf2 expression levels were not significantly different between MsrB1+/+ and MsrB1−/− livers. We also analyzed the expression of an Nrf2 target gene, HO-1. The basal levels of HO-1 were similar in MsrB1−/− and MsrB1+/+ livers (Fig. 4A and C). Although the APAP challenge induced the expression of HO-1 in both livers, the expression levels were significantly lower in MsrB1-deficient livers.
Fig. 4. Expression levels of Nrf2 and its target protein HO-1.
Male MsrB1+/+ (WT) and MsrB1−/− (KO) mice were intraperitoneally challenged with APAP (300 mg/kg) or saline control for 6 h. (A) Total lysates from livers were subjected to Western blot analysis of Nrf2 and HO-1. GAPDH was used as the loading control. (B, C) Quantitative analysis of Nrf2 (B) and HO-1 (C) levels normalized to GAPDH. Data are expressed as mean ± SE (n = 6 for each group). *p < 0.05 and **p < 0.01 vs. WT or KO controls; #p < 0.05, based on comparisons with WT mice.
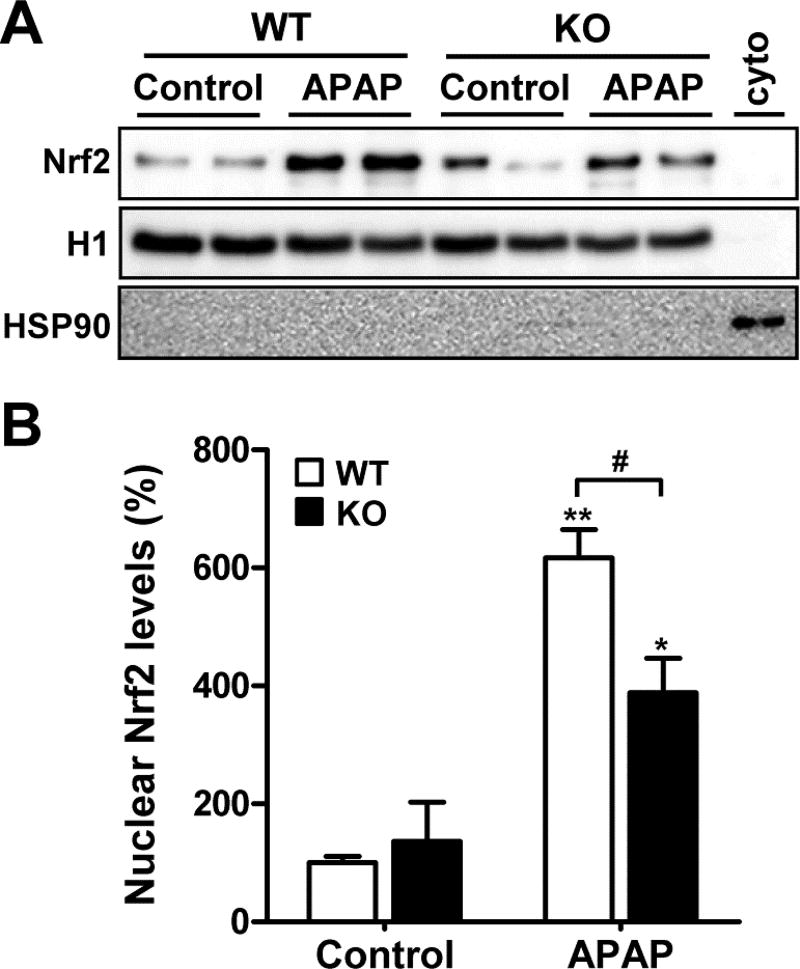
Nrf2 is translocated from cytoplasm to nucleus when activated. We thus analyzed the nuclear accumulation of Nrf2 in response to APAP through the fractionation of cytoplasmic and nuclear proteins from liver tissues. Cytoplasmic Nrf2 proteins were hardly detectable in all samples obtained from control and APAP-treated MsrB1+/+ or MsrB1−/− mice, while nuclear Nrf2 proteins were readily detectable (Supplementary Fig. S2). The basal nuclear Nrf2 protein levels were similar in both MsrB1+/+ and MsrB1−/− mice (Fig. 5A and B). APAP challenge significantly increased the nuclear accumulation of Nrf2 in both MsrB1+/+ and MsrB1−/− livers. However, MsrB1−/− mice showed significantly lower nuclear Nrf2 levels than MsrB1+/+ mice after APAP challenge (Fig. 5A and B). Taken together, these results suggest that MsrB1 deficiency attenuates APAP-induced Nrf2 activation.
Fig. 5. Analysis of nuclear accumulation of hepatic Nrf2 upon APAP challenge.
Male MsrB1+/+ (WT) and MsrB1−/− (KO) mice were intraperitoneally challenged with APAP (300 mg/kg) or saline control for 6 h. (A) Cytoplasmic and nuclear proteins were fractionated from livers and the nuclear Nrf2 protein levels were analyzed through Western blot analysis. Ten micrograms of proteins was loaded onto SDS–PAGE. Histone 1 (H1) and HSP90 were used as nuclear and cytoplasmic marker proteins, respectively. Cyto, a cytoplasmic sample of APAP-treated WT liver. (B) Quantitative analysis of nuclear Nrf2 normalized to GAPDH. All data are representative of two independent experiments and shown as mean ± SE (n = 3 for each group). *p < 0.05 and **p < 0.01 vs. WT or KO controls; #p < 0.05, based on comparisons with WT mice.
MsrB1 deficiency does not affect APAP-induced expression of thioredoxin reductase 1
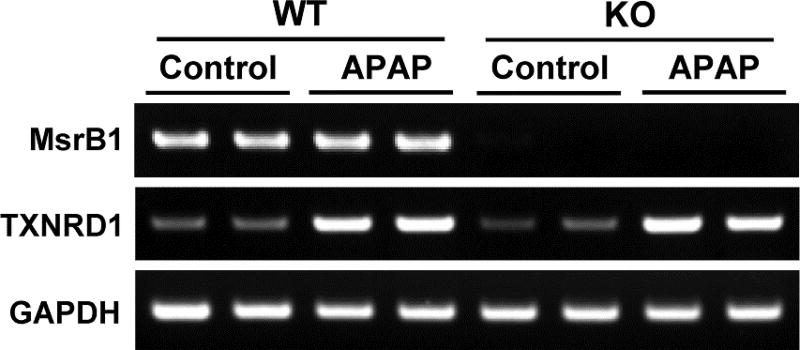
Thioredoxin reductase 1 (TXNRD1), a key redox regulator included in the cytosolic thioredoxin system, is involved in the mechanism modulating the susceptibility to APAP-induced hepatotoxicity. Depletion of TXNRD1 induces resistance to APAP-induced toxicity in livers and hepatocytes [22,28,29]. We recently reported that MsrA can modulate hepatic TXNRD1 expression upon APAP challenge [22]. MsrA deficiency increased APAP-induced TXNRD1 expression levels in hepatocytes, whereas overexpression of MsrA reduced the elevated TXNRD1 levels in APAP-treated MsrA-deficient cells [22]. Therefore, we tested whether MsrB1 deficiency would affect the expression of hepatic TXNRD1 under APAP conditions. RT-PCR analysis showed that APAP challenge increased the TXNRD1 expression levels in both MsrB1+/+ and MsrB1−/− livers, and that no significant difference could be observed between the two liver samples upon APAP challenge (Fig. 6). These results suggest that MsrB1 deficiency does not affect the expression of hepatic TXNRD1 upon APAP challenge.
Fig. 6. Effect of MsrB1 deficiency on APAP-induced thioredoxin reductase 1 (TXNRD1) expression levels.
Liver samples from male MsrB1+/+ (WT) and MsrB1−/− (KO) mice intraperitoneally challenged with APAP (300 mg/kg for 6 h) or saline control were subjected to RT-PCR to assess TXNRD1 expression. GAPDH mRNA expression was used as the internal control.
Discussion
In this study, we demonstrated the protective role of MsrB1, which is the major hepatic MsrB enzyme, against APAP-induced hepatotoxicity, using MsrB1 knockout mice and primary hepatocytes. MsrB1 deficiency promoted APAP-induced liver injury and cytotoxicity in mice and hepatocytes, respectively. Increased oxidative stress with GSH depletion is thought to be a major mechanism through which APAP induces hepatotoxicity [11]. MsrB1 deficiency led to an increase in APAP-induced oxidative stress processes, such as lipid peroxidation and protein oxidation. In addition, basal GSH/GSSG levels in liver were significantly lower in MsrB1-deficient mice than in wild-type mice, which was also the case for APAP-induced GSH/GSSG levels. Therefore, the results obtained from this study suggest that the antioxidative function of MsrB1 plays a key role in protecting hepatic cells from APAP-induced toxicity. A decrease in basal GSH/GSSG ratios in livers harvested from MsrB1-deficient mice has also been reported in a previous study [16].
We previously demonstrated that a deficiency in MsrA, which is specific to the methionine S-sulfoxide reduction process, aggravates APAP-induced acute liver injury in mice [19]. MsrA deficiency significantly accelerates APAP-induced hepatic oxidative stress processes, such as GSH depletion and lipid peroxidation [19]. The present study demonstrates that a deficiency in MsrB1, responsible for methionine R-sulfoxide reduction, induces a higher susceptibility to APAP-induced hepatic damage. Therefore, this result suggests that both methionine S- and R-sulfoxide reduction systems, harboring antioxidative activities, are key toward conferring hepatic protection against APAP-induced toxicity.
Nrf2 is a key transcription factor that modulates the responses to oxidative and electrophilic challenges. Generally, the activation of Nrf2 has a beneficial effect on hepatic protection processes against oxidative challenge. For example, Nrf2-deficient mice exhibit an enhanced susceptibility to liver damage induced by ischemia/reperfusion challenge [30]. Liver-specific TXNRD1-deficient mice show enhanced hepatic Nrf2 activity, providing them with resistance to APAP-induced liver injury [28]. In the present study, APAP-induced nuclear accumulation levels of Nrf2 were shown to be attenuated in MsrB1−/− livers. This impact on Nrf2 activation might be responsible for the increased APAP-induced hepatotoxicity in MsrB1-deficient mice.
There have been several reports describing the effects of antioxidant enzymes, including selenoproteins, on APAP-induced hepatotoxicity in vivo. For example, mice overexpressing selenoprotein GSH peroxidase 1 show increased sensitivity to APAP toxicity [31], whereas there is little impact of knockout of this gene [32]. Knockout of cytosolic superoxide dismutase 1 (SOD1) provides mice with resistance to APAP toxicity [32], as does selenoprotein TXNRD1 knockout [28,29]. In contrast, a deficiency in mitochondrial SOD2 aggravates APAP toxicity [33], as does MsrB1 deficiency. Therefore, it is appropriate to conclude that the antioxidant enzymes have different effects on APAP toxicity, which can be protective or deleterious, or neither.
Supplementary Material
Highlights.
-
➢
MsrB1 deficiency increases APAP-induced hepatotoxicity.
-
➢
MsrB1 depletion enhances hepatic oxidative stress including lipid peroxidation levels.
-
➢
MsrB1 deficiency accelerates glutathione oxidation in response to APAP.
-
➢
MsrB1 deficiency attenuates APAP-induced Nrf2 activation.
Acknowledgments
We thank Prof. Sang Gyu Kwak (Catholic University of Daegu, Korea) for help with statistical analysis. This work was supported by a grant from the National Research Foundation of Korea (2016R1D1A1A02936942 to HYK) and a grant from the National Institutes of Health (AG021518 to VNG).
Abbreviations
- ALT
alanine transaminase
- APAP
acetaminophen
- AST
aspartate transaminase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- H&E
hematoxylin and eosin
- HO
heme oxygenase
- HNE
4-hydroxynonenal
- LDH
lactate dehydrogenase
- Msr
methionine sulfoxide reductase
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-PCR
- TXNRD1
thioredoxin reductase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflicts of interest exist.
References
- 1.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY. The methionine sulfoxide reduction system: selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxid Redox Signal. 2013;19:958–969. doi: 10.1089/ars.2012.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo S, Levine RL. Methionine in proteins defends against oxidative stress. FASEB J. 2009;23:464–472. doi: 10.1096/fj.08-118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci U S A. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabreiro F, Picot CR, Perichon M, Castel J, Friguet B, Petropoulos I. Overexpression of mitochondrial methionine sulfoxide reductase B2 protects leukemia cells from oxidative stress-induced cell death and protein damage. J Biol Chem. 2008;283:16673–16681. doi: 10.1074/jbc.M708580200. [DOI] [PubMed] [Google Scholar]
- 6.Sharov VS, Ferrington DA, Squier TC, Schoneich C. Diastereoselective reduction of protein-bound methionine sulfoxide by methionine sulfoxide reductase. FEBS Lett. 1999;455:247–250. doi: 10.1016/s0014-5793(99)00888-1. [DOI] [PubMed] [Google Scholar]
- 7.Grimaud R, Ezraty B, Mitchell JK, Lafitte D, Briand C, Derrick PJ, Barras F. Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J Biol Chem. 2001;276:48915–48920. doi: 10.1074/jbc.M105509200. [DOI] [PubMed] [Google Scholar]
- 8.Rumack BH. Acetaminophen misconceptions. Hepatology. 2004;40:10–15. doi: 10.1002/hep.20300. [DOI] [PubMed] [Google Scholar]
- 9.Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 10.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 11.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HY, Gladyshev VN. Methionine sulfoxide reduction in mammals: characterization of methionine-R-sulfoxide reductases. Mol Biol Cell. 2004;15:1055–1064. doi: 10.1091/mbc.E03-08-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HY, Gladyshev VN. Different catalytic mechanisms in mammalian selenocysteine- and cysteine-containing methionine-R-sulfoxide reductases. PLoS Biol. 2005;3:e375. doi: 10.1371/journal.pbio.0030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladyshev VN, Arner ES, Berry MJ, Brigelius-Flohe R, Bruford EA, Burk RF, Carlson BA, Castellano S, Chavatte L, Conrad M, Copeland PR, Diamond AM, Driscoll DM, Ferreiro A, Flohe L, Green FR, Guigo R, Handy DE, Hatfield DL, Hesketh J, Hoffmann PR, Holmgren A, Hondal RJ, Howard MT, Huang K, Kim HY, Kim IY, Kohrle J, Krol A, Kryukov GV, Lee BJ, Lee BC, Lei XG, Liu Q, Lescure A, Lobanov AV, Loscalzo J, Maiorino M, Mariotti M, Sandeep Prabhu K, Rayman MP, Rozovsky S, Salinas G, Schmidt EE, Schomburg L, Schweizer U, Simonovic M, Sunde RA, Tsuji PA, Tweedie S, Ursini F, Whanger PD, Zhang Y. Selenoprotein gene nomenclature. J Biol Chem. 2016;291:24036–24040. doi: 10.1074/jbc.M116.756155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novoselov SV, Kim HY, Hua D, Lee BC, Astle CM, Harrison DE, Friguet B, Moustafa ME, Carlson BA, Hatfield DL, Gladyshev VN. Regulation of selenoproteins and methionine sulfoxide reductases A and B1 by age, calorie restriction, and dietary selenium in mice. Antioxid Redox Signal. 2010;12:829–838. doi: 10.1089/ars.2009.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fomenko DE, Novoselov SV, Natarajan SK, Lee BC, Koc A, Carlson BA, Lee TH, Kim HY, Hatfield DL, Gladyshev VN. MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form. J Biol Chem. 2009;284:5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Jia Y, Zhou J, Huang K. Effect of methionine sulfoxide reductase B1 silencing on high-glucose-induced apoptosis of human lens epithelial cells. Life Sci. 2013;92:193–201. doi: 10.1016/j.lfs.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Tang JY, He AH, Jia G, Liu GM, Chen XL, Cai JY, Shang HY, Liao JQ, Zhao H. Protective effect of selenoprotein X against oxidative stress-induced cell apoptosis in human hepatocyte (LO2) cells via the p38 pathway. Biol Trace Elem Res. 2017 doi: 10.1007/s12011-017-1025-z. [DOI] [PubMed] [Google Scholar]
- 19.Singh MP, Kim KY, Kim HY. Methionine sulfoxide reductase A deficiency exacerbates acute liver injury induced by acetaminophen. Biochem Biophys Res Commun. 2017;484:189–194. doi: 10.1016/j.bbrc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Heo JY, Cha HN, Kim KY, Lee E, Kim SJ, Kim YW, Kim JY, Lee IK, Gladyshev VN, Kim HY, Park SY. Methionine sulfoxide reductase B1 deficiency does not increase high-fat diet-induced insulin resistance in mice. Free Radic Res. 2017;51:24–37. doi: 10.1080/10715762.2016.1261133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro. 1981;17:913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- 22.Singh MP, Kwak GH, Kim KY, Kim HY. Methionine sulfoxide reductase A protects hepatocytes against acetaminophen-induced toxicity via regulation of thioredoxin reductase 1 expression. Biochem Biophys Res Commun. 2017;487:695–701. doi: 10.1016/j.bbrc.2017.04.119. [DOI] [PubMed] [Google Scholar]
- 23.Kwak GH, Kim KY, Kim HY. Methionine sulfoxide reductase B3 deficiency stimulates heme oxygenase-1 expression via ROS-dependent and Nrf2 activation pathways. Biochem Biophys Res Commun. 2016;473:1033–1038. doi: 10.1016/j.bbrc.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Kim JI, Choi SH, Jung KJ, Lee E, Kim HY, Park KM. Protective role of methionine sulfoxide reductase A against ischemia/reperfusion injury in mouse kidney and its involvement in the regulation of trans-sulfuration pathway. Antioxid Redox Signal. 2013;18:2241–2250. doi: 10.1089/ars.2012.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, McMahon M, Hayes JD, Itoh K, Yamamoto M, Park BK. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39:1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- 27.Copple IM, Goldring CE, Jenkins RE, Chia AJ, Randle LE, Hayes JD, Kitteringham NR, Park BK. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 2008;48:1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 28.Patterson AD, Carlson BA, Li F, Bonzo JA, Yoo MH, Krausz KW, Conrad M, Chen C, Gonzalez FJ, Hatfield DL. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem Res Toxicol. 2013;26:1088–1096. doi: 10.1021/tx4001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iverson SV, Eriksson S, Xu J, Prigge JR, Talago EA, Meade TA, Meade ES, Capecchi MR, Arner ES, Schmidt EE. A Txnrd1-dependent metabolic switch alters hepatic lipogenesis, glycogen storage, and detoxification. Free Radic Biol Med. 2013;63:369–380. doi: 10.1016/j.freeradbiomed.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudoh K, Uchinami H, Yoshioka M, Seki E, Yamamoto Y. Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Ann Surg. 2014;260:118–127. doi: 10.1097/SLA.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirochnitchenko O, Weisbrot-Lefkowitz M, Reuhl K, Chen L, Yang C, Inouye M. Acetaminophen toxicity. Opposite effects of two forms of glutathione peroxidase. J Biol Chem. 1999;274:10349–10355. doi: 10.1074/jbc.274.15.10349. [DOI] [PubMed] [Google Scholar]
- 32.Lei XG, Zhu JH, McClung JP, Aregullin M, Roneker CA. Mice deficient in Cu,Zn-superoxide dismutase are resistant to acetaminophen toxicity. Biochem J. 2006;399:455–461. doi: 10.1042/BJ20060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa Y, Morita M, Hosomi H, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Knockdown of superoxide dismutase 2 enhances acetaminophen-induced hepatotoxicity in rat. Toxicology. 2009;264:89–95. doi: 10.1016/j.tox.2009.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.