Abstract
Growing evidence suggests that adverse environmental stimuli, especially during sensitive periods in early life, may lead to cardiometabolic disease in later life. However, the underlying biological mechanisms remain a mystery. Recent studies inferred that epigenetic modifications are likely involved. We review recent studies, primarily focused on the findings from human studies, to indicate the role of DNA methylation in the associations between childhood adversity and cardiometabolic disease in adulthood. In particular, we focused on DNA methylation modifications in genes regulating the hypothalamus pituitary adrenal axis as well as the immune system.
Keywords: DNA methylation, childhood adversity, cardiometabolic disease
1. Introduction
Childhood adversity, including verbal, physical, or sexual abuse, neglect, as well as family dysfunction (e.g., an incarcerated, mentally ill, or substance-abusing family member; domestic violence; absence of a parent because of divorce or separation etc.), is a global problem, and exerts substantial burden on the children themselves and on society.[1] A mounting body of evidence suggests that adverse experiences in childhood are associated with cardiometabolic disease in later life.[2, 3] However, little is known about the underlying biological mechanisms. In the past decade, the search for these mechanisms has progressed rapidly and therein found that epigenetic modifications are likely involved. Emerging evidence from human and animal research suggests that early life stress could lead to lasting, broad, and functionally organized signatures in DNA methylation.[4] For example, mice that were exposed to chronic and unpredictable maternal separation from postnatal day 1 to 14 showed differential methylation in several candidate genes.[5] Subsequent studies in humans also identified differential methylation of NR3C1 gene promoter not only in postmortem hippocampal tissue among adult suicide victims with a history of childhood abuse, but also in peripheral blood from adults with exposure to childhood maltreatment[6–8]. Several reviews have described the association between childhood adversity and DNA methylation,[9, 10] and the association between DNA methylation and cardiometabolic disease.[11, 12] The present review, however, is primarily focused on the findings from human studies to indicate the role of DNA methylation in the relationship between childhood adversity and cardiometabolic disease in adulthood. While cardiometabolic diseases are caused by a combination of genetic and environmental factors, in this review, we focus on childhood adversity.
Epigenetic modifications are molecular mechanisms that regulate gene expression without changing DNA sequences, including DNA methylation, posttranslational histone modification, small RNA signaling and chromatin conformation changes.[13] Previous studies have demonstrated that the epigenetic modifications take place from the early embryo stage, and could persist across the life course, thereby leading to disease in adulthood.[14] DNA methylation is one of best-studied epigenetic modifications and is essential to mammalian development and cell differentiation. The best-known DNA methylation mechanism is the attachment of a methyl group to cytosine, typically at the fifth carbon position. The primary target of cytosine methylation in mammals is the C-phosphate-G (CpG) dinucleotide.[15] These sites are relatively rare in the genome but more common at promoter regions of genes, also referred to as CpG islands. Generally, increased methylation of CpG islands is associated with gene repression.[16] In addition, methylation at enhancers, insulators and gene bodies were also observed, but the mechanisms by which these influence the binding and function of regulatory proteins are not completely understood.[17] Three active DNA methyltransferases (DNMT1, DNMT3A and DNMT3B) that are responsible for methylation deposition and maintenance have been identified in mammals.[18]
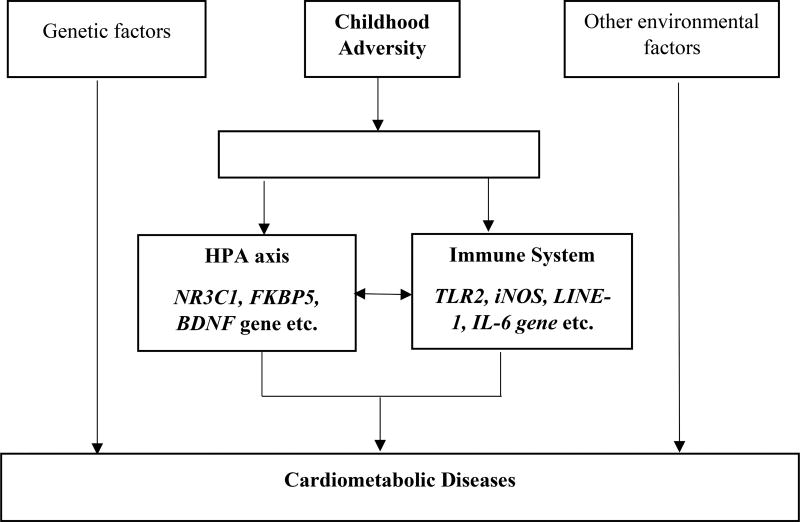
Most data concerning DNA methylation of various pathologies have been obtained from animal models. However, comparable human epigenetic studies are still limited. DNA methylation may hold potential to identify new etiology through which childhood adversity becomes biologically embedded and leads to cardiometabolic disease in adulthood. This knowledge may aid in developing novel prevention and intervention strategies to reduce the burden associated with stress-related health problems. Candidate gene studies and recent preliminary epigenome-wide association studies (EWAS) in early life stress have identified multiple genes involved in the development of obesity, fatty acid synthase, hypothalamus–pituitary–adrenal (HPA) axis, immune system, cellular and neuronal projection etc.(Supplementary Table 1).[19–21] In particular, DNA methylation alterations in genes HPA axis as well as the immune system in human studies are of most interest. Here we first review the DNA methylation involved in the HPA axis and immune system caused by childhood adversity, then explore the associations of those genes with cardiometabolic disease. Figure 1 displays the schematic model showing how DNA methylation modifications in some genes related to the HPA axis and immune system could mediate the effect of childhood adversity on cardiometabolic disease in later life.
Figure 1.
Childhood adversity associated with later life cardiometabolic disease risk mediated through DNA methylation modifications in HPA axis and immune system.
2. Methods
In the first-stage, we performed a systematic search of PubMed, Embase, and PsycINFO databases through Oct 2017 for relevant studies of the association between childhood adversity and DNA methylation. The following key words were used: (‘child abuse’ OR ‘physical abuse’ OR ‘sexual abuse’ OR ‘psychological abuse’ OR ‘emotional abuse’ OR ‘neglect*’ OR ‘trauma*’ OR ‘advers*’ OR ‘maltreat*’ OR ‘bully*’ OR ‘bullied’ OR ‘victim*’ OR ‘expressed emotion’ OR ‘communication deviance’ OR ‘parental loss’ OR ‘separate*’ OR ‘discrimination’) AND ‘child*’ AND ‘methylation’. The titles, abstracts and full-texts were reviewed respectively. After excluding 242 duplicated records, we initially retrieved 1576 abstracts (517 from PubMed, 1171 from Embase, and 130 form PsycINFO) (Supplementary Figure 1). A majority of those references were excluded after reviewing the abstracts or titles and 42 articles were identified. Of those 33 articles identified genes in the HPA axis or immune system (Supplementary Table 1). The following information from each study is presented in Supplementary Table 1: first author, years of publication, country of origin, definition of childhood adversity, research design, sample size, age of sample size, and main results.
In the second stage, based on the genes identified in the first stage, we searched the databases and identified the genes not only related to childhood adversity, but also associated with cardiometabolic diseases. The following key words for cardiometabolic disease were used: ‘cardiovascular diseases’ OR ‘cardiovascular’ OR ‘coronary artery disease’ OR ‘atherosclerosis’, ‘coronary disease’ OR ‘coronary heart disease’ OR ‘ischemic heart disease’ OR ‘heart failure’, ‘myocardial infarction’ OR ‘stroke’ OR ‘brain vascular accident’ OR ‘hypertension’ OR ‘metabolic syndrome’ OR ‘metabolic cardiovascular syndrome’ OR ‘diabetes type 2’ OR ‘diabetes mellitus’.
3. Results
3.1. Hypothalamus–pituitary–adrenal axis
The HPA axis is a biological system particularly affected by early adverse experiences, such as child abuse and neglect[22] or being reared in harsh early environments.[23] The HPA axis is one of the primary stress response systems.[24] Upon exposure to stress, the paraventricular nucleus of the hypothalamus activates and secretes corticotrophin-releasing hormone that promotes the release of adrenocorticotropic hormone from the anterior pituitary to the adrenal glands, which finally stimulates the release of glucocorticoids.[25] The activity and regulation of this system are driven by adrenal cortisol release, which via a negative feedback loop, inhibits the HPA axis activity initiated in the hypothalamus and pituitary. The association between adversity and later health outcomes mediated by HPA axis feedback regulation have been observed not only in childhood,[26] but also long after the cessation of the early adverse experience, in adolescence and adulthood.[27, 28]
3.1.1. Childhood adversity and DNA methylation in HPA axis
Changes in methylation levels of stress reactivity genes can be induced by childhood adversity. Candidate or genome-wide epigenetic studies in humans have found many HPA axis related genes were affected by childhood adversity through DNA methylation, such as glucocorticoid receptor gene (GR),[7] Serotonin transporter gene (SLC6A4),[29] proopiomelanocortin gene (POMC, encodes a preproprotein),[30] potassium voltage-gated channel subfamily Q member 2 (KCNQ2), Ephrin B1 (EFNB1),[31] alsin Rho guanine nucleotide exchange factor (ALS2, involved in small GTPase regulation),[32] leucine rich glioma inactivated 1 (LGI1),[33] brain-derived neurotrophic factor (BDNF, a stress and activity-dependent factor involved in many activities modulated by the HPA axis),[34], Kit ligand gene (KITLG, encodes the ligand of the tyrosine-kinase receptor),[30] and FK506-binding protein 5 (FKBP5, an important regulator of the stress hormone system) gene.[35] One of the most studied is the GR gene, also known as NR3C1 gene, which codes for the glucocorticoid receptor and is located in the reverse strand of chromosome 5q31. glucocorticoid receptor is a key element involved in several steps of HPA axis modulation.[25] Higher NR3C1 methylation levels have been associated with a reduced NR3C1 expression and a flattened cortisol recovery slope, possibly leading to impaired negative feedback regulation of the HPA axis.[36] Chronic perturbations in the HPA axis system can have a widespread effect on long lasting health outcomes.[37]
Epigenetic changes of NR3C1 gene caused by early adversity have been observed in brain tissues and peripheral blood samples. Methylation levels of CpG sites measured in the exon region of the NR3C1 gene in leukocyte cells were associated with early adverse experiences in healthy adults [7] and adults with borderline personality disorder.[8] Methylation differences in leukocyte CpG sites located around the nerve growth factor-inducible protein A (NGFI-A) binding regions seem to be particularly affected by early adverse experiences.[7, 8] Similar changes were observed in human brain tissue. McGowan and colleagues demonstrated that individuals with histories of child abuse have higher methylation levels at specific CpG sites in the exon 1F of the promoter region of the NR3C1 gene in a seminal examination of postmortem hippocampal brain tissue of adult suicide victims.[6] Oberlander and colleagues demonstrated the associations between prenatal maternal depression symptoms and site-specific methylation levels of the NR3C1 gene in mixed mononuclear cells from human cord blood.[38] Meanwhile, this study also found that methylation levels were associated with infants’ salivary cortisol levels at 3 months of age. In another study, the authors found that prenatal psychological stress can have an impact on the methylation pattern of the children's NR3C1 gene, and not due to direct maternal transmission.[39] These results further suggested the connections between DNA methylation profiles and ongoing HPA axis activity.[38]
3.1.2. DNA methylation and cardiometabolic disease in HPA axis
The associations between polymorphisms of the NR3C1 gene and cardiometabolic disease have been confirmed in many studies.[40–42] Recently, a study reported a link between the epigenetic modifications of the NR3C1 promoter, receptor gene, and physiological measures of the stress response.[43] The researchers found clear association between certain epigenetic alternations and blood pressure. In addition, the socially evaluated cold pressor test (completely immerse the hand in ice-cold (2–3 °C) water while watched by a woman and videotaped) induced a strong cardiovascular and HPA axis response.[44] Both cardiovascular systems and HPA axis were affected by functional genetic variants and methylation patterns. In a twin study, Zhao and colleagues observed an association between methylation of the promoter region of the NR3C1 gene in peripheral blood leukocytes and subclinical atherosclerosis. This association was independent of genetic, early family environmental and other coronary risk factors.[45]
Besides epigenetic alternations of the NR3C1 gene, an association of BDNF genotype and promoter methylation with acute and long-term stroke outcomes was also found in an East Asian cohort.[46] Many studies suggested that BDNF plays an important role in regulating energy homeostasis and body weight.[47, 48] Pereira and colleagues found that FKBP5 gene expression in subcutaneous adipose tissue was correlated to markers of insulin resistance.[49] These studies provide an important illustration that expression of HPA axis related genes altered by an epigenetic mechanism may contribute to susceptibility to cardiometabolic disease, and posit a possible mechanism underlying the link between childhood adversity and cardiometabolic risk. Rooij and colleagues found that variation in methylation status in the NR3C1 promoter was associated with physical and perceived acute stress responses, and these associations could largely be explained by differences in lifestyle and education in a large population of healthy adults. [50] This result also supported the hypothesis of DNA methylation acting as a mediator between adverse environment and disease to some extent. The HPA axis is also known to interact with the immune system,[51] which is another pivotal mechanism to mediate the relationship between adversity and cardiometabolic disease.
3.2. Immune System
Accumulating research has suggested that childhood adversity may promote states of heightened proinflammatory signaling that may increase risk for cardiometabolic disease.[52] Individuals who experience childhood adversity have been found to show elevated immune system activity. For example, childhood maltreatment may contribute to higher levels of fibrinogen, white blood cell counts.[53], IL-6, and NF-κB.[54, 55] Lifetime stress, trauma, low socioeconomic conditions, and child abuse have all been associated with elevated levels of IL-4, IL-2, and TNF-α in peripheral blood samples.[56] It was reported that early childhood adversity (deprivation and neglect) affected the long-term functioning of the immune system in adolescents, specifically evinced by a secretion of higher levels of herpes simplex virus secretory Ig-A into saliva.[57] Low socioeconomic status, harsh early environmental circumstances and maltreatment have also been associated with elevated levels of C-reactive protein in adults.[58, 59] In addition to affecting inflammatory markers in peripheral blood and saliva, adversity early in life has been found to affect patterns of gene expression involved in immune system responses. For example, unfavorable socioeconomic circumstances in the early years of life presage the expression of NR3C1 and toll-like receptor 4 (TLR-4) gene expression in adolescence.[60] Further, posttraumatic stress disorder has been shown to have distinct expression patterns in genes involved in immune activation in peripheral blood cells.[61] Individuals exposed to low socioeconomic status early in life showed an up-regulation of genes involved in inflammatory activity, along with a down-regulation of genes involved in glucocorticoid signaling, which plays a critical role in anti-inflammatory activity.[27]
The important role of inflammation in cardiometabolic disease has been noted for several decades.[62] Many previous studies have demonstrated that patients with elevated inflammatory factors are at increased risk of diabetes and cardiovascular disease.[62] A meta-analysis by Wang and colleagues showed that elevated C-reactive protein levels were significantly associated with increased risk of type 2 diabetes based on results of 22 studies, and a significant dose–response association was also observed between the levels of its inducer IL-6 and later occurrence of type 2 diabetes.[63] Kaptoge and colleagues performed a meta-analysis based on 29 studies and found that several different pro-inflammatory cytokines, including IL-6, IL-18, and TNF-α, were each associated with coronary heart disease risk independent of conventional risk factors and in an approximately log-linear manner.[64] In the past few decades, several studies have also strengthened the concept that hypertension has an immunologic basis.[65]
3.2.1. Childhood adversity and DNA methylation in the immune system
Associations between early-life adversities with many inflammation related genes have been found in candidate or genome-wide epigenetic studies. Uddin and colleagues examined the associations between posttraumatic stress disorder and methylation patterns from DNA extracted from peripheral blood in adults, and found that externally experienced traumatic events induced downstream alterations in immune function by reducing methylation levels of immune-related genes.[66] A study by Janusek et al. showed that reduced methylation of the IL6 promoter was related to increased exposure to childhood trauma and greater TSST-induced IL-6 levels in African American men.[20] Another prospective cohort study demonstrated a consistent association between psychological factors with higher average leucocyte DNA methylation in the intercellular adhesion molecule-1 (ICAM-1) promoter region and in the coagulation factor III (F3) promoter region. The authors also found that hostility was positively associated with toll-like receptor 2 (TLR-2) promoter methylation, and that life satisfaction was inversely associated with both TLR-2 and iNOS promoter methylation.[67] A case-control study by Misiak and colleagues showed that emotional abuse and total trauma score predicted lower LINE-1 methylation in first-episode schizophrenia patients.[68] The New York Women’s Birth Cohort study demonstrated that growing up in a single parent family was associated with higher Alu methylation after adjusting for other early life factors.[69] A genome-wide methylation study by Prados and colleagues found that early life events were associated with methylation levels of the CpGs located near the IL17RA gene.[31] Methylation of other immune-related genes, such as TLR8, tartrate-resistant acid phosphatase (ACP5), and neuropeptide FF receptor 2 (NPFFR2), were also found to be associated with posttraumatic stress disorder.[56] These emerging studies are interesting in suggesting a link between trauma and adversity, especially in early life, and DNA methylation related to the immune system. However, further studies are needed to replicate these findings.
3.2.2. DNA methylation and cardiometabolic disease in immune system
Several studies have demonstrated associations between inflammation gene methylation and cardiometabolic risk. Zuo et al. found that hypomethylation of IL-6 promoter is associated with the increased risk for coronary heart disease, especially for acute myocardial infarction.[70] Studies by Cash et al. and Baccarelli et al. showed that cardiovascular risk factors, including higher serum vascular cell adhesion molecule, higher low-density lipoprotein and lower high-density lipoprotein cholesterol were associated with LINE-1 methylation.[71, 72] Baccarelli and Lin et al. have demonstrated that LINE-1 hypomethylation in blood DNA was associated with risk of ischemic heart disease, stroke, and total mortality.[73, 74] Turcot and colleagues found that LINE-1 hypomethylation in visceral adipose tissue samples from severely obese individuals was associated with higher prevalence of metabolic syndrome, and therefore elevated risk for cardiovascular disease.[75] The Normative Aging Study (NAS) found that increases in the degree of methylation of Alu elements were associated with increases in blood pressure. Positive associations between BP and the degree of methylation of the genes for TLR2 and iNOS and negative associations of BP with methylation of the gene for IFN-γ were also found in this study.[76] One cross-sectional study by Kim and colleagues examined Alu methylation in relation to myocardial infarction and reported a higher degree of methylation in cases compared to healthy controls.[77]
These studies aforementioned indicate that childhood adversity affects DNA methylation of selected genes involved in inflammatory processes, which are in turn associated with increased risk of cardiometabolic disease. Such epigenetic changes may represent biological pathways that mediate the effects of psychological factors on cardiometabolic disease.
3.3. Methylation of other possible genes related to childhood adversity
Beside HPA axis and immune systems, childhood adversity have also been associated with DNA methylation of Peptidase M20 Domain Containing 1 which is linked with energy homeostasis regulation (PM20D1, a bidirectional enzyme that catalyzes both condensation of fatty acids and amino acids to generate N-acyl amino acids and the reverse hydrolytic reaction),[78] Opioid Receptor Kappa 1 (OPRK1, encodes an opioid receptor),[79] oxytocin receptor (OXTR),[80] cytochrome p450 family 2 subfamily e member 1 (CYP2E1, encodes a member of the cytochrome P450 superfamily of enzymes),[81] and protein phosphatase 1 regulatory subunit 3g (PPP1R3G, Involved in glucose homeostasis and glycogenesis in the liver)[82] etc. (supplementary Table 1) However, the roles of these in cardiometablic diseases remain unclear.
4. Summary and Future Directions
Although childhood adversity has been associated with cardiometabolic disease, the underlying pathways for this associations have yet to be fully elucidated. For the past few years, epigenetic processes, particularly the DNA methylation, have been considered among the crucial mediators of the effects of childhood adversity.[83] However, there is limited direct evidence that early adversity could increase the risk of cardiometabolic disease through DNA methylation. In this review, we focused on DNA methylation modifications in genes regulating the HPA axis as well as the immune system (Table 1). It is likely that more than one biological mechanism is responsible for the poor cardiometabolic outcomes observed among individuals exposed to childhood adversity.
Table 1.
Genes whose methylation may mediate the association between childhood adversities and cardiometabolic disease risk
| Function | Childhood adversity | Methylation status |
Cardiometabolic disease | |
|---|---|---|---|---|
| HPA axis | ||||
| NR3C1 | Located on 5q31, encodes glucocorticoid receptor protein | childhood maltreatment or adversity[7] | ↑ | Blood pressure,[43] subclinical atherosclerosis[45] |
| BDNF | Located on 11q13, encodes a member of the nerve growth factor family of proteins | childhood maternal care[98] | ↑ | Stroke outcome[46] |
| POMC | Located on 2p23.3, encodes a preproprotein that undergoes extensive, tissue-specific, post-translational processing via cleavage by subtilisin-like enzymes known as prohormone convertases | Maltreatment[30] | ↑ | Higher triglycerides and higher insulin concentrations[99] |
| FKBP5* | Located on 6p21, encodes a member of the immunophilin protein family | Early trauma[100] | ↓ | Insulin resistance[49] |
| Immune system | ||||
| TLR2# | Located on 4q32, encodes a member of the Toll-like receptor family which plays a fundamental role in pathogen recognition and activation of innate immunity | Negative psychological factors[67] | ↑ | Blood pressure[76] |
| iNOS# | Located on 17q11, encodes a nitric oxide synthase which is expressed in liver and is inducible by a combination of lipopolysaccharide and certain cytokines | Negative psychological factors[67] | ↓ | Blood pressure[76] |
| LINE-1 | Belongs to the group of long interspersed nuclear elements | Emotional abuse and total trauma score[68] | ↓ | Cardiovascular disease[75] |
| Alu | A member of the short interspersed repetitive DNA elements family of repetitive elements | Single parent family[69] | ↑ | Blood pressure[76] [77] |
| IL6 | Located on 7p15.3, encodes a cytokine that functions in inflammation and the maturation of B cells | Childhood trauma [20] | ↓ | Coronary heart disease[70] |
Only found that higher FKBP5 gene expression was positively correlated with serum insulin to date.
Only found that TLR2 and iNOS gene methylation were correlated with negative psychological in older population to date.
In order to better characterize the role of DNA methylation between childhood adversity and cardiometabolic disease, a few points should be considered in future research.
Epigenetics inheritance and preadaptation theory: There is growing evidence in humans that prenatal maternal emotional state, such as depression, anxiety, trauma or stress, is associated with the methylation state and adverse health outcomes in offspring,[39] which suggests that epigenetic information could be transmitted from one generation to the next. However, the fetus may adapt to adverse environmental cues in utero with permanent adjustments in homeostatic systems to aid survival.[84] In other words, organisms could “inform” their progeny about prevailing conditions and reprogram the gene expression through epigenetics modifications to preadapt the child to the current environment. Once these adaptations are inconsistent with the postnatal environment, they may ultimately be disadvantageous and result in an increased risk of disease. Therefore, studies including at least two generations are warranted to confirm this theory in humans and to understand the underlying inheritance patterns. Twin studies offer another promising design to explore the mediation effect of DNA methylation between child adversity and cardiometabolic outcomes, as the twins share 100% (for identical twins) or average 50% (for non-identical twins) of genetics and the same familiar environments when grow up together, which could rule out heterogeneity due to genetic and familiar environmental confounding.[85]
Methylation changes with age: Mounting evidence from both animal and human studies suggests that the epigenome is in constant drift throughout the lifespan in response to stochastic and environmental factors.[86] DNA methylation and demethylation are likely modified by early-life unfavorable experiences,[83] but yet there is modification that also occurs throughout life. The monozygotic twin design, completely matched for genetics, age, sex, cohort effects, maternal influences and common environment, can be applied in longitudinal studies to explore the age-dependent patterns in genetic and environmental contributions to epigenetic modification and gene activity, which can be linked to aging-related phenotypes.[87]
Cause and consequence: Comparing with the stable and the conservative of DNA sequence, epigenetic processes are developmentally dynamic. It is fundamentally important to identify the methylation tags showing corresponding changes to the changes of psychosocial stress. This will not only serve as solid evidence supporting the direct link for the observed associations but also indicate that the reduction in stress may reverse the DNA methylation, a strong statement for public health initiative. On the other hand, because of the plastic nature of DNA methylation, it is difficult to distinguish whether the identified DNA methylation changes are causal or secondary to the cardiometabolic disease. Therefore, a longitudinal study in healthy population with chronic stress, DNA methylation, and preclinical markers of cardiometabolic disease assessed in multiple times is warranted to address these critical issues.
Cell and tissue heterogeneity: DNA methylation patterns may vary between cells and tissue types. This may make study design a challenge because relevant tissues may not be accessible from living individuals. Previous studies have found that both brain and blood can respond to certain environmental stimuli through epigenetically-mediated changes and that these changes are indeed to some extent concordant between both tissue types.[88] Some tools have been developed to aid interpretation of blood-based DNA methylation results in the context of brain tissue.[89] In regards to cell subtypes, there have been several approaches well developed to estimate the percentage of major cell compositions based on genome-wide DNA methylation data, and being adjusted for in the subsequent statistical analysis.[90] However, studies focusing on epigenetic signatures of specific cell types rather than measuring methylation in mixed cells may elucidate which cell types are more relevant to early life stress and thus involved in the mechanisms leading to cardio-metabolic disease.
Epigenome wide association studies (EWAS): With new technologies and approaches constantly being invented, such as comprehensive DNA methylation microarrays, more opportunities have been granted to explore these intricate mechanisms. Epigenetic research (especially the EWAS and GWAS) leads to complex data structures. This complicated gene-gene, and gene-environment interactions involved in the relationship between child adversity and cardio-metabolic outcomes [91, 92] induce a great challenges to current statistical methods. Therefore, advanced methodologies are needed to address these knotty issues and to reduce the false positive results. In addition, the majority of previous EWAS studies on childhood adversity did not have an independent replication cohort (Supplementary Table 1). Future research should follow the current recommendations with respect to the study design and data analysis for EWAS.[93]
Other related factors: Many studies have shown that adverse exposures in early life were associated with higher rates of smoking, alcohol and drug consumption.[94, 95] The effects of unfavorable lifestyle due to adversity are difficult to disentangle from the effects of the adversity per se. Therefore, the complex interplay of genetics, lifestyle changes and environment exposures should be considered when designing future studies to disentangle the relationships among childhood adversity, epigenetic changes and cardiometabolic outcomes. In addition, resilience, defined as the capacity to resist negative consequences resulting from adverse events or to bounce back from adversities, has become an important area of research. Resilient coping may buffer the effects of childhood adversity on long-term health outcomes in adulthood.[96, 97] Therefore, studies on resilience may provide a better understanding of the mechanisms linking childhood adversity and cardiometabolic diseases, and inform approaches to prevent disease in those affected by childhood adversity.
This review summarized the current state of the literature regarding the effect of childhood adversity on DNA methylation status in genes related to the HPA axis and immune system, and the possible mediation effects of DNA methylation in these two pathways on cardiometabolic disease risk. Shared biological pathways suggest that interdisciplinary research may be a promising strategy to uncover the mechanisms through which childhood adversity contributes to the development of cardiometabolic disease. Future research is warranted to directly target these mechanisms with measures of childhood adversity and cardiometabolic outcomes, which may aid in developing novel prevention and intervention strategies to reduce the burden associated with stress-related health problems.
Supplementary Material
DNA methylation involved in the HPA axis and immune system caused by childhood adversity was reviewed.
The associations of those genes with cardiometabolic disease were explored.
This narrative system review gives comprehensive view of childhood adversity, DNA methylation, and cardiometabolic disease.
Acknowledgments
Funding
The present study was supported by NIH/NHLBI HL125577
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors' contributions
GH and SS conceived the idea for the manuscript and produced the first draft. NAY and CLD were involved in critical review and also in rewriting of subsequent drafts. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Guang Hao, Department of Population Health Sciences, Medical College of Georgia, Augusta University, Augusta, GA; ghao@augusta.edu.
Nagy A. Youssef, Department of Psychiatry & Health Behavior, Medical College of Georgia, Augusta University, Augusta, GA; nyoussef@augusta.edu.
Catherine L. Davis, Department of Population Health Sciences, Georgia Prevention Institute, Medical College of Georgia, Augusta University, Augusta, GA; katie.davis@augusta.edu.
Shaoyong Su, Department of Population Health Sciences, Medical College of Georgia, Augusta University, 1120 15th Street, HS 1721 Augusta, GA 30912.
References
- 1.Centers for Disease C, Prevention. Adverse childhood experiences reported by adults --- five states, 2009. MMWR Morbidity and mortality weekly report. 2010;59:1609–13. [PubMed] [Google Scholar]
- 2.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 3.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS medicine. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell C, Schneper LM, Notterman DA. DNA methylation, early life environment, and health outcomes. Pediatric research. 2016;79:212–9. doi: 10.1038/pr.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biological psychiatry. 2010;68:408–15. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 6.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PloS one. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perroud N, Paoloni-Giacobino A, Prada P, Olie E, Salzmann A, Nicastro R, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bick J, Naumova O, Hunter S, Barbot B, Lee M, Luthar SS, et al. Childhood adversity and DNA methylation of genes involved in the hypothalamus-pituitary-adrenal axis and immune system: whole-genome and candidate-gene associations. Dev Psychopathol. 2012;24:1417–25. doi: 10.1017/S0954579412000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szyf M. DNA methylation, behavior and early life adversity. Journal of genetics and genomics = Yi chuan xue bao. 2013;40:331–8. doi: 10.1016/j.jgg.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zeng C. Role of DNA methylation in cardiovascular diseases. Clinical and experimental hypertension. 2016;38:261–7. doi: 10.3109/10641963.2015.1107087. [DOI] [PubMed] [Google Scholar]
- 12.Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–25. doi: 10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–32. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Cunliffe VT. Experience-sensitive epigenetic mechanisms, developmental plasticity, and the biological embedding of chronic disease risk. Wiley interdisciplinary reviews Systems biology and medicine. 2015;7:53–71. doi: 10.1002/wsbm.1291. [DOI] [PubMed] [Google Scholar]
- 15.Ziller MJ, Muller F, Liao J, Zhang Y, Gu H, Bock C, et al. Genomic distribution and inter-sample variation of non-CpG methylation across human cell types. PLoS genetics. 2011;7:e1002389. doi: 10.1371/journal.pgen.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sengupta N, Seto E. Regulation of histone deacetylase activities. Journal of cellular biochemistry. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- 17.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13:484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 18.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 19.Marinova Z, Maercker A, Kuffer A, Robinson MD, Wojdacz TK, Walitza S, et al. DNA methylation profiles of elderly individuals subjected to indentured childhood labor and trauma. BMC Med Genet. 2017;18:21. doi: 10.1186/s12881-017-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janusek LW, Tell D, Gaylord-Harden N, Mathews HL. Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: An epigenetic link. Brain, Behavior, and Immunity. 2017;60:126–35. doi: 10.1016/j.bbi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Loucks EB, Huang YT, Agha G, Chu S, Eaton CB, Gilman SE, et al. Epigenetic Mediators Between Childhood Socioeconomic Disadvantage and Mid-Life Body Mass Index: The New England Family Study. Psychosom Med. 2016;78:1053–65. doi: 10.1097/PSY.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child development. 2010;81:252–69. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of personality. 2004;72:1365–93. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 24.Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neuroscience and biobehavioral reviews. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Palma-Gudiel H, Cordova-Palomera A, Leza JC, Fananas L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neuroscience and biobehavioral reviews. 2015;55:520–35. doi: 10.1016/j.neubiorev.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Romens SE, McDonald J, Svaren J, Pollak SD. Associations between early life stress and gene methylation in children. Child Dev. 2015;86:303–9. doi: 10.1111/cdev.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological psychiatry. 2008;63:1147–54. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swartz JR, Hariri AR, Williamson DE. An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Molecular psychiatry. 2017;22:209–14. doi: 10.1038/mp.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houtepen LC, Vinkers CH, Carrillo-Roa T, Hiemstra M, Van Lier PA, Meeus W, et al. Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans. Nature Communications. 2016:7. doi: 10.1038/ncomms10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prados J, Stenz L, Courtet P, Prada P, Nicastro R, Adouan W, et al. Borderline personality disorder and childhood maltreatment: a genome-wide methylation analysis. Genes Brain Behav. 2015;14:177–88. doi: 10.1111/gbb.12197. [DOI] [PubMed] [Google Scholar]
- 32.Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. Genome-wide epigenetic regulation by early-life trauma. JAMA Psychiatry. 2012;69:722–31. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khulan B, Manning JR, Dunbar DR, Seckl JR, Raikkonen K, Eriksson JG, et al. Epigenomic profiling of men exposed to early-life stress reveals DNA methylation differences in association with current mental state. Transl Psychiatry. 2014;4:e448. doi: 10.1038/tp.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unternaehrer E, Meyer AH, Burkhardt SC, Dempster E, Staehli S, Theill N, et al. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress. 2015;18:451–61. doi: 10.3109/10253890.2015.1038992. [DOI] [PubMed] [Google Scholar]
- 35.Bustamante AC, Aiello AE, Guffanti G, Galea S, Wildman DE, Uddin M. FKBP5 DNA methylation does not mediate the association between childhood maltreatment and depression symptom severity in the Detroit Neighborhood Health Study. Journal of Psychiatric research. 2018;96:39–48. doi: 10.1016/j.jpsychires.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Knaap LJ, Oldehinkel AJ, Verhulst FC, van Oort FV, Riese H. Glucocorticoid receptor gene methylation and HPA-axis regulation in adolescents. The TRAILS study. Psychoneuroendocrinology. 2015;58:46–50. doi: 10.1016/j.psyneuen.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Frontiers in neuroendocrinology. 2013;34:27–46. doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. EpiGenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 39.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan YX, Dong J, Wu LJ, Shao S, Zhang J, Zhang L, et al. Associations between polymorphisms in the glucocorticoid-receptor gene and cardiovascular risk factors in a Chinese population. Journal of epidemiology / Japan Epidemiological Association. 2013;23:389–95. doi: 10.2188/jea.JE20130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huizenga NA, Koper JW, de Lange P, Pols HA, Stolk RP, Grobbee DE, et al. Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. The Journal of clinical endocrinology and metabolism. 1998;83:47–54. doi: 10.1210/jcem.83.1.4498. [DOI] [PubMed] [Google Scholar]
- 42.Lin RC, Wang WY, Morris BJ. High penetrance, overweight, and glucocorticoid receptor variant: case-control study. Bmj. 1999;319:1337–8. doi: 10.1136/bmj.319.7221.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li-Tempel T, Larra MF, Sandt E, Meriaux SB, Schote AB, Schachinger H, et al. The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clinical epiGenetics. 2016;8:12. doi: 10.1186/s13148-016-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–5. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, An Q, Goldberg J, Quyyumi AA, Vaccarino V. Promoter methylation of glucocorticoid receptor gene is associated with subclinical atherosclerosis: A monozygotic twin study. Atherosclerosis. 2015;242:71–6. doi: 10.1016/j.atherosclerosis.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JM, Stewart R, Park MS, Kang HJ, Kim SW, Shin IS, et al. Associations of BDNF genotype and promoter methylation with acute and long-term stroke outcomes in an East Asian cohort. PloS one. 2012;7:e51280. doi: 10.1371/journal.pone.0051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai A, Bruno D, Sarreal AS, Hernando RT, Saint-Louis LA, Nierenberg J, et al. Plasma BDNF levels vary in relation to body weight in females. PloS one. 2012;7:e39358. doi: 10.1371/journal.pone.0039358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300:R1053–69. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira MJ, Palming J, Svensson MK, Rizell M, Dalenback J, Hammar M, et al. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metabolism-Clinical and Experimental. 2014;63:1198–208. doi: 10.1016/j.metabol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 50.de Rooij SR, Costello PM, Veenendaal MV, Lillycrop KA, Gluckman PD, Hanson MA, et al. Associations between DNA methylation of a glucocorticoid receptor promoter and acute stress responses in a large healthy adult population are largely explained by lifestyle and educational differences. Psychoneuroendocrinology. 2012;37:782–8. doi: 10.1016/j.psyneuen.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspectives in psychiatric care. 2009;45:262–77. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 52.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular psychiatry. 2011;16:729–37. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of general psychiatry. 2008;65:409–15. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. The American journal of psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 55.Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain, behavior, and immunity. 2011;25:1468–74. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156b:700–8. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2963–7. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European journal of epidemiology. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- 59.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic medicine. 2007;69:402–9. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 61.Zieker J, Zieker D, Jatzko A, Dietzsch J, Nieselt K, Schmitt A, et al. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Molecular psychiatry. 2007;12:116–8. doi: 10.1038/sj.mp.4001905. [DOI] [PubMed] [Google Scholar]
- 62.Esser N, Paquot N, Scheen AJ. Inflammatory markers and cardiometabolic diseases. Acta clinica Belgica. 2015;70:193–9. doi: 10.1179/2295333715Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes care. 2013;36:166–75. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. European heart journal. 2014;35:578–89. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agita A, Alsagaff MT. Inflammation, Immunity, and Hypertension. Acta medica Indonesiana. 2017;49:158–65. [PubMed] [Google Scholar]
- 66.Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9470–5. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim D, Kubzansky LD, Baccarelli A, Sparrow D, Spiro A, 3rd, Tarantini L, et al. Psychological factors and DNA methylation of genes related to immune/inflammatory system markers: the VA Normative Aging Study. BMJ open. 2016;6:e009790. doi: 10.1136/bmjopen-2015-009790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Misiak B, Szmida E, Karpinski P, Loska O, Sasiadek MM, Frydecka D. Lower LINE-1 methylation in first-episode schizophrenia patients with the history of childhood trauma. Epigenomics. 2015;7:1275–85. doi: 10.2217/epi.15.68. [DOI] [PubMed] [Google Scholar]
- 69.Tehranifar P, Wu HC, Fan X, Flom JD, Ferris JS, Cho YH, et al. Early life socioeconomic factors and genomic DNA methylation in mid-life. EpiGenetics. 2013;8:23–7. doi: 10.4161/epi.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuo HP, Guo YY, Che L, Wu XZ. Hypomethylation of Interleukin-6 Promoter is Associated with the Risk of Coronary Heart Disease. Arquivos brasileiros de cardiologia. 2016;107:131–6. doi: 10.5935/abc.20160124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cash HL, McGarvey ST, Houseman EA, Marsit CJ, Hawley NL, Lambert-Messerlian GM, et al. Cardiovascular disease risk factors and DNA methylation at the LINE-1 repeat region in peripheral blood from Samoan Islanders. EpiGenetics. 2011;6:1257–64. doi: 10.4161/epi.6.10.17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baccarelli A, Tarantini L, Wright RO, Bollati V, Litonjua AA, Zanobetti A, et al. Repetitive element DNA methylation and circulating endothelial and inflammation markers in the VA normative aging study. EpiGenetics. 2010;5:222–8. doi: 10.4161/epi.5.3.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Tarantini L, et al. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–28. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin RT, Hsi E, Lin HF, Liao YC, Wang YS, Juo SH. LINE-1 methylation is associated with an increased risk of ischemic stroke in men. Current neurovascular research. 2014;11:4–9. doi: 10.2174/1567202610666131202145530. [DOI] [PubMed] [Google Scholar]
- 75.Turcot V, Tchernof A, Deshaies Y, Perusse L, Belisle A, Marceau S, et al. LINE-1 methylation in visceral adipose tissue of severely obese individuals is associated with metabolic syndrome status and related phenotypes. Clinical epiGenetics. 2012;4:10. doi: 10.1186/1868-7083-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexeeff SE, Baccarelli AA, Halonen J, Coull BA, Wright RO, Tarantini L, et al. Association between blood pressure and DNA methylation of retrotransposons and pro-inflammatory genes. International journal of epidemiology. 2013;42:270–80. doi: 10.1093/ije/dys220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim M, Long TI, Arakawa K, Wang R, Yu MC, Laird PW. DNA methylation as a biomarker for cardiovascular disease risk. PloS one. 2010;5:e9692. doi: 10.1371/journal.pone.0009692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suderman M, Borghol N, Pappas JJ, Pinto Pereira SM, Pembrey M, Hertzman C, et al. Childhood abuse is associated with methylation of multiple loci in adult DNA. BMC Med Genomics. 2014;7:13. doi: 10.1186/1755-8794-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz PE, Gross JA, Dhir SK, Maussion G, Yang J, Bramoulle A, et al. Epigenetic Regulation of the Kappa Opioid Receptor by Child Abuse. Biological psychiatry. 2017 doi: 10.1016/j.biopsych.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 80.Smearman EL, Almli LM, Conneely KN, Brody GH, Sales JM, Bradley B, et al. Oxytocin Receptor Genetic and Epigenetic Variations: Association With Child Abuse and Adult Psychiatric Symptoms. Child Dev. 2016;87:122–34. doi: 10.1111/cdev.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumsta R, Marzi SJ, Viana J, Dempster EL, Crawford B, Rutter M, et al. Severe psychosocial deprivation in early childhood is associated with increased DNA methylation across a region spanning the transcription start site of CYP2E1. Translational Psychiatry. 2016:6. doi: 10.1038/tp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esposito EA, Jones MJ, Doom JR, MacIsaac JL, Gunnar MR, Kobor MS. Differential DNA methylation in peripheral blood mononuclear cells in adolescents exposed to significant early but not later childhood adversity. Dev Psychopathol. 2016;28:1385–99. doi: 10.1017/S0954579416000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lutz PE, Turecki G. DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience. 2014;264:142–56. doi: 10.1016/j.neuroscience.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285:R271–3. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 85.Vogler GP. Methodology for Genetic-Studies of Twins and Families - Neale,Mc, Cardon,Lr. Behav Genet. 1993;23:107–8. [Google Scholar]
- 86.Ollikainen M, Smith KR, Joo EJ, Ng HK, Andronikos R, Novakovic B, et al. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Human molecular Genetics. 2010;19:4176–88. doi: 10.1093/hmg/ddq336. [DOI] [PubMed] [Google Scholar]
- 87.Tan Q, Christiansen L, von Bornemann Hjelmborg J, Christensen K. Twin methodology in epigenetic studies. The Journal of experimental biology. 2015;218:134–9. doi: 10.1242/jeb.107151. [DOI] [PubMed] [Google Scholar]
- 88.Hannon E, Lunnon K, Schalkwyk L, Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: implications for epigenetic studies of neurological and neuropsychiatric phenotypes. EpiGenetics. 2015;10:1024–32. doi: 10.1080/15592294.2015.1100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edgar RD, Jones MJ, Meaney MJ, Turecki G, Kobor MS. BECon: a tool for interpreting DNA methylation findings from blood in the context of brain. Translational psychiatry. 2017;7:e1187. doi: 10.1038/tp.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kubota T, Miyake K, Hirasawa T. Epigenetic understanding of gene-environment interactions in psychiatric disorders: a new concept of clinical genetics. Clinical epiGenetics. 2012;4:1. doi: 10.1186/1868-7083-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cordell HJ. Detecting gene-gene interactions that underlie human diseases. Nature reviews Genetics. 2009;10:392–404. doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michels KB, Binder AM, Dedeurwaerder S, Epstein CB, Greally JM, Gut I, et al. Recommendations for the design and analysis of epigenome-wide association studies. Nature methods. 2013;10:949–55. doi: 10.1038/nmeth.2632. [DOI] [PubMed] [Google Scholar]
- 94.Sartor CE, Grant JD, Duncan AE, McCutcheon VV, Nelson EC, Calvert WJ, et al. Childhood sexual abuse and two stages of cigarette smoking in African-American and European-American young women. Addictive behaviors. 2016;60:131–6. doi: 10.1016/j.addbeh.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fothergill K, Ensminger ME, Doherty EE, Juon HS, Green KM. Pathways from Early Childhood Adversity to Later Adult Drug Use and Psychological Distress: A Prospective Study of a Cohort of African Americans. Journal of health and social behavior. 2016;57:223–39. doi: 10.1177/0022146516646808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Traub F, Boynton-Jarrett R. Modifiable Resilience Factors to Childhood Adversity for Clinical Pediatric Practice. Pediatrics. 2017:139. doi: 10.1542/peds.2016-2569. [DOI] [PubMed] [Google Scholar]
- 97.Beutel ME, Tibubos AN, Klein EM, Schmutzer G, Reiner I, Kocalevent RD, et al. Childhood adversities and distress - The role of resilience in a representative sample. PloS one. 2017;12:e0173826. doi: 10.1371/journal.pone.0173826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kundakovic M, Gudsnuk K, Herbstman JB, Tang DL, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6807–13. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoo JY, Lee S, Lee HA, Park H, Park YJ, Ha EH, et al. Can proopiomelanocortin methylation be used as an early predictor of metabolic syndrome? Diabetes care. 2014;37:734–9. doi: 10.2337/dc13-1012. [DOI] [PubMed] [Google Scholar]
- 100.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature neuroscience. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.