Abstract
Background
The use of antibiotics in the primary prophylaxis for spontaneous bacterial peritonitis (SBP) in patients with cirrhosis is controversial.
Purpose
To determine the beneficial effect of fluoroquinolones as compared to placebo in primary prophylaxis of SBP in high-risk patients with cirrhosis using meta-analysis.
Data Sources
Medline, Embase, Cochrane, and Web of Science databases were searched in all languages until August 2008.
Study Selection
Randomized-placebo controlled studies evaluating the role of fluoroquinolones in primary prevention of SBP in patients with low protein ascites (total ascitic protein <1.5 g/dL) and without prior history of SBP.
Data Extraction
Two investigators independently performed literature search and data extraction, and then another investigator independently reviewed whether the studies met pre-specified criteria and rechecked data extraction. Odds ratios (Peto method) for the risk reduction with fluoroquinolones were calculated for each study and combined using a random-effects model.
Results
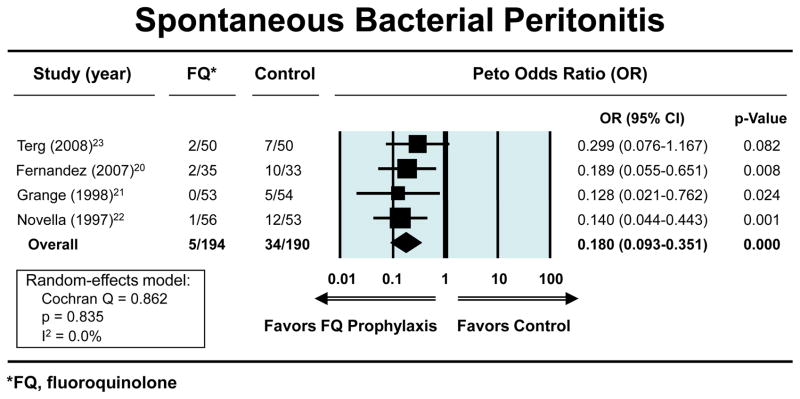
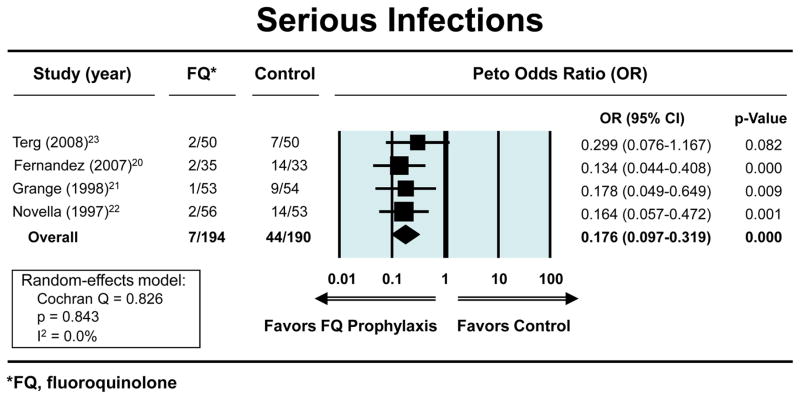
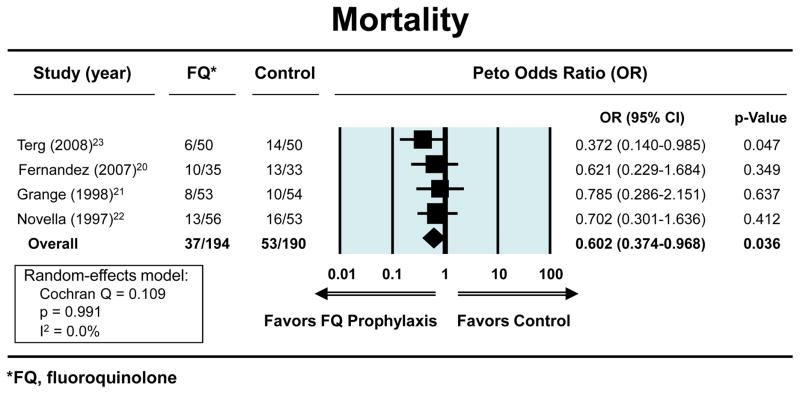
Four randomized-controlled studies met predefined criteria. The odds ratios for developing first episode of SBP, serious infections and mortality with fluoroquinolone prophylaxis (n=194) vs. placebo (n=190) were 0.18 (95% CI, 0.09–0.35), 0.18 (95% CI, 0.10–0.32) and 0.60 (95% CI, 0.37–0.97), respectively. All studies were unidirectional in showing the beneficial effect of fluoroquinolone prophylaxis.
Limitations
Few studies with relatively small sample sizes.
Conclusions
Daily oral fluoroquinolone prophylaxis reduces the risk of development of first episode of SBP and mortality in cirrhotic patients with low total protein in the ascitic fluid. Fluoroquinolones may be advisable for the primary prophylaxis of SBP in selected high-risk patients with cirrhosis.
Search terms: Spontaneous bacterial peritonitis, low protein ascites, primary prophylaxis, norfloxacin, ciprofloxacin, prevention, jaundice, liver failure, complication of cirrhosis, ascites, hepatorenal syndrome, mortality, meta-analysis
Introduction
Cirrhosis is an important cause of morbidity and mortality in both developed and developing countries. Cirrhosis of the liver leads to a significant decrease in life expectancy and reduces quality of life 1. Based upon data from the National Center for Health Statistics (NCHS), chronic liver disease and cirrhosis are estimated to result in 25,000 deaths and 353,000 hospital discharges each year in the United States 1. Complications of cirrhosis include hepatocellular carcinoma, variceal hemorrhage, hepatic encephalopathy, and ascites 2. Patients with cirrhosis and ascites have a median survival of 2 years 3,4.
Spontaneous bacterial peritonitis (SBP) is a serious, life-threatening complication of ascites and may lead to premature death in 30–50% of patients4. Gram negative bacteria are the most common pathogen causing SBP, in the setting of no obvious surgical cause of peritonitis in a patient with ascites3. Prompt recognition and institution of appropriate antibiotic therapy for the treatment of SBP is warranted4. Any delay in starting therapy may lead to higher mortality because SBP may trigger a cascade of events mediated by cytokines5. Renal failure and hepatic encephalopathy may ensue and both of these events significantly shorten survival5. Fluoroquinolones, and third generation cephalosporins have been commonly used for the treatment of SBP6. Development of the first episode of SBP is an important risk factor for future episodes of SBP, and is a predictor of mortality7. Secondary prophylaxis after the first episode of SBP is the standard of care in the field of liver disease and it is well accepted8,9. Both the International Ascites Club (IAC) consensus statement and the American Association for the Study of Liver Diseases (AASLD) guidelines support the use of antibiotics for secondary prophylaxis of SBP3,10. The role of antibiotic prophylaxis in the setting of variceal bleeding in patients with cirrhosis also is well-accepted and universally recommended by major societies and expert panels in the field, including the American Gastorenterology Association, IAC, and the AASLD3,4,10.
In turn, the role of antibiotics in primary prophylaxis for SBP is controversial11. Currently, IAC and most expert panels do not support the routine use of antibiotics in this setting3,4. Since 30–50% of deaths in patients with cirrhosis are attributable to infections, this is an area of intense research interest. Several groups have identified patients who are at high risk of developing their first episode of SBP. The goal is to identify a subset of patients with decompensated cirrhosis and ascites who may be candidates for primary prophylaxis for SBP to possibly reduce mortality due to infections. Multivariate analyses provided the following variables to be predictive of new onset SBP: low ascitic fluid protein levels (< 1.5 gm/dL), serum bilirubin > 3.2 mg/dL, and platelet count < 98,000 /cu mm7,12,13. Most experts consider low protein levels in the ascitic fluid as the most significant factors in determining the high risk of first episode of SBP12.
Current recommendations and expert opinion do not support routine use of fluoroquinolones or any other antibiotic for primary prophylaxis in this subset of high-risk patients for SBP3. This prompted us to conduct a meta-analysis to examine the beneficial effect of fluoroquinolones vs. placebo in the primary prophylaxis for SBP in high-risk patients who do not have a history of prior episode of SBP.
Methods
Retrieval of clinical trials
We searched the following databases in all languages until August 2008 and followed QUOROM guidelines: Medline from 1966, Embase from 1966, COCHRANE (Issue 3, 2008), and Web of Science from 1955. Indexing terms included (Primary prophylaxis) AND (spontaneous bacterial peritonitis). A manual review of the bibliographies of seminal primary and review articles was also performed to identify additional relevant studies. Additionally, manual search of AGA, and AASLD abstracts from 2007 were done to identify relevant studies that are not yet published in full articles.
Criteria for inclusion of studies in the meta-analysis included 1) randomized-controlled clinical trials, evaluating the efficacy of fluoroquinolones vs. placebo in the primary prophylaxis for SBP in patients who are at high risk of developing first episode of SBP based upon a predefined criteria (derived from previously published studies); 2) high risk for first episode defined as ascitic fluid protein levels < 1.5 g/dL; 3) well-defined outcomes by reporting at least one of the following: SBP, severe infections (bacteremia and/or SBP), and death.
Exclusion criteria included studies in post-transplant patients, studies with overlap between patient populations such as case-mix with secondary prophylaxis, case-reports or series, ascites due to non-liver related causes such as ovarian cancer etc., and epidemiologic studies lacking intervention of interest.
Definitions
Primary outcome measures: development of SBP during the study period defined as >250 polymorphonuclear leukocytes/cu mm (culture negative neutrocytic ascites), culture positivity or positive for gram-stain in the ascitic fluid.
Secondary outcome measures: severe infections, defined as SBP or bacteremia during the study period, and all-cause mortality, defined as death due to any cause during the study period.
Data Extraction and Quality Assessment
Two investigators (RL, FP) independently performed the initial literature search and data extraction. Subsequently an additional investigator (GC) confirmed whether eligible studies met inclusion criteria and independently assessed the accuracy of data extraction. Any conflict was resolved with consensus. Quality assessment of the studies has been described in the table 1.
Table 1.
Characteristics of studies included in the analysis.
| Author | Year | Country | Type of study |
Number of patients Rx group |
Medication Rx Group |
Number of patients placebo group |
Medication Placebo Group |
Study design |
Blinding | Randomization method reported |
Allocation concealment (Yes/no) |
Sample size calculations in manuscript (Yes/no) |
Intention to treat analysis (Yes/no) |
Mean Follow- up (weeks) Rx Group |
Mean Follow- up (weeks) Placebo Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terg23 | 2008 | Argentina | Multi- center | 50 | Ciprofloxacin 500 mg /day | 50 | Placebo pills | RCT | Double-blind | Yes | No | Yes | Yes | 31 ±18 | 30 ±18 |
| Novella22 | 1997 | Spain | Multi-center | 56 | Norfloxacin 400 mg/day | 53 | Norfloxacin 400 mg/day only when hospitalized | RCT | Single-blind | Yes | No | No | Yes | 47 ± 5 | 40 ± 5 |
| Grange21 | 1998 | France | Multi- center | 53 | Norfloxacon 400 mg/day | 54 | Placebo pills | RCT | Single- blind | No | Yes (identical Norfloxacin and placebo pills) | Yes | Yes | 18 ± 10 | 19 ± 10 |
| Fernandez20 | 2007 | Spain | Single- center | 35 | Norfloxacin 400 mg/day | 33 | Placebo pills | RCT | Single- blind | Yes | Yes (identical Norfloxacin and placebo pills) | Yes | Yes | 52# | 52# |
Abbreviations: Rx, treatment; RCT, randomized-controlled trial
equivalent to 1 year
Statistical analysis
For each eligible study, odds ratios (OR) and their respective 95% confidence intervals (CI) were estimated to evaluate effect sizes of primary and secondary outcome. Since some of the trials had few primary and secondary outcome events, the Peto method was used14,15. An OR less than 1.00 indicated risk reduction by fluoroquinolone vs. placebo. Because patient populations may have differed among studies (eg, fluoroquinolone regimens), a random effects-model incorporating the variance between study findings in a weighted average of rate ratios (weighted according to sample size), was used to estimate the overall (summary) OR and its 95% CI. As confirmation for the stratified OR estimates, P values, and confidence limits, exact stratified methods with STATXACT software, version 6 (Cytel Inc, Cambridge, MA) were also computed. In order to frame the pooled risk reduction for the primary and secondary endpoints in a more clinically relevant format, the number needed to treat (NNT) was calculated as the reciprocal of the absolute risk reduction16.
Cochran Q statistic17 and Inconsistency Index (I2) were used to examine the heterogeneity among studies18. Publication bias was examined by the Egger test to determine whether there was an association between test accuracy estimates and their precision. Except for the exact methods, all statistical procedures were performed using Comprehensive Meta Analysis software, version 2 (Biostat™, Englewood, NJ).19 Statistical significance for the two-sided p-values was set, a priori, as <0.05.
Role of the Funding Sources
The funding sources did not have any role in manuscript preparation and submission, and have no potential conflict of interest.
Results
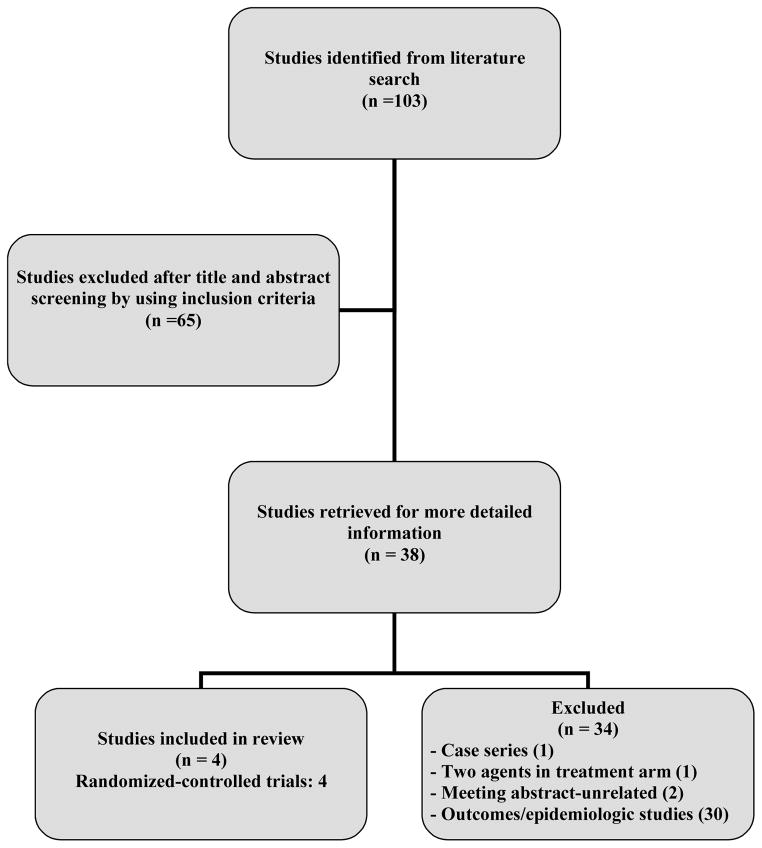
Four randomized-controlled studies were eligible for the meta-analysis and Table 1 shows the characteristics of the studies included in the meta-analysis. Figure 1 shows the literature search and data retrieval protocol. All four studies were published in the English language. Two studies were conducted in Spain20,21 and one each in France22 and Argentina23. The average age of patients included in the four studies was 58 years ; 67% were men in the three studies that reported sex of the patients. The laboratory and baseline characteristics of patients in individual studies are shown in Table 2. The most common cause of liver disease in the patients included in the four studies was alcoholic cirrhosis (Table 3).
Figure 1.
Literature search protocol and derivation of studies included in the meta-analysis
Table 2.
Biochemical characteristics of patients in clinical trials assessing the efficacy of primary antibiotic prophylaxis in patients with low total protein ascitic fluid.
| Author | Total Bilirubin (mg/dL) Rx Group | Total Bilirubin (mg/dL) Placebo Group | Prothrombin time (%)Rx Group | Prothrombin time (%) Placebo Group | Albumin (g/dL) Rx Group | Albumin (g/dL) Placebo Group | Platelet Count (per mm^3) Rx Group | Platelet Count (per mm^3) Placebo Group | Creatinine (mg/dL) Rx Group | Creatinine (mg/dL) Placebo Group | Total Protein, Ascites (g/dL) Rx Group | Total Protein, Ascites (g/dL) Placebo Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terg23 | 2.9±4.6 | 2.7±3.2 | 58±19 | 57±14 | 2.7±0.5 | 2.9±0.6 | 117,809±72,001 | 135,655±70,618 | 0.9±0.3 | 0.9±0.2 | 0.84±0.01 | 0.85±0.36 |
| Novella22 | 3.8±0.3 | 4.1±0.3 | 60±2 | 55±3 | 2.7±0.1 | 2.7±0.1 | N/A | N/A | 1.1±0.08 | 0.9±0.06 | 1±0.2 | 0.9±0.1 |
| Grange21 | 5.1±0.8 | 3.8±0.6 | 53.1±16.5 | 55.±14.6 | 3.29±3.37 | 2.95±0.55 | N/A | N/A | 0.8±0.2 | 0.8±0.1 | 0.93±0.29 | 1.04±0.28 |
| Fernandez20 | 3.5±2.3 | 4.4±4.6 | N/A (INR: 1.49±0.30) | N/A (INR: 1.56±0.36) | 2.8±0.6 | 2.6±0.5 | 120,314±74,952 | 94,061±53,571 | 1.2±0.4 | 1.2±0.4 | 0.9±0.4 | 0.9±0.3 |
Table 3.
Clinical characteristics of patients in clinical trials assessing the efficacy of primary antibiotic prophylaxis in patients with low total protein in the ascitic fluid.
| Author | Cause of Cirrhosis Rx Group | Cause of Cirrhosis Placebo Group | MELD Score* Rx Group | MELD Score* Placebo Group | Child Pugh Score** Rx Group | Child Pugh Score** Placebo Group |
|---|---|---|---|---|---|---|
| Terg23 | N/A | N/A | NA | NA | 8.5±1.5 | 8.3±1.3 |
| Novella22 | Alcohol 31, Hep C 22, Other 3 | Alcohol 31, Hep C 19, Hep B 3 | NA | NA | CP class B/C 29/27 | CP class B/C 24/29 |
| Grange21 | Alcohol 48, Viral 4, Other 1 | Alcohol 45, Viral 6, Other 3 | NA | NA | NA | NA |
| Fernandez20 | Alcohol 20, Other 15 | Alcohol 16, Other 17 | 16.7±3.0 | 10.4±1.5 | 9.9±1.5 | 10.4±1.5 |
Abbreviations: Rx, treatment;
MELD, model for end stage liver disease (range: 6–40)
Child Pugh Score (range: 0–15)
Quality Assessment
All four studies were randomized-controlled trials. Table 1. describes the important quality characteristics of the studies included in the meta-analysis. Based upon the Jadad 3-item scoring system for quality assessment of the randomized-controlled studies, all studies were of fair to good quality24. Overall quality score of studies included in the meta-analysis was 2.25 out of a maximum score of 3.
Meta-analysis
The Peto OR for the odds of developing a first episode of SBP with fluoroquinolone prophylaxis (n=194) vs. placebo (n=190), in patients with low total protein in the ascitic fluid and who had no history of prior episodes of SBP was 0.18 (95% CI, 0.09–0.35) (figure 1). The ORs showing a reduction in the risks for both severe infections and mortality with fluoroquinolone prophylaxis were 0.18 (95% CI, 0.10–0.32) (figure 2a) and 0.60 (95% CI, 0.37–0.97) (figure. 2b), respectively, Each study separately showed a beneficial effect for fluoroquinolones in this clinical setting in one or more outcomes. The exact methods gave similar results for: reduction in first episode of SBP (OR 0.12; 95% CI, 0.04–0.31); severe infection (OR 0.12; 95% CI, 0.04–0.28); and mortality (OR 0.60; 95% CI, 0.36–1.00). The respective NNTs to prevent first SBP episode, bacteremia, and mortality were: 7 (95% CI, 4.7–10.6), 6 (95% CI, 3.8 to 7.7), and 12 (95% CI, 5.8 to 261.2). The average duration of follow-up was 40 weeks (range 18–52 weeks) and 297 patient-years.
Figure 2.
Figure 2a. Forest plots showing beneficial effect of fluoroquinolones vs. placebo in reducing the risk of spontaneous bacterial peritonitis. An OR less than 1.00 indicates risk reduction by fluoroquinolone vs. placebo
Figure 2b. Forest plots showing beneficial effects of fluoroquinolones vs. placebo in reducing the risk of severe infections. An OR less than 1.00 indicates risk reduction by fluoroquinolone vs. placebo.
Figure 2c. Forest plots showing the beneficial effects of fluoroquinolones vs. placebo in reducing the risk of mortality. An OR less than 1.00 indicates risk reduction by fluoroquinolone vs. placebo.
Heterogeneity and Publication Bias
For both the primary and secondary outcomes, the P values for the Q statistic were nonsignificant (all P > 0.71), indicating a lack of heterogeneity across studies. Although the sample of only four studies was small, there was no obvious publication bias for any of these outcomes of interest among the studies, based on the Egger regression method (all P > 0.22).
Infection and Mortality Rate
The rate of first episode of SBP, bacteremia and mortality in fluoroquinolone vs. placebo arm was 2.5% vs. 17.9% (P < 0.01), 1.0 % vs. 5.3% (P < 0.05) and 19.1% vs. 27.9% (P < 0.05) over 297 patient-years of follow-up, respectively (Table 4). Gram-negative organisms were the most common pathogens isolated from the ascitic fluid in both the treatment and placebo arms (Table 4). There was no statistically significant difference in the rate of infections due to gram-positive bacteria.
Table 4.
Bacterial infections and organisms identified in clinical trials assessing the efficacy of primary antibiotic prophylaxis in patients with low total protein ascitic fluid.
| Author | Infection Rx/Placebo Group |
Gram Negative Bacteria Rx/Placebo Group |
Gram Positive Bacteria Rx/Placebo Group |
SBP Rx/Placebo Group |
Bacteremia Rx/Placebo Group |
Severe Infections Rx/Placebo Group |
Urinary Infections Rx/Placebo Group |
Respiratory Infections Rx/Placebo Group |
Other Infections Rx/Placebo Group |
HRS Rx/Placebo Group |
Death Rx/Placebo Group |
Causes of Death Rx Group |
Causes of Death Placebo Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terg23 | 8 / 16 | NA / NA | 1 / 1 | 2 / 7 | 0 / 0* | 2 / 7 | 2 / 4 | 2 / 4 | 2 (cellulitis,otitis) v. 1 (cellulitis) | 1 / 2 | 6 / 14 | Liver failure 2, GI bleed 2, Sepsis 1, HRS 1 | Liver failure 2, GI bleed 3, Sepsis 3, HRS 2, SBP 3, Other 1 |
| Novella22 | 14 / 18 | 11 / 13 | 10 / 8 | 1 / 12 | 1 / 2 | 2 / 14 | 8 / 14 | 4 / 3 | 1 (meningitis) v. 2 (cellulitis) | NA / NA | 13 / 16 | Liver failure 7, GI bleed 4, Other 2 | Liver failure 12, GI bleed 1, SBP 2, Other 1 |
| Grange21 | 4 / 12 | 0 / 6 | 4 / 4 | 0 / 5 | 1 / 4 | 1 / 9 | 2 / 1 | 1 / 1 | 0 v. 1(ENT) | NA / NA | 8 / 10 | Liver failure 4, GI bleed 2, Hepatocellular Carcinoma 2 | Liver failure 1, GI bleed 1, Sepsis 4, Hepatocellular carcinoma 2, Other 2 |
| Fernandez20 | 14 / 19 | 13 / 6 | 2 / 7 | 2 / 10 | 0 / 4 | 2 / 14 | 6 / 4 | 1 /3 | 7 v 4 (catheter sepsis, cellulitis, cholangitis, and bronchitis in both groups without specifying how many per group) | 5 / 8 | 10 / 13 | Liver failure 4, GI bleed 1, HRS 5 | Liver failure 1, GI bleed 2, Septic shock 1, HRS 8, Stroke 1 |
Not reported (assumed 0)
Adverse effects
No serious adverse event that could be directly attributable to the medication was reported (Table 5). There was no statistically significant increase in the rate of gram-positive infections in the fluoroquinolone arm. Two patients discontinued therapy in the active treatment arm due to nausea.
Table 5.
Adverse effects in the treatment and placebo arm of the studies included in the meta-analysis.
| Author | Adverse Effects Treatment Group | Adverse Effects Placebo group |
|---|---|---|
| Terg23 | Renal failure (7) | Renal failure (9) |
| Upper gastrointestinal bleed (4) | Upper gastrointestinal bleed (7) | |
| Hepatic encephalopathy (7) | ||
| Nausea requiring withdrawal of treatment (1) | Hepatic encephalopathy (8) | |
| Novella22 | Oral candidiasis (1) | NA |
| Grange21 | Nausea requiring withdrawal of treatment (1) | Gastrointestinal bleed (1) |
| Hypersomnia (1) | Surgery (1) | |
| Surgery (2) | ||
| Fernandez20 | Renal failure (7) | Renal failure (16) |
Discussion
The main finding of this meta-analysis is that primary prophylaxis with oral fluoroquinolone (norfloxacin 400 mg or ciprofloxacin 500 mg) taken once daily reduces the risk of first episode of SBP, bacteremia, and death in patients with low total protein (<1.5 gm/dL) levels in the ascitic fluid. On average, number needed to treat to prevent one episode of SBP and death are 7, and 12, respectively. These findings may have important clinical implications in the management of patients with decompensated cirrhosis as SBP is one of the leading causes of mortality in these patients.
Most experts believe that bacterial translocation across the gut may be the underlying mechanism responsible for increased rate of infection in patients with low total protein ascites3. This hypothesis is also supported by the fact that enteric pathogens especially gram-negative bacteria are the leading cause of infection in patients with ascites and cirrhosis. Therefore, it is plausible that the use of antibiotics that are active against gram-negative pathogens such as norfloxacin and ciprofloxacin may lead to reduction in the infection rate in this subset of patients.
Most experts caution against widespread or in-judicious use of antibiotics in patients with ascites for prevention of first episode SBP due to concerns regarding development of drug resistance strains of gram-negative bacteria or changing the spectrum of pathogens from gram-negative to gram-positive pathogens in this setting3,11,25. However, selective use of antibiotics in high-risk patients may be appropriate in certain setting. Experts have identified a sub-group of patients with ascites and high risk of SBP who could be candidates for antibiotic prophylaxis. The three key factors that are considered to be significant in predicting the first episode of SBP include low total (<1.5 g/dL) protein in ascitic fluid, bilirubin (>3.2 mg/dL) and platelets below 98,000/cu mm. Individual studies lacked the power to reveal a strong beneficial effect of primary prophylaxis with fluoroquinolones in high-risk patients on reducing all three outcomes. Our meta-analysis shows that, in a select group of patients with ascites due to cirrhosis of liver, primary prophylaxis with fluoroquinolones is efficacious.
There are several strengths that should be acknowledged with our study. The quality of evidence of the studies included in the meta-analysis was high, suggesting strong internal validity of the findings. The results are generalizable to most liver centers in the United States as the laboratory and demographics were typical of patients with cirrhosis in the Western World. The beneficial effect of intervention was unidirectional and there is good biological plausibility to support these results. Additionally, consistency of results between two accepted statistical methods used for pooling sparce outcomes data helps ensure the reliability of our findings.
We also acknowledge some limitations of this study. Only a few studies with relatively small sample sizes were eligible for meta-analysis. The results obtained are applicable only to a select group of patients with ascites. One study protocol allowed for the use of norfloxacin for the placebo-patients while the patients were hospitalized during the course of the study period. Use of norfloxacin in the placebo-arm would bias the results of the meta-analysis towards the null hypothesis, therefore we think that our results, although they may underestimate the benefit, are valid. We conducted a sensitivity analysis by excluding this study but the results remained consistent. The average follow-up of these studies was less than a year so its unclear if the benefits of primary prophylaxis in SBP continue beyond a year. Despite the relatively lower MELD scores of patients being included in individual studies our meta-analysis suggests a mortality benefits.
Conclusions
Primary prophylaxis with once daily oral fluoroquinolone is effective in reducing the risk of first episode of SBP, severe infections and mortality in cirrhotic patients with low protein concentration in the ascitic fluid. These findings provide evidence that may be helpful in refining current guidelines for the management of infectious complications in patients with decompensated cirrhosis and ascites especially those who are awaiting liver transplant. The results of our meta-analysis are directly applicable to patient care in a wide-range of clinical settings, including both in-patient admissions by residents, internists, and hospital physicians and out-patient settings, when these patients are evaluated by either internists, family practitioners, gastroenterologists or hepatologists. The proposed intervention may reduce the burden of death from complications of cirrhosis. Future studies are needed to address issues related to the duration of primary prophylaxis, and management of drug-resistance in this potentially fatal clinical setting.
Acknowledgments
Grant Support: This project was supported by the intramural research program of the Clinical Center, National Institutes of Health.
Authors would like to thank Drs. David Kravetz and Tarek Hassenein for critical review of the manuscript.
Abbreviations
- SBP
Spontaneous bacterial peritonitis
Footnotes
Potential Financial Conflicts of Interest:
None disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Williams JG, Roberts SE, Ali MF, Cheung WY, Cohen DR, Demery G, Edwards A, Greer M, Hellier MD, Hutchings HA, Ip B, Longo MF, Russell IT, Snooks HA, Williams JC. Gut. 2007;56(Suppl 1):1–113. doi: 10.1136/gut.2006.117598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Gut. 2005;54:718–25. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G. Gastroenterology. 2001;120:726–48. doi: 10.1053/gast.2001.22580. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-del-Arbol L, Urman J, Fernandez J, Gonzalez M, Navasa M, Monescillo A, Albillos A, Jimenez W, Arroyo V. Hepatology. 2003;38:1210–8. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 6.Soares-Weiser K, Brezis M, Leibovici L. Cochrane Database Syst Rev. 2001:CD002232. doi: 10.1002/14651858.CD002232. [DOI] [PubMed] [Google Scholar]
- 7.Andreu M, Sola R, Sitges-Serra A, Alia C, Gallen M, Vila MC, Coll S, Oliver MI. Gastroenterology. 1993;104:1133–8. doi: 10.1016/0016-5085(93)90284-j. [DOI] [PubMed] [Google Scholar]
- 8.Guarner C, Runyon BA. Gastroenterologist. 1995;3:311–28. [PubMed] [Google Scholar]
- 9.Younossi ZM, McHutchison JG, Ganiats TG. J Hepatol. 1997;27:295–8. doi: 10.1016/s0168-8278(97)80174-2. [DOI] [PubMed] [Google Scholar]
- 10.Runyon BA. Hepatology. 2004;39:841–56. doi: 10.1002/hep.20066. [DOI] [PubMed] [Google Scholar]
- 11.Evans LT, Kim WR, Poterucha JJ, Kamath PS. Hepatology. 2003;37:897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 12.Runyon BA. Gastroenterology. 1986;91:1343–6. doi: 10.1016/0016-5085(86)90185-x. [DOI] [PubMed] [Google Scholar]
- 13.Guarner C, Sola R, Soriano G, Andreu M, Novella MT, Vila MC, Sabat M, Coll S, Ortiz J, Gomez C, Balanzo J. Gastroenterology. 1999;117:414–9. doi: 10.1053/gast.1999.0029900414. [DOI] [PubMed] [Google Scholar]
- 14.Cooper NJ, Jones DR, Sutton AJ. Clin Trials. 2005;2:260–4. doi: 10.1191/1740774505cn090oa. [DOI] [PubMed] [Google Scholar]
- 15.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 16.Moriarty PM. Am J Cardiol. 2001;87:1206–8. A7. doi: 10.1016/s0002-9149(01)01497-7. [DOI] [PubMed] [Google Scholar]
- 17.Cochran WG. Biometrics. 1954;10:101–21. [Google Scholar]
- 18.Higgins JP, Thompson SG. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Biostat; Engelwood, NJ: 2005. [Google Scholar]
- 20.Fernandez J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V, Such J, Gines P, Arroyo V. Gastroenterology. 2007;133:818–24. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 21.Grange JD, Roulot D, Pelletier G, Pariente EA, Denis J, Ink O, Blanc P, Richardet JP, Vinel JP, Delisle F, Fischer D, Flahault A, Amiot X. J Hepatol. 1998;29:430–6. doi: 10.1016/s0168-8278(98)80061-5. [DOI] [PubMed] [Google Scholar]
- 22.Novella M, Sola R, Soriano G, Andreu M, Gana J, Ortiz J, Coll S, Sabat M, Vila MC, Guarner C, Vilardell F. Hepatology. 1997;25:532–6. doi: 10.1002/hep.510250306. [DOI] [PubMed] [Google Scholar]
- 23.Terg R, Fassio E, Guevara M, Cartier M, Longo C, Lucero R, Landeira C, Romero G, Dominguez N, Munoz A, Levi D, Miguez C, Abecasis R. J Hepatol. 2008;48:774–9. doi: 10.1016/j.jhep.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Jadad AR, Tugwell P. Int J Technol Assess Health Care. 1996;12:195–208. doi: 10.1017/s0266462300009570. [DOI] [PubMed] [Google Scholar]
- 25.Park YH, Lee HC, Song HG, Jung S, Ryu SH, Shin JW, Chung YH, Lee YS, Suh DJ. J Gastroenterol Hepatol. 2003;18:927–33. doi: 10.1046/j.1440-1746.2003.03086.x. [DOI] [PubMed] [Google Scholar]