Abstract
Objective
To characterize the audiometric natural progression in patient-ears with small volume (<1000mm3), treatment-naïve cochleovestibular schwannomas (CVSs) in Neurofibromatosis Type 2 (NF2).
Study Design
Prospective, longitudinal cohort study.
Setting
Quaternary medical research institute.
Patients
111 ears in 71 NF2 patients with small, treatment-naïve CVSs observed from July 2006 to July 2016.
Intervention
Serial audiometric testing, including pure tone audiometry, speech audiometry, and magnetic resonance imaging (MRI).
Outcome measures
four-frequency pure tone average (4f-PTA) of 0.5, 1, 2, & 4 K Hz and word recognition score (WRS) were recorded. Their changes were compared with MRI changes in CVS volume over time. Times to significant hearing loss (10 dB loss in 4f-PTA) and WRS based on 95% critical difference were measured.
Results
Linear regression analysis showed a significant correlation with baseline hearing level (4f-PTA) and internal auditory canal (IAC) tumor volume to annual hearing decrease rate (AHDR) (p=0.003, p=0.0004). Hearing level at baseline and tumor volume correlate with AHDR while tumor volume growth rate does not. Two-way analysis of variance found significant differences in AHDR, risk of significant hearing loss and risk of critical difference in WRS based on baseline hearing level (abnormal or normal) and IAC tumor volume (greater or less than 200 mm3)
Conclusion
Subjects with normal baseline hearing and small IAC tumor component had a low AHDR and low risk of significant hearing loss and may warrant conservative management while the presence of baseline hearing loss and large IAC volume resulted in higher ADHR and greater risk for further hearing loss and may benefit from early treatment interventions.
INTRODUCTION
Neurofibromatosis type 2 (NF2) is a hereditary multiple neoplasia syndrome characterized by bilateral cochleovestibular schwannomas (CVSs). The disease predisposes patients to developing multiple central and peripheral nervous system tumors and is inherited in an autosomal dominant pattern.1 Hearing loss is a major morbidity of NF2 patients, however, the timing and pattern of hearing loss as well as their relation to tumor growth seem highly variable, multifactorial, and difficult to predict. Within two years of diagnosis, the majority of persons with NF2 experience no significant change in hearing.2 In contrast, others may have rapid hearing loss seemingly unrelated to tumor size or growth rate. Even within a single individual, the tumor growth rate and rate of hearing loss can often vary between ears.3
The ideal time to offer intervention has not been well established for CVSs in NF2, especially for small tumors.4 Because it is unclear which patients and which ears will develop significant hearing loss or tumor growth over time, conservative management is commonly offered. This consists of watchful waiting with regular exams, audiometric testing, and imaging. Alternatively, others propose early intervention with total tumor removal and hearing preservation in hopes of preventing future hearing loss due to tumor growth.5 However, hearing preservation rates range between 42% and 63% while recurrence can be as high as 58% after surgery for small tumors.5,6 These outcomes must be weighed against the possibility that the tumors may go years without meaningful growth or hearing loss, especially in the case of small tumors.
Other studies have looked at CVSs of all sizes in NF2. The purpose of the current study was to characterize audiometric properties of small volume (<1000 mm3), treatment-naïve tumors and their changes over time. We have previously correlated audiovestibular characteristics of small volume, treatment-naïve tumors in NF2 with MRI findings in a cross-sectional manner.7 In the current study, we look at pure tone thresholds and word recognition scores (WRS) and correlate audiometric changes in these measures with tumor volume on magnetic resonance imaging (MRI) in a longitudinal manner. We compare characteristics of ears with progressive hearing loss through the duration of follow-up to those with stable hearing. We also specifically test the associations of baseline hearing level and baseline intracanalicular tumor volume with the development of hearing loss. We hypothesized that internal auditory canal (IAC) tumor volume > 200 mm3 would be associated with the development of hearing loss based on a study which indicated median internal auditory canal (IAC) volume of adults ranged between 191 and 202 mm3 on computerized tomography (CT) scan.8 Understanding the relationship of these audiometric and imaging characteristics with hearing loss may be useful in predicting which patients are at risk of developing significant hearing loss over time and therefore help guide the decision to offer future intervention.
MATERIALS AND METHODS
Patients
Audiometric and imaging data were obtained from patients prospectively enrolled in the National Institutes of Health (NIH) NF2 natural history study (08-N-0044, clinicaltrials.gov identifier NCT00598351) approved by the Combined Neuroscience Institutional Review Board. Signed informed consent, and when applicable, assent was obtained for all participants. The diagnosis of NF2 was made by clinical and/or genetic criteria.9 Individuals with at least one treatment-naïve CVS less than 1000 mm3 (intracanalicular and posterior fossa components combined) on initial MRI were included in this study. Patients with only a single visit were excluded due to the inability to measure longitudinal outcomes. Patient data with middle ear disease or nondetectable hearing were excluded from analysis. In the scenario where a patient underwent surgery, only data prior to surgery were included. Any post-treatment measurements were excluded from analysis.
Audiometric testing
Baseline and serial pure tone audiometric testing was performed on all patients including air conduction thresholds from 250 to 8000 Hz and bone conduction thresholds from 250 to 4000 Hz. Patients were followed with serial audiograms at an approximately annual interval. Hearing was analyzed using a 4-frequency air-conduction threshold pure tone average (4f-PTA) of 0.5, 1, 2, and 4 kHz. A 4f-PTA ≤ 20 dB hearing level (HL) was considered normal hearing. Abnormal hearing or hearing loss at enrollment was defined as 4f-PTA >20 dB HL. Change in hearing was calculated as the difference between the 4f-PTA of the most recent audiogram and the baseline. Annual hearing decrease rate (AHDR) was determined by accounting for the time interval between audiograms and was expressed in decibels per year (dB/year). A significant loss of hearing was defined as a decrease in the 4f-PTA ≥10 dB. The time interval in which an ear experienced a 10 dB or greater decrease in hearing from baseline was also gathered from the serial audiograms. Word recognition ability was also tested at baseline and serially at the time of pure tone testing.
Imaging
Patients underwent MRI with and without gadolinium contrast at approximately annual intervals that corresponded with the date of the audiogram. Inner ear MRI was performed with <1-mm in-plane resolution using a 3T MR-scanner (Philips, Andover, MA). The volume of the tumors was determined using post-contrast T1-weighted images and the following formula: volume = (maximum anteroposterior dimension × maximum mediolateral dimension × maximum craniocaudal dimension)/2.10,11 The internal auditory canal (IAC) and posterior fossa (PF) components, if present, were summed for total CVS volume. Change in tumor volume was calculated as the difference between the more recent and baseline MRI. The time interval between scans was used to determine a rate of tumor growth expressed as cubic millimeters per year (mm3/year). This was performed for IAC, PF, and total volumes.
Statistical Analysis
Statistical analysis was performed using XLSTAT Version 2017.2 (Addinsoft, New York, NY). A p-value ≤0.05 was considered significant in all statistical tests. One-way analysis of variance (ANOVA) was used to compare characteristics of ears that experienced progressive hearing loss during the time of follow-up to those which did not (stable hearing). Multiple linear regression analysis was used to evaluate the relation of baseline hearing level and IAC tumor volume to AHDR. Subsequently, ears were divided into four groups for analysis: 1) those with baseline hearing ≤20 dB HL 4f-PTA and IAC tumor volume ≤200 mm3 (normal hearing, small IAC tumor), 2) normal hearing and IAC tumor volume >200 mm3 but ≤1000 mm3 (normal hearing, large IAC tumor), 3) abnormal baseline hearing >20 dB HL and small IAC tumor (abnormal hearing, small IAC tumor), and 4) abnormal hearing, large IAC tumor. Differences in AHDR for these groups were analyzed with a two-way factorial ANOVA. Tukey HSD (Honestly Significantly Different) post hoc analysis was performed to determine the differences between groups within 95% confidence intervals. A chi-square test of association was used for analysis of association between categorical variables. A Kaplan-Meier analysis with log-rank testing was used to determine risk and time to significant hearing loss in these four groups.
Word recognition scores (WRS) were treated as a binomial variable as described by Thornton and Raffin to account for test-retest variability.12 Interval changes in WRS score were determined to be significant if they fell out of the 95% critical difference level from baseline score. A one-way ANOVA was used to compare characteristics between ears which had a critical difference from baseline WRS score. Survival analysis for risk and time to critical difference in WRS was also performed in the four previously defined groups.
RESULTS
Patients and baseline characteristics
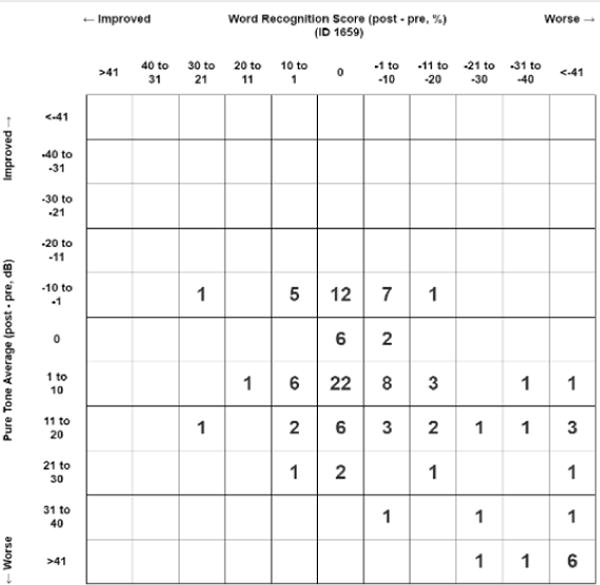
One hundred thirty-three ears in 88 patients met initial inclusion criteria. After application of exclusion criteria, 111 ears in 71 patients were included for analysis. Mean age at enrollment was 30.9 years (SD 19.2, range 8-68). Mean number of audiograms was 5.0 (SD 2.1) over an average of 3.9 years (SD 1.22). Forty-three female and 28 male patients were enrolled, including 55 left ears and 56 right ears. Overall AHDR for all ears was 2.56 dB/year (SD 4.4). Baseline 4f-PTA and WRS (Figure 1a) and change in 4f-PTA and WRS at the end of follow-up (Figure 1b) are presented in standard scattergrams. A summary of patient and tumor characteristics are presented in Table 1.
FIGURE 1.
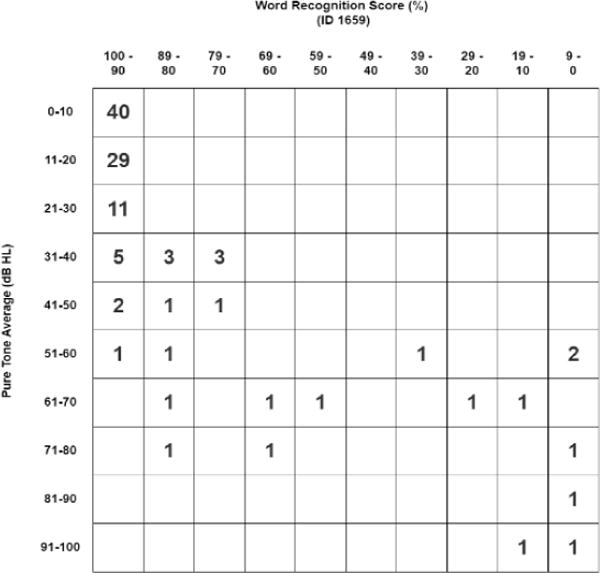
A: Scattergram of baseline pure tone average (PTA) against word recognition score (WRS).
B: Scattergram of change in pure tone average (PTA against change in word recognition score (WRS) at the end of follow-up
TABLE 1.
Summary of patient characteristics
| Mean (SD) |
Audiometric Data |
||||||
|
|
|||||||
| Age, years | 30.9 (19.2) | Baseline, mean (SD) | End, mean (SD) | Difference, mean (SD) | Rate, dB/yr, mean (SD) | ||
|
|
|||||||
| Follow-up, years | 3.9 (1.2) | 4f-PTA, dB | 23.8 (22.4) | 33.7 (30.3) | 9.9 (16.9) | 2.6 (4.4) | |
| Gender | N (%) | SRT, dB | 17.3 (16.9) | 24.0 (22.6) | 6.6 (11.9) | 1.7 (3.4) | |
|
|
|
||||||
| Female | 43 (60.6) | Volumetric Data | |||||
| Male | 28 (39.4) | Baseline, mean (SD) | End, mean (SD) | Difference, mean (SD) | Rate, mm3/yr, mean (SD) | ||
|
|
|||||||
| Ear | N (%) | IAC volume, mm3 | 165.1 (144.5) | 270.4 (190.4) | 105.2 (107.4) | 31.0 (38.4) | |
|
|
|||||||
| Left | 55 (49.5) | PF volume, mm3 | 90.1 (153.0) | 534.8 (1217.7) | 444.7 (1186.2) | 119.4 (304.3) | |
| Right | 56 (50.5) | Total volume, mm3 | 255.3 (247.3) | 805.2 (1312.3) | 549.9 (1212.4) | 150.4 (312.4) | |
4f-PTA: Four frequency pure tone average (0.5, 1, 2, 4 kHz), IAC: internal auditory canal, PF: posterior fossa
Factors associated with 10 dB or greater hearing decline from baseline
The 111 ears were initially divided into two categorical groups comprised of those ears with stable hearing (<10 dB net decrease in 4f-PTA, n=75) and those ears with progressive hearing loss (≥10 dB net decrease in 4f-PTA, n=36) through the course of the study. One-way ANOVA tests were performed to compare differences in mean characteristics (Table 2) between these two groups. Overall mean net decrease in 4f-PTA was 27.4 dB (SD 19.9) in the group with progressive hearing loss as compared to 1.5 dB (SD 4.2) in the stable hearing group (p<0.0001). Mean duration of follow-up was similar in the two groups (4.0 years, SD 1.0 vs 3.9 years, SD 1.3, p=0.554). There was no difference in distribution of age (30.6 years SD 19.7 vs 31.4 years SD 18.5, p=0.84), sex (χ2(1)=1.503, p=0.22), or tumor laterality (χ2(1)=0.769, p=0.381) between the two groups.
TABLE 2.
One Way Analysis of Variance (ANOVA) of tumor-ears associated with hearing loss progression (> 10 dB) compared to those with stable hearing (<10 dB)
| All ears (n=111) | < <10 dB hearing loss (n=75) | ≥ 10 dB hearing loss (n=36) | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F-value | p-value | |
| Age (years) | 30.9 | 19.2 | 30.6 | 19.7 | 31.4 | 18.5 | 0.0 | 0.83586 |
| 4f-PTA | ||||||||
| Baseline | 23.8 | 22.4 | 19.5 | 21.8 | 32.6 | 21.2 | 8.9 | 0.00343 |
| Net decrease | 9.9 | 16.9 | 1.5 | 4.2 | 27.4 | 19.9 | 117.3 | < 0.0001 |
| AHDR | 2.6 | 4.4 | 0.3 | 1.7 | 7.2 | 4.7 | 124.7 | < 0.0001 |
| IAC tumor volume (mm3) | ||||||||
| Baseline | 165.1 | 144.5 | 132.4 | 124.2 | 233.2 | 161.0 | 13.1 | 0.00044 |
| Net growth | 105.2 | 107.4 | 101.0 | 97.9 | 114.1 | 126.1 | 0.4 | 0.55012 |
| Growth rate (mm3/year) | 31.0 | 38.4 | 31.5 | 39.8 | 30.0 | 35.6 | 0.0 | 0.84877 |
| PF tumor volume (mm3) | ||||||||
| Baseline | 90.1 | 153.0 | 89.2 | 159.0 | 92.1 | 141.7 | 0.0 | 0.92575 |
| Net growth | 444.7 | 1186.2 | 313.5 | 883.6 | 718.0 | 1630.4 | 2.9 | 0.09273 |
| Growth rate (mm3/year) | 119.4 | 304.3 | 94.7 | 265.8 | 170.9 | 370.9 | 1.5 | 0.21871 |
| Total tumor volume (mm3) | ||||||||
| Baseline | 255.3 | 247.3 | 221.7 | 240.6 | 325.3 | 249.8 | 4.4 | 0.03810 |
| Net growth | 549.9 | 1212.4 | 414.5 | 912.7 | 832.1 | 1654.5 | 2.9 | 0.08942 |
| Growth rate (mm3/year) | 150.4 | 312.4 | 126.2 | 278.0 | 200.9 | 373.4 | 1.4 | 0.24023 |
4f-PTA: Four frequency pure tone average (0.5, 1, 2, 4 kHz), AHDR: annual hearing decrease rate (dB/year), IAC: internal auditory canal, PF: posterior fossa
In the progressive hearing loss group, mean hearing level at baseline was 32.6 dB HL (SD 21.2) compared to 19.5 dB HL (SD 21.8) in the group with stable hearing (p=0.003). Mean IAC tumor volume at baseline was significantly larger (p=0.0004) in the group that experienced hearing loss progression (233.2 mm3; SD 161.0) compared to the stable hearing group (132.4 mm3; SD 124.2) Mean baseline total CVS volume was significantly larger (p=0.04) in the progressive hearing loss group (325.3 mm3; SD 249.8) compared to the stable hearing group (221.7 mm3; SD 240.6). There were no significant differences in the PF volume measurements between the two groups.
Effects of baseline hearing level and IAC tumor volume on hearing decrease rate
Multiple linear regression analysis was performed to evaluate the relationship between baseline hearing level and IAC tumor volume on AHDR (Table 3). Both worse baseline hearing and larger IAC tumor volume were associated with a faster AHDR (p=0.044 and 0.001 respectively). There was no significant interaction between baseline 4f-PTA and IAC tumor volume (p=0.196) indicating that main effects of both factors on rate of hearing loss are independent of each other.
TABLE 3.
Multiple linear regression coefficients for baseline 4f-PTA and IAC tumor volume on AHDR
| Standard | Lower bound | Upper bound | |||
|---|---|---|---|---|---|
| Regression Coefficient | error | p-value | (5%) | (95%) | |
| Baseline 4f-PTA | 0.327 | 0.161 | 0.044 | 0.009 | 0.646 |
| Baseline IAC tumor volume | 0.443 | 0.129 | 0.001 | 0.188 | 0.699 |
| Interaction between 4f-PTA and IAC tumor volume | −0.247 | 0.196 | 0.21 | −0.636 | 0.142 |
4f-PTA: Four frequency pure tone average (0.5, 1, 2, 4 kHz), AHDR: annual hearing decrease rate (dB/year), IAC: internal auditory canal
Two-way factorial ANOVAs were performed to compare AHDR in four groups previously described groups. Age, PF and total CVS volume metrics were also evaluated to assess for collinearity (Table 4). The normal hearing, small IAC tumor group was the largest in the study (n=52 ears). In this group, AHDR was 0.7 dB/year (SD 2.6 dB/year), which was at a significantly lower rate of change than that observed for all other groups (p=0.001-0.02). There was no significant difference in AHDR among the other groups (3.6-5.0 dB/year, p=0.7-0.95).
TABLE 4.
Two-way Analysis of Variance (ANOVA) of baseline hearing and IAC tumor volume on rate of hearing loss
| Baseline hearing | Normal (≤20 dB HL, n=69) | Abnormal (>20 dB HL, n=42) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline IAC tumor volume | ≤200 mm3 (n=52) | >200 mm3 (n=17) | ≤200 mm3 (n=23) | >200 mm3 (n=19) | R2 | F | p-value | ||||
| M | SD | M | SD | M | SD | M | SD | ||||
| AHDR (dB/year) | 0.7 | 2.6 | 4.3 | 6.0 | 3.6 | 3.4 | 5.0 | 5.6 | 0.18 | 7.65 | 0.0001 |
|
| |||||||||||
| Baseline 4f-PTA (dB) | 9.7 | 5.1 | 9.3 | 4.8 | 47.6 | 17.4 | 46.4 | 23.9 | |||
| Baseline IAC tumor volume (mm3) | 70.7 | 55.7 | 330.0 | 133.1 | 120.0 | 55.8 | 330.6 | 127.6 | |||
|
| |||||||||||
| Age (years) | 25.0 | 18.3 | 21.6 | 9.8 | 49.8 | 14.2 | 32.3 | 18.4 | 0.28 | 14.10 | <0.0001 |
| IAC tumor growth rate (mm3/year) | 34.4 | 35.7 | 23.1 | 21.6 | 30.7 | 49.3 | 29.4 | 43.6 | 0.01 | 0.38 | 0.768 |
| PF tumor volume (mm3) | |||||||||||
| Baseline | 41.8 | 120.9 | 201.5 | 201.7 | 85.4 | 159.5 | 128.5 | 119.4 | 0.14 | 5.82 | 0.001 |
| Growth rate (mm3/year) | 47.9 | 140.6 | 435.8 | 540.4 | 24.3 | 63.5 | 147.3 | 351.7 | 0.22 | 9.77 | <0.0001 |
| Total tumor volume (mm3) | |||||||||||
| Baseline | 112.5 | 154.2 | 531.5 | 224.2 | 205.4 | 188.8 | 459.1 | 200.5 | 0.48 | 32.42 | <0.0001 |
| Growth rate (mm3/year) | 82.3 | 160.6 | 458.9 | 550.5 | 55.0 | 74.4 | 176.6 | 362.4 | 0.19 | 8.59 | <0.0001 |
4f-PTA: Four frequency pure tone average (0.5, 1, 2, 4 kHz), AHDR: annual hearing decrease rate (dB/year), IAC: internal auditory canal, PF: posterior fossa.
R2: 0.18,
R2 adjusted: 0.133
Tumors with the most growth over time were those with IAC tumor components >200 mm3 at time of enrollment. In the group with large IAC tumor components and abnormal hearing (n=19), mean total tumor volume was 459.1 mm3 (SD 200.5) and the mean growth rate was 176.6 mm3/year (SD 362.4) in total tumor volume. In the group with large IAC tumor components and normal baseline hearing (n=17), mean total volume was 531.5 mm3 (SD 224.2) and mean growth rate was 458.9 mm3/year (SD 550.5). These growth rates were significantly faster than groups with small baseline IAC tumor components (55.0 and 82.3 mm3/year, p<0.0001).
The slowest overall tumor growth rate was observed in the abnormal hearing, small IAC tumor group (n=23), with a rate of 55 mm3/year (SD 74.4 mm3/year). It should be noted that this group’s mean age of 47.6 years (SD 17.4 years) was statistically higher than that of the other groups (21.6-32.3 years; p<0.0001-0.005). There was no significant difference in age among the other three groups (p=0.22-0.88). Notably, there was no statistically significant difference in growth rate of the IAC tumor component among all groups (p=0.725-1.0).
Kaplan-Meier analysis was performed to look at time to hearing outcomes among the four groups based on baseline hearing level and IAC tumor volume. The event studied was significant hearing loss progression defined as ≥10 dB change in 4f-PTA (Figure 2a). Only 36 of 111 ears (32%) had ≥10 dB hearing loss progression over the period of follow-up. The log-rank test demonstrated a significant difference in time to hearing loss progression among these groups (p<0.0001). Median time to significant hearing loss progression in ears with abnormal baseline hearing and small IAC tumor component was 3.0 years (n=23). Median time to significant hearing loss in ears with abnormal baseline hearing and larger tumors was 3.2 years (n=19). Median time to hearing loss was not reached in ears with baseline normal hearing (n=69) regardless of tumor size over the 4.8-year time-period studied. However, the percentage of ears developing hearing loss was higher in the group with larger IAC tumor component (44%) compared to those with small IAC components (19%) at 4.8 years.
FIGURE 2.

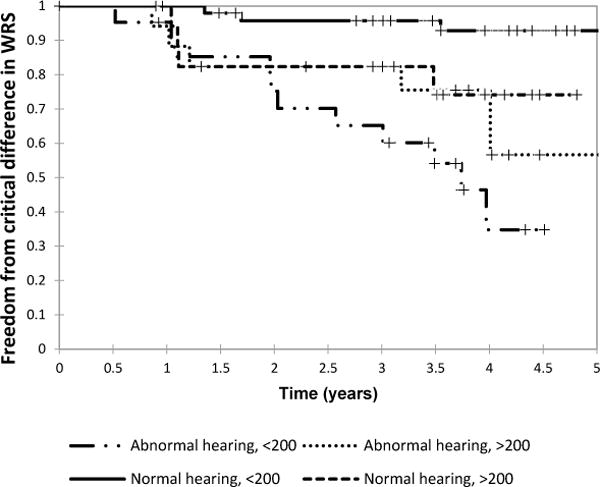
A: Kaplan-Meier curve of time to 10 dB four-frequency pure tone average (4f-PTA) hearing loss stratified by baseline abnormal hearing (greater or less than 20 dB hearing level) and baseline internal auditory canal (IAC) tumor component (greater or less than 200 mm3).
B: Kaplan-Meier curve of time to critical difference in word recognition score (WRS) stratified by baseline abnormal hearing (greater or less than 20 dB hearing level) and baseline internal auditory canal (IAC) tumor component (greater or less than 200 mm3).
Word recognition scores
A critical difference in WRS was seen in 24 of 107 ears over the observed time. One-way ANOVA tests were performed to compare differences in mean characteristics between tumor-ears that experienced a critical difference in WRS compared to those that did not. Ears that experienced a critical difference in WRS had a baseline hearing level of 34.0 dB HL (SD 21.9) compared to 17.4 dB HL (SD 16.8) in ears which did not have a change in WRS (p<0.0002). Ears with a critical difference in WRS had a faster AHDR at 5.6 dB/year (SD 5.7) compared to 1.6 dB/year (SD 3.4) in those with stable WRSs (p<0.0001). There was no statistically significant difference in age, any tumor volume metrics, or baseline WRS (data not shown).
A Kaplan-Meier analysis was performed to examine the effects of baseline IAC tumor volume and hearing level on survival time to a critical difference in WRS (Figure 2b). A log-rank test showed a statistically significant difference in survival among the four groups (p=0.0002). In ears with abnormal baseline hearing and a small IAC tumor component, median WRS survival was 3.7 years (n=22), which was similar to the time until 10 dB 4f-PTA loss in the same group (3.0 years). Median survival of WRS was not met in the other groups. At 4.8 years, in ears with small IAC tumors components and normal baseline hearing, only 4% developed a critical difference in WRS, 43% for large IAC tumors with abnormal baseline hearing, and 26% in ears with normal baseline hearing but larger IAC tumor volume.
DISCUSSION
The objective of this study was to characterize the audiometric natural history of small volume, treatment-naïve CVSs in Neurofibromatosis type 2. The decision to offer surgery, other treatments, or observation in CVSs is complex and multifactorial. Although presenting hearing loss, risk of future loss, and tumor growth are contributing factors, there are no well-defined standards or guidelines. This is especially true in the case of NF2-related CVSs less than 1000mm3.3 Many studies have looked at “small” tumors as a subset of NF2 patients with tumors of varying sizes. Further, size has been defined variably including the using single largest linear dimension (diameter) or by calculated volumes.2,7,10,13 Consensus from the literature regarding small tumors is that they are associated with better PTA, SRT and ABR,4 hearing preservation can be good (65%) when offered surgery,13 and risk of developing significant hearing loss is less than 0.5 after 2 to 6 years.3,11 Within our cohort of 111 treatment-naïve ears with tumors <1000 mm3, we observed heterogeneity in patient-tumor characteristics.
In our patients, we found that mean baseline hearing level was worse (32.6 dB) and IAC tumor volume was larger (233.2 mm3) in ears which had progressive hearing loss through our follow-up period compared to ears with stable hearing (19.5 dB, 132.4 mm3). There was a statistically significant difference in mean baseline total tumor volume (325.3 mm3 vs 221.7 mm3) between these groups, however, this is more likely due to a contributing effect of baseline IAC tumor volume because baseline PF tumor volume was not significantly different between those with stable versus progressive hearing loss. There was no significant difference in age, sex distribution, or laterality indicating that baseline hearing level and IAC tumor size may be associated with developing significant hearing loss independent of these other factors.
The largest subset in our study (n=52) had normal hearing at baseline (mean 9.7 dB HL) and small intracanalicular components (<200 mm3). The annual hearing decrease rate in this subset was 0.7 dB/year. In this group, 19% developed a significant decline in hearing while only 4% developed a critical difference in WRS. Mean total tumor volume was 112.5 mm3 in this group and could expect to grow at a mean rate 82.3 mm3/year. Patients in this group were young with a mean age of 25 years. This supports the use of watchful waiting in patients who are younger with normal hearing and smaller baseline tumors because their risk of developing significant change in 4f-PTA or WRS is low over our observation period of 4.8 years.
The group that experienced the greatest AHDR (5.0 dB/year, SD 5.6) was the abnormal hearing, large IAC tumor group (n=19). Mean age at enrollment was 32.3, baseline 4f-PTA was 46.4 dB and IAC tumor volume was 330.6 mm3. Median time to significant hearing progression was 3.2 years and overall risk of developing significant hearing decline was 0.66 in 5 years. This may be a group who would benefit from early counseling and discussion of interventions to maintain their current hearing status.
We found that patients with abnormal baseline hearing and small IAC tumor volume were significantly older (49.8 years) than other groups (21.6-32.3 years). Generally, increasing age at presentation correlated with worse baseline hearing (R2=0.249; data not shown). This group had a higher risk of developing a significant hearing change despite a smaller tumor size (205.4 mm3) and slower total tumor growth rate (55 mm3/year vs 82.3-458.9 mm3/year), supporting current literature that shows CVS growth rate tends to decrease with increasing age.9,13 The biology of tumors in patients who present at an older age may be more benign and slower growing than in younger patients. We believe that hearing loss progression in the abnormal baseline hearing/small tumor group may be age-related loss in addition to chronic changes from having a longstanding tumor. Alternatively, this may be an artifact of selection bias because patients with more aggressive tumor growth in this age group may have been more likely to have undergone intervention and been excluded from the study.
In our study, we found that tumors experiencing the most growth were those that were already larger at baseline independent of hearing level. This agrees with current literature which shows that rate of hearing loss is independent of rate of total tumor growth. In the group with normal baseline hearing and larger IAC volume tumor (n=17), AHDR was 4.3 dB/year. The percentages of ears developing a significant hearing change in 4f-PTA or WRS were 44% and 26%, respectively. Compared to other groups, they had a larger mean baseline total tumor volume (531.5 mm3) and faster mean total tumor growth rate (458.9 mm3/year).
We chose IAC tumor volume greater or less than 200 mm3 as a categorical parameter in assessing AHDR for two reasons. 1) Our initial analysis of ears that experienced significant hearing loss progression compared to those with stable hearing had a larger IAC tumor volume component (233.2 mm3 vs 132 mm3) while no other volume metrics were significantly associated with the difference in hearing. 2) A recent study of computed tomography (CT) scans of the IAC found that the median IAC volume of adults was 191 mm3 and 202 mm3 in the right and left ears respectively, including both sexes.8 Additionally, a study of sporadic non-NF2 intracanalicular tumors found that AHDR was higher in tumors that had a growing IAC component.14
We hypothesized that as tumors approached and exceeded 200 mm3, or the limits of the IAC volume, hearing loss would increase due to pressure effects of the tumor and the bony limits on the cochlear nerve. Proposed mechanisms for hearing loss include compression of the blood supply to the cochlea, disruption of cochlear neuron axonal transport, or direct effects on the cochlea itself.7 A significant correlation was seen between baseline IAC tumor volume and AHDR, however the association could also be generalized to overall tumor volume when accounting for hearing level at baseline and volume as categorical rather than continuous variables. Further, the rate of IAC tumor growth was consistent among all groups regardless of baseline IAC tumor volume size, hearing level or PF extension (23.1-34.4 mm3/year, p=0.77). A potential future study may involve looking at the percentage of the IAC occupied by tumor with hearing loss or risk of hearing loss. The infiltrative nature of the tumor in the IAC and its tendency to remodel bone are other factors that may affect hearing and warrant study over time in patients with CVSs.13,15-17
A limitation of our study is the short duration of follow-up. In life-long diseases such as NF2, the clinical information from follow-up greater than 4.8 years may be needed to fully understand natural history of CVSs. Another limitation was the omission of MRI fluid-attenuated inversion recovery (FLAIR) sequences which have been used to identify intralabrynthine protein and cochlear aperture obstruction in relation to hearing loss.18 This data was currently not available for enough MRIs to make statistically powerful longitudinal analyses in our study. Finally, prospective studies of patient outcomes will be needed to validate our recommendations. Our study found a correlation between baseline characteristics of age, hearing level, tumor size and annual hearing decrease rate. While these parameters may have some predictive value in stratifying patients at risk of developing changes in hearing, they clearly are not the only factors associated with changes in hearing. In the future, the natural history of small volume treatment-naïve tumors with additional test outcomes including auditory brainstem response testing and vestibular testing (caloric responses, rotatory chair testing, and vestibular evoked myogenic potentials) will be examined longitudinally as well.
Young patients with small tumors and normal hearing may be safely observed with regular follow-up, audiologic testing, and imaging because the risk of significant hearing loss in 4.8 years is low. Our patients with abnormal hearing levels at baseline and IAC tumor components >200 mm3 had the fastest AHDR (5.0 dB/year) and a median time to significant hearing change of 3.2 years. We propose that this progression in hearing loss occurs as the tumor fills the IAC. Patients who fall into this category may benefit from hearing preservation interventions such as bevacizumab or surgical IAC decompression.19,20 Our findings support the current understanding that tumor growth alone is not predictive of hearing loss in CVSs related to NF2.21-22 While baseline hearing level and tumor size seem to correlate significantly with hearing loss, these must be interpreted within the context of the patient’s broader clinical picture.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neurologic Disorders and Stroke (Z99 NS999999), National Institute on Deafness and Other Communication Disorders (ZIA-DC000064), and the Clinical Center at the National Institutes of Health.
References
- 1.Mautner VF, Lindenau M, Baser ME, et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996;38(5):880–5. doi: 10.1097/00006123-199605000-00004. discussion 885–6. [DOI] [PubMed] [Google Scholar]
- 2.Masuda A, Fisher LM, Oppenheimer ML, Iqbal Z, Slattery WH, Natural History Consortium Hearing changes after diagnosis in neurofibromatosis type 2. Otol Neurotol. 2004;25(2):150–154. doi: 10.1097/00129492-200403000-00012. doi: 00129492-200403000-00012 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974–1986. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalwani AK, Abaza MM, Makariou EV, Armstrong M. Audiologic presentation of vestibular schwannomas in neurofibromatosis type 2. Am J Otol. 1998;19(3):352–357. [PubMed] [Google Scholar]
- 5.Slattery WH, 3rd, Brackmann DE, Hitselberger W. Hearing preservation in neurofibromatosis type 2. Am J Otol. 1998;19(5):638–643. [PubMed] [Google Scholar]
- 6.Friedman RA, Goddard JC, Wilkinson EP, et al. Hearing preservation with the middle cranial fossa approach for neurofibromatosis type 2. Otol Neurotol. 2011;32(9):1530–1537. doi: 10.1097/MAO.0b013e3182355855. [DOI] [PubMed] [Google Scholar]
- 7.Holliday MA, Kim HJ, Zalewski CK, et al. Audiovestibular characteristics of small cochleovestibular schwannomas in neurofibromatosis type 2. Otolaryngol Head Neck Surg. 2014;151(1):117–124. doi: 10.1177/0194599814529081. [DOI] [PubMed] [Google Scholar]
- 8.Essbaiheen F, Hegazi T, Rosenbloom L. The normal adult human internal auditory canal: A volumetric multidetector computed tomography study. Otol Neurotol. 2017 doi: 10.1097/MAO.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 9.Baser ME, Mautner VF, Parry DM, Evans DG. Methodological issues in longitudinal studies: Vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42(12):903–906. doi: 10.1136/jmg.2005.031302. doi: jmg.2005.031302 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62(6):1314–9. doi: 10.1227/01.neu.0000333303.79931.83. discussion 1319–20. [DOI] [PubMed] [Google Scholar]
- 11.Yu YL, Lee MS, Juan CJ, Hueng DY. Calculating the tumor volume of acoustic neuromas: Comparison of ABC/2 formula with planimetry method. Clin Neurol Neurosurg. 2013;115(8):1371–1374. doi: 10.1016/j.clineuro.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21(3):507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- 13.Fisher LM, Doherty JK, Lev MH, Slattery WH. Concordance of bilateral vestibular schwannoma growth and hearing changes in neurofibromatosis 2: Neurofibromatosis 2 natural history consortium. Otol Neurotol. 2009;30(6):835–841. doi: 10.1097/MAO.0b013e3181b2364c. [DOI] [PubMed] [Google Scholar]
- 14.van Linge A, Borsboom GJ, Wieringa MH, Goedegebure A. Hearing loss progresses faster in patients with growing intracanalicular vestibular schwannomas. Otol Neurotol. 2016;37(9):1442–1448. doi: 10.1097/MAO.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 15.Doherty J, Go JL, Linthicum FH., Jr Neurofibromatosis 2 invasion of the internal auditory canal wall: Clinical significance. Otol Neurotol. 2014;35(9):1662–1668. doi: 10.1097/MAO.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bathla G, Case BM, Berbaum K, Hansen MR, Policeni B. Vestibular schwannomas: Do linear and volumetric parameters on MRI correlate with hearing loss? Otol Neurotol. 2016;37(8):1168–1173. doi: 10.1097/MAO.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 17.Asthagiri AR, Vasquez RA, Butman JA, et al. Mechanisms of hearing loss in neurofibromatosis type 2. PLoS One. 2012;7(9):e46132. doi: 10.1371/journal.pone.0046132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: A retrospective review of 31 patients. Otol Neurotol. 2012;33(6):1046–1052. doi: 10.1097/MAO.0b013e31825e73f5. [DOI] [PubMed] [Google Scholar]
- 19.Bernardeschi D, Peyre M, Collin M, Smail M, Sterkers O, Kalamarides M. Internal auditory canal decompression for hearing maintenance in neurofibromatosis type 2 patients. Neurosurgery. 2016;79(3):370–377. doi: 10.1227/NEU.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 20.Peyeare M, Goutagny S, Bah A, et al. Conservative management of bilateral vestibular schwannomas in neurofibromatosis type 2 patients: Hearing and tumor growth results. Neurosurgery. 2013;72(6):907–13. doi: 10.1227/NEU.0b013e31828bae28. discussion 914; quiz 914. [DOI] [PubMed] [Google Scholar]
- 21.Patel NB, Nieman CL, Redleaf M. Hearing in static unilateral vestibular schwannoma declines more than in the contralateral ear. Ann Otol Rhinol Laryngol. 2015;124(6):490–494. doi: 10.1177/0003489414566181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonne N, Aboukais R, Baroncini M, et al. Pediatric neurofibromatosis type 2: Clinical and molecular presentation, management of vestibular schwannomas, and hearing rehabilitation. Childs Nerv Syst. 2016;32(12):2403–2413. doi: 10.1007/s00381-016-3257-1. [DOI] [PubMed] [Google Scholar]


