Abstract
Objective
To assess the effect of prophylactic negative pressure wound therapy on surgical site infections and other wound complications in women after cesarean delivery.
Data Sources
We searched Ovid Medline, Embase, SCOPUS, Cochrane Database of Systematic Reviews and ClinicalTrials.gov.
Study Eligibility Criteria
We included randomized controlled trials and observational studies comparing prophylactic negative pressure wound therapy to standard wound dressing for cesarean delivery.
Study Appraisal and Synthesis Methods
The primary outcome was surgical site infection after cesarean. Secondary outcomes were composite wound complications, wound dehiscence, wound seroma, endometritis, and hospital re-admission. Heterogeneity was assessed using Higgin’s I2. Relative risks with 95% confidence intervals were calculated using random effects models.
Results
Six randomized controlled trials and three cohort studies in high-risk mostly obese women met inclusion criteria and were included in the meta-analysis. Six were full-text articles, two published abstracts, and one report of trial results in ClinicalTrials.gov. Studies were also heterogeneous in the patients included and type of negative pressure wound therapy device. The risk of surgical site infection was significantly lower with use of prophylactic negative pressure wound therapy compared with standard wound dressing (7 studies: pooled RR 0.45; 95% CI 0.31, 0.66; ARR −6.0%, 95% CI −10.0%, −3.0%; NNT 17, 95% CI 10, 34). There was no evidence of significant statistical heterogeneity (I2=9.9%) or publication bias (Egger P=0.532). Of the secondary outcomes, only composite wound complications were significantly reduced in patients receiving prophylactic negative pressure wound therapy compared to standard dressing (9 studies: pooled RR 0.68, 95% CI 0.49, 0.94).
Conclusions
Studies on the effectiveness of prophylactic negative pressure wound therapy at cesarean delivery are heterogeneous, but suggest a reduction in surgical site infection and overall wound complications. Larger definitive trials are needed to clarify the clinical utility of prophylactic negative pressure wound therapy after cesarean.
Keywords: Cesarean delivery, meta-analysis, prophylactic negative pressure wound therapy, surgical site infection, dehiscence, seroma, endometritis, hospital readmission, antibiotics
Introduction
Cesarean delivery is the most common major surgical procedure among women in the United States. In 2015 over 1.2 million cesarean deliveries were performed in the United States, representing 32% of all births1. The overall rate of cesarean delivery has increased dramatically since 1996, although starting in 2009 this rate has been slowly decreasing, in part due to efforts to reduce non-medically indicated cesareans2. Postoperative complications remain a significant and costly contributor to maternal morbidity, particularly among high-risk patients3. Obesity (body mass index [BMI] >30 kg/m2) exacerbates the problem of surgical site infection after cesarean delivery4,5. The impact of obesity has received particular attention given the rising global levels of obesity6.
Modern techniques for prevention of wound complications include proper pre-operative skin preparation, antiseptic surgical techniques, prophylactic antibiotics, and sterile postoperative dressings7. Despite these measures, wound complications after cesarean remain common. More recently, prophylactic negative pressure wound therapy (NPWT) has emerged as a possible intervention for reducing surgical wound complications. This type of dressing, first approved by the FDA in 1995, uses negative pressure at the wound site to reduce edema, remove exudate, increase localized blood flow, stimulate granulation tissue growth, and ultimately accelerate wound healing8. Although most commonly utilized in the treatment of wounds, emerging research suggests that NPWT may be beneficial as prophylaxis among high-risk patients.
In 2010, two brands of modified, single-use, battery powered, portable NPWT devices, Prevena™ (KCI USA, San Antonio, TX) and PICO™ (Smith & Nephew, St. Petersburg, FL), were FDA-cleared for prophylactic application after wound closure at the time of surgery. A recent meta-analysis of randomized controlled trials investigating the use of NPWT for closed surgical incisions showed significant reductions in wound infection, seroma formation, and wound exudate compared to a standard surgical dressing9. However, none of the included studies reported data for cesarean deliveries. While several observational studies and pilot randomized trials (RCTs) have supported the use of NPWT to reduce wound complications after cesarean delivery, the relatively small sample sizes in these studies limit their impact on clinical practice.
The objective of this systematic review and meta-analysis was to evaluate the effectiveness of prophylactic NPWT on the rate of surgical site infections and other wound complications in women undergoing cesarean delivery compared with standard surgical dressings.
Methods
This study did not involve any patient health information, human or animal experimentation, and was therefore exempt from IRB review. Acelity played no role in the design, the analysis, or the interpretation of this study.
Search strategy and study selection
This systematic review and meta-analysis was conducted based on a pre-defined study protocol following the Preferred Reporting Items for Systematic Reviews and Meta-analysis criteria10. A medical librarian searched the published and grey literature for records discussing cesarean delivery and prophylactic NPWT in March of 2017. The librarian (LS) created search strategies using a combination of keywords and controlled vocabulary in Ovid Medline 1946-, Embase 1947-, Scopus 1823-, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrials.gov 1997-. If studies in Clinicaltrials.gov were reported as completed but did not provide results and a related publication was not found, the corresponding author was queried for unpublished results. The full search strategies can be found in the Appendix.
Two authors (RK, LY) independently reviewed the search results to identify relevant studies. Titles and abstracts were screened, and articles deemed potentially relevant were retrieved for full-text review. Studies that did not involve NPWT and cesarean delivery in human subjects were excluded. Studies that investigated non-prophylactic use of NPWT or did not include outcome data relevant to wound infections or complications were also excluded. Reviews, commentaries, and case reports were also excluded. Given that the use of prophylactic NPWT at cesarean delivery is still relatively novel, including only RCTs would likely be too restrictive and potentially introduce publication bias. Therefore, we included both RCTs and cohort studies. The bibliographies of included studies were searched for additional eligible studies. Lastly, an expert in the field (MT) was queried for any additional studies, which led to retrieval of a PhD thesis with interim results from an RCT.
Data extraction
Two reviewers (RK, LY) independently reviewed eligible articles to extract data regarding study characteristics including design and location, inclusion and exclusion criteria, number of patients, patient demographics and comorbidities, frequency of wound complications, and hospital readmissions. The type of NPWT device and length of treatment in both the intervention and control groups was collected. In case of published abstracts, the first author was contacted for additional information regarding methods, baseline demographics, unpublished results and detailed outcome information, although no additional results were obtained from this correspondence. The two reviewers assessed the quality of each study based on criteria adapted from the Cochrane Handbook11. Individual study quality was assessed using pre-defined criteria. High quality studies were defined as randomized trials with appropriate randomization method, clear definition of outcomes and use of intention to treat analysis, while low quality studies were missing one or more of these attributes. Outcomes were considered clearly defined if the authors provided an adequate level of detail about the criteria and timing of outcome data collection for this metric to be reproducible. Disagreements were resolved through arbitration and discussion with a third author (MT).
Outcomes
The primary outcome for this analysis was surgical site infection after cesarean. This was chosen because of its clinical significance and the biologic plausibility of NPWT on its prevention. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for wound complications, and hospital re-admission. In studies for which there were both overall complication rates and rates stratified by complication type, the overall complication rate as reported was used for the outcome of wound complications to avoid counting patients multiple times.
Statistical Analysis
All statistical analyses were performed in the METAN add-on program in STATA version 14.2 (StataCorp LP, College Station, TX). Statistical heterogeneity was assessed using the Higgin’s I2, with a value >30% considered to represent significant heterogeneity12. With the exception of a sensitivity analysis described below, all risk estimates were reported as pooled relative risks with 95% confidence intervals (CI). Additionally, we estimated pooled absolute risks for the primary outcome of surgical site infection in the NPWT and standard dressing groups using meta-analysis of proportions, and the associated absolute risk reduction and number needed to treat. A random effects model13 was used for all meta-analyses even when statistical heterogeneity was not evident, given the likelihood of clinical heterogeneity between studies. One study also reported adjusted odds ratios for surgical site infection and overall wound complications15. We conducted a sensitivity analysis to assess if use of the adjusted odds ratios would have an impact on the pooled estimates. Analyses were also stratified by study design, abstract versus full-text, NPWT device type, and study quality to assess their impact on our estimates. All secondary analyses were pre-specified. Publication bias was assessed by visual inspection of a funnel plot and symmetry was tested statistically using the Egger’s test16,17.
Results
Study selection
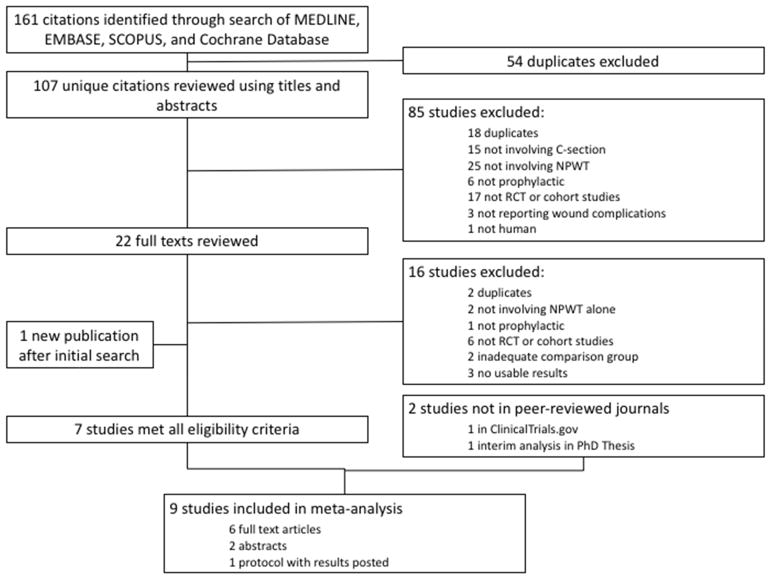
A total of 161 results were identified in the initial search and exported to EndNote. Following the removal of duplicates, a total of 107 unique citations remained. The titles and abstracts were screened for initial inclusion. Eighty-five studies were excluded for being additional duplicates (n=18), not related to the use of prophylactic NPWT after cesarean delivery (n=46), ineligible study designs (n=17), not reporting appropriate outcomes (n=3), and non-human subjects (n=1). Twenty-two remaining studies were reviewed in full-text or published abstract if no full-text was available. Of these, 16 studies were excluded for duplicate publication of results (n=2), not involving prophylactic NPWT alone (n=3), ineligible study designs (n=6), no usable results (n=3), and not having a valid comparison group (n=2) (Figure 1). One cohort study comparing prophylactic NPWT to standard dressing with regard to overall wound complications was not included because the authors reported odds ratios only and did not present the necessary count data for calculating absolute and relative risks14. In addition to the six remaining studies15,18–22, two unpublished studies reported outcomes in ClinicalTrials.gov23,24, one of which was published during manuscript preparation23, and one RCT were available as part of a published PhD thesis25, resulting in a total of nine studies included in the analysis.
Figure 1.
Flow diagram for study selection.
Study characteristics
Of the nine studies meeting inclusion criteria, six studies were RCTs, while three were cohort studies (two retrospective, one prospective with a historical control group22). Six studies were full text publications, two were published abstracts, and one was presented as results in ClinicalTrials.gov. Five studies were determined to be high quality and four were low quality. Seven studies were conducted in the United States and two were conducted in Australia18 and Denmark25. Inclusion and exclusion criteria differed significantly across studies, with the majority including high risk obese women above a given BMI threshold. Inclusion of scheduled or emergent cesarean deliveries in the studies was variable. Various prophylactic NPWT devices were employed, with Prevena ™ and PICO ™ systems being the most common. Sample sizes ranged from 54 to 535 patients (Table 1).
Table 1.
Characteristics of included studies
| Author (et al.) | Year | Country | Study Design | Text Form | Inclusion Criteria | Exclusion Criteria | Sample Size | NPWT Device Used | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Chaboyer | 2014 | Australia | RCT | Full text |
|
|
87 | PICO | High |
| Mark | 2014 | USA | RC | Full text |
|
Not reported | 69 | KCI | Low |
| Ruhstaller | 2017 | USA | RCT | Full text |
|
|
119 | Prevena | High |
| Swift | 2015 | USA | Mixed | Full text | Not reported | Not reported | 319 | Prevena | Low |
| Tuuli | 2017 | USA | RCT | Abstract |
|
|
120 | PICO | High |
| Villers | 2017 | USA | RC | Abstract |
|
Not reported | 317 | Not reported | Low |
| Hyldig | 2016 | Denmark | RCT | PhD Thesis |
|
|
535 | PICO | High |
| Heine | 2017 | USA | RCT | Full text |
|
|
82 | Prevena | High |
| Stitely | N/A | USA | RCT | Clinical Trials.gov (NCT0065 4641) |
|
|
54 | Not reported | Low |
Abbreviations: NPWT, negative pressure wound therapy; RCT, randomized controlled trial; RC, retrospective cohort.
Reporting of baseline characteristics varied across studies, with several studies lacking any information (Table 2). The most commonly reported characteristics were age and BMI, while diabetes was the most commonly reported comorbidity. Only two studies reported race. While the age distribution appeared similar across studies, average BMI was highly variable, ranging from 35 to 54 kg/m2 in the intervention groups. Other potentially important comorbidities such as smoking history, parity, cesarean history, and chorioamnionitis were reported by four or fewer studies. Surgical characteristics including length of surgery and closure technique were infrequently reported.
Table 2.
Baseline characteristics of patient populations in the included studies.
| Characteristic | Chaboyer | Mark | Ruhstaller | Swift | Villers | Heine | Hyldig |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age | |||||||
| Intervention | 30.6 (5.5)a | 26.1 (4.2)b | 27 (8)a | 30.8 (6.0)b | -- | 30.35 (5.724)b | 32 (5)b |
| Control | 30.7 (5.0) | 29.5 (6.6) | 29 (10) | 29.4 (5.8) | 29.67 (4.953) | 32 (5) | |
|
| |||||||
| BMI (kg/m2) | |||||||
| Intervention | 35.7 (4.5)a | 53.8 (11.1)b | 36.1 (8.6)a | 37.7 (9.0)b | 48.2c | 46.25 (7.319)b | 34.7 (6.7)a |
| Control | 36.8 (5.8) | 51.3 (5.8) | 35.1 (9.5) | 33.6 (8.5) | 44.6 | 46.79 (5.608) | 33.9 (6.5) |
|
| |||||||
| Race* (n[%]) | |||||||
| White | -- | -- | 26 (21.8) | -- | -- | 27 (29.3) | |
| African American | 91 (76.4) | 64 (69.6) | |||||
| Other | 2 (1.7) | 1 (1.1) | |||||
|
| |||||||
| Gestational Age (weeks) | |||||||
| Intervention | -- | 37.8 (2.9)b | 39 (2)a | 39 (2.6)a | -- | 38.08 (1.983)b | |
| Control | 36.9 (3.8) | 39 (2) | 39 (4.3) | 37.87 (1.976) | |||
|
| |||||||
| Planned CS (%) | |||||||
| Intervention | 100 | 52.4 | 0 | 52 | |||
| Control | 100 | 77.1 | 0 | 53 | |||
|
| |||||||
| Comorbidities (%) | |||||||
|
| |||||||
| Any | |||||||
| Intervention | 68.1 | -- | -- | 100 | -- | -- | |
| Control | 69.7 | 100 | |||||
|
| |||||||
| Diabetes | |||||||
| Intervention | 29.5 | 35 | 8.2 | 19.1 | -- | -- | 19 |
| Control | 27.9 | 27.1 | 7.0 | 16.3 | 19 | ||
|
| |||||||
| Smoker | |||||||
| Intervention | 6.8 | 14.3 | 8.2 | -- | -- | -- | 8 |
| Control | 23.3 | 16.7 | 5.2 | 9 | |||
|
| |||||||
| Multiparous | |||||||
| Intervention | -- | 61.9 | 62.3 | -- | -- | -- | |
| Control | 70.8 | 60.3 | |||||
|
| |||||||
| Previous CS | |||||||
| Intervention | 84 | -- | 18.0 | 43.6 | -- | -- | |
| Control | 93 | 19.0 | 36.8 | ||||
|
| |||||||
| Chorioamnionitis | |||||||
| Intervention | -- | 0 | 16.4 | 15.4 | 10 | -- | |
| Control | 4.2 | 12.1 | 2.9 | 1 | |||
|
| |||||||
| Surgical characteristics | |||||||
|
| |||||||
| Length of surgery, m | |||||||
| Intervention | -- | 76.3 (18.8)b | 61 (22)a | 64.6 (24.2)b | -- | -- | 37 (15)a |
| Control | 63.9 (21.6) | 62 (18) | 60.3 (20.1) | 36 (17) | |||
|
| |||||||
| Closed with staples (%) | |||||||
| Intervention | -- | 4.8 | -- | 3 | -- | -- | 61 |
| Control | 85.4 | 12 | 60 | ||||
Note: Tuuli, and Stitely did not provide baseline characteristics.
Race was reported as a combined term for the intervention and control groups.
Values reported as median(IQR)
Values reported as mean (SD)
Values reported as median
Abbreviations: BMI, body mass index; CS, cesarean section; m, minutes; IQR, interquartile range; SD, standard deviation.
Meta-analysis results and risk of bias
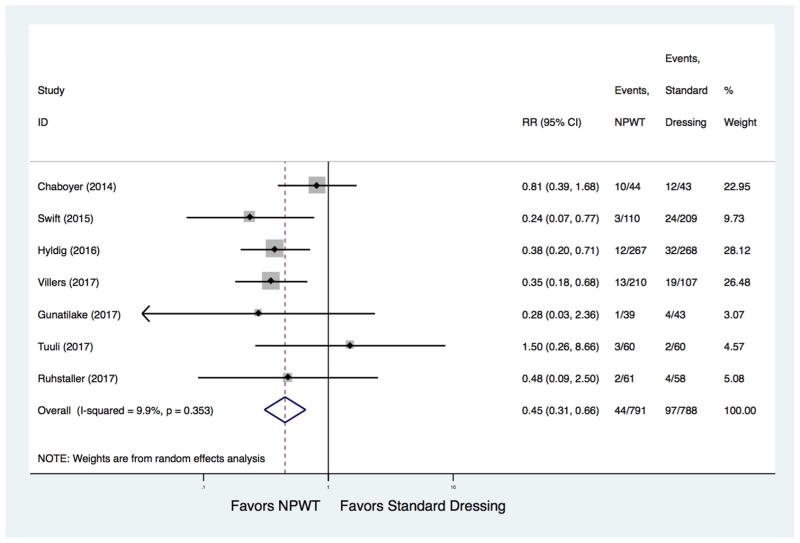
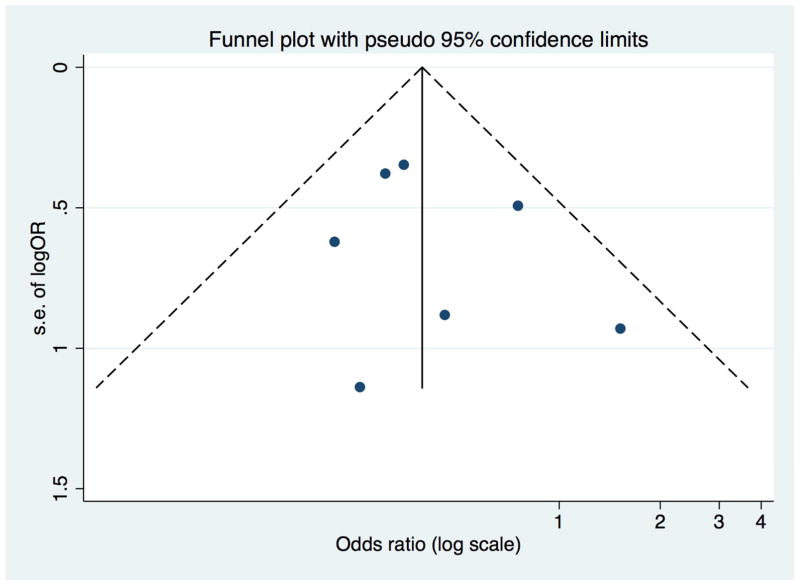
The absolute risk of developing surgical site infection was 5.0% (95% CI 2.0%, 7.0%) with prophylactic NPWT and 11% (95% CI 7.0%, 16.0%) with standard wound dressing. Compared with standard wound dressing, prophylactic NPWT was associated with a significantly lower risk of surgical site infection (7 studies, pooled RR 0.45, 95% CI [0.31, 0.66]) (Figure 2, Table 3). The absolute risk reduction was −6.0 % (95% CI −10.0 %, −3.0 %), with a number needed to treat of 17 (95% CI 10, 34). There was no evidence of significant statistical heterogeneity (I2=9.9%). In stratified analyses the risk estimate suggested a lower rate of surgical site infection with use of prophylactic NPWT across type of estimates used, study design, NPWT device, reporting as full-text or abstract, and study quality, although not statistically significant in some subgroups (Table 4). There was no evidence of publication bias (Egger test P=0.532) (Figure 3).
Figure 2.
Forest plot of the effect of prophylactic negative pressure wound therapy on surgical site infection after cesarean delivery.
Table 3.
Pooled estimates of the effect of prophylactic NPWT on the primary and secondary outcomes.
| Outcome | # Studies | Total N | Pooled RR (95% CI) | I2 (%) |
|---|---|---|---|---|
| Surgical site infection | 7 | 1579 | 0.45 (0.31, 0.66) | 9.9 |
| Composite wound complications | 9 | 1702 | 0.68 (0.49, 0.94) | 44.2 |
| Dehiscence | 5 | 1175 | 0.86 (0.61, 1.23) | 0 |
| Seroma | 2 | 437 | 1.21 (0.93, 1.57) | 0 |
| Endometritis | 3 | 1171 | 0.37 (0.13, 1.07) | 36.5 |
| Hospital re-admission | 2 | 156 | 0.80 (0.23, 2.76) | 0 |
Abbreviations: RR, relative risk; CI, confidence interval.
Table 4.
Stratified analysis of the effect of prophylactic NPWT on surgical site infection, composite wound complications and wound dehiscence
| Stratification | Surgical Site Infection (pooled RR[95% CI]) | I2(%) | Composite wound complications (pooled RR [95% CI]) | I2(%) | Dehiscence (pooled RR [95% CI]) | I2 (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Estimates | ||||||
| Unadjusted | 0.45 (0.31, 0.66) | 9.9 | 0.68 (0.49, 0.94) | 44.2 | 0.86 (0.61, 1.23) | 0 |
| Adjusted* | 0.42 (0.28, 0.63) | 0 | - | - | - | - |
|
| ||||||
| Study Design | ||||||
| RCT | 0.55 (0.35, 0.87) | 4.8 | 0.82 (0.57, 1.18) | 38.6 | 0.88 (0.52, 1.50) | 7.2 |
| Cohort | 0.32 (0.18, 0.57) | 0 | 0.45 (0.26, 0.78) | 24.7 | 0.71 (0.19, 2.63) | . |
|
| ||||||
| Device | ||||||
| PICO | 0.62 (0.31, 1.26) | 46.6 | 0.69 (0.54, 0.90) | 5.8 | 1.08 (0.35, 3.31) | 21.7 |
| Prevena | 0.30 (0.12, 0.71) | 0 | 0.36 (0.19, 0.66) | 0 | 0.69 (0.27, 1.76) | 0 |
| KCI | - | 0.20 (0.01, 3.50) | . | |||
| Unknown | 0.35 (0.18, 0.68) | . | 0.90 (0.39, 2.08) | 78.3 | ||
|
| ||||||
| Reported form | ||||||
| Full-text | 0.46 (0.29, 0.72) | 8.4 | 0.66 (0.44, 0.99) | 52.5 | 0.82 (0.57, 1.19) | 0 |
| Abstract | 0.57 (0.15, 2.23) | 57.2 | 0.81 (0.32, 2.03) | 46.5 | 1.96 (0.43, 8.91) | 0 |
|
| ||||||
| Quality | ||||||
| High | 0.55 (0.35, 0.87) | 4.8 | 0.67 (0.54, 0.84) | 0.0 | 0.81 (0.28, 2.32) | 31.6 |
| Low | 0.32 (0.18, 0.57) | 0 | 0.60 (0.27, 1.32) | 74.6 | 0.91 (0.32, 2.60) | 0 |
Denotes the adjusted OR
Figure 3.
Funnel plot of the effect of prophylactic negative pressure wound therapy on surgical site infection after cesarean delivery.
Prophylactic NPWT was associated with a statistically significant reduction in composite wound complications (9 studies, pooled RR 0.68, 95% CI 0.49, 0.94), but not in the other secondary outcomes, including dehiscence (5 studies, pooled RR 0.86 95% CI 0.61, 1,23), seroma (2 studies, pooled RR 1.21, 95% CI 0.93, 1.57), endometritis (3 studies, pooled RR 0.37, 95% CI 0.13, 1.07) or hospital re-admission (2 studies, pooled RR 0.80, 95% CI 0.23, 2.76) (Table 3)
Comment
Main Findings
We conducted this systematic review and meta-analysis to comprehensively synthesize evidence on the effectiveness of prophylactic NPWT on the risk of surgical site infections and other would complications after cesarean. Studies were heterogeneous in their design, patients included, type of NPWT device, and publication type. Nonetheless, our results show that prophylactic NPWT was associated with a statistically significant 55% reduction in surgical site infection and overall wound complications after cesarean. The number needed to treat to prevent one surgical site infection was 17. We observed no statistically significant reduction in secondary outcomes including dehiscence, seroma, endometritis, or hospital re-admission.
Clinical Implications
Our findings are consistent with previous studies showing reduction in infection rates with the use of prophylactic NPWT after non-OBGYN surgical procedures. A meta-analysis of prophylactic NPWT after general surgical procedures found a significant reduction in surgical site infections9. In contrast to our study, the authors also reported a significant reduction in seroma. Cesarean deliveries were not included in that study. Another meta-analysis of ventral hernia repair including five retrospective cohort studies reported a reduction in surgical site infection and wound dehiscence with the use of prophylactic NPWT, but not in the rate of seroma26.
A reduction in surgical site infection with prophylactic NPWT is biologically plausible. Proposed mechanisms of prophylactic NPWT include wound shrinkage, induction of cellular stretch that promotes wound healing, removal of extracellular fluid, creation of a favorable environment for healing and promotion of angiogenesis and neurogenesis27. It may also serve as a microbial barrier, increase blood flow, and improve tissue oxygenation. Cellular deformation may also release cytokines and inflammatory factors that promote chemotaxis of other cells, including leukocytes, into the area8,28.
Of our secondary outcomes, only overall wound complications were significantly reduced with prophylactic NPWT. The inclusion of surgical site infection in the composite wound complication measure likely explains this result in the absence of other significant differences in dehiscence, seroma, endometritis and hospital re-admission. However, these secondary outcomes were only reported by a subset of studies and thus may be limited by small overall sample sizes. Additionally, composite wound complication measures are difficult to interpret due to a lack of consistent definitions across studies. Nevertheless, these results are encouraging with regard to the potential ability of prophylactic NPWT to alter patient outcomes postoperatively.
Strengths and Weaknesses
Strengths of this review include the predesigned protocol, comprehensive search strategy involving an expert librarian (LS), two investigators independently screening all articles for eligibility and extracting data to reduce bias. We used a random effects model to pool data in order to take into account heterogeneity between studies even in the absence of demonstrable statistical heterogeneity. We included published abstracts to avoid publication bias since full-text articles represented only a proportion of studies. Finally, we conducted sensitivity and stratified analyses to assess the impact of various factors on our findings.
There are limitations that should be considered. Our findings carry forward the limitations of the primary studies. The relatively small number of studies and significant variability in outcome reporting are important limitations. While the inclusion of published abstracts and unpublished studies reduced publication bias, it carries the risk of including lower quality non-peer reviewed data. Moreover, there was significant heterogeneity between the studies included. The inclusion of cohort studies carries a risk of confounding, especially since many potential confounders were not consistently assessed in the primary studies. However, sensitivity analysis including adjusted estimates produced similar findings, suggesting robustness of our findings. Definitions of surgical site infections and other wound complication were unclear in some studies14,21. Side effects were not consistently reported in the studies and could not be synthesized. This is important because some studies have reported high rates of side effects including skin blisters, erythema, and wound bleeding with use of prophylactic NPWT after other types of surgical procedures29.
Finally, we did not include a cost-effectiveness analysis as part of the current study. Given that prophylactic NPWT devices cost between $200 and $500 USD, this is an important consideration in applying these results in a clinical setting30. Since 2015, three cost-effectiveness analyses of prophylactic NPWT for post-cesarean delivery have been performed using various methods but with inconclusive results30–32. Two studies based in Australia suggested that prophylactic NPWT was cost-effective in obese women undergoing cesarean delivery, although the degree of uncertainty around these estimates was high31,32. A U.S.-based decision-analytic model favored the standard postoperative dressing as the most cost-effective strategy in a patient population with a surgical site infection rate of 14% or less30. On the other hand, prophylactic NPWT was potentially cost-effective in populations with a higher risk of surgical site infection. Based on this model and our pooled estimates of absolute risk, it would seem that prophylactic NPWT would not be cost-effective in the patient population represented in our meta-analysis. However, as previously noted, patient characteristics were heterogeneous between studies and may not truly reflect a “high-risk” population as defined in these cost-effectiveness analyses. Clinical heterogeneity may also explain why the conclusions differed between the Australian analyses predicated on a specific group of women and the U.S.-based model that potentially involved a broader population of interest. Nevertheless, several additional studies, including those in our sample, have been conducted since the publication of these cost-effectiveness analyses, necessitating an updated assessment to incorporate these findings as well as changes in device pricing over time.
Summary and Future Research Direction
In conclusion, results of this meta-analysis suggest that use of prophylactic NPWT after cesarean delivery in high-risk patients is associated with a significant reduction in the risk of surgical site infection. However, because of the limited number and clinical heterogeneity between studies, further research is needed. Results of ongoing clinical trials33,34 powered to assess effectiveness, side effects and cost-effectiveness will help clarify the role of prophylactic NPWT after cesarean.
Acknowledgments
Dr Colditz is supported in part by the Foundation for Barnes Jewish Hospital. Dr. Tuuli is supported by NIH U01 (U01HD077384-03) and R01 (1R01HD086007-01) grants. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Financial Disclosure
Dr Tuuli received supplemental research grant funding from Acelity, manufacturers of Prevena™.
The other authors did not report any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2015. Natl Cent Heal Stat Data Br. 2016;2015(258):1–8. http://www.ncbi.nlm.nih.gov/pubmed/27648876. [PubMed] [Google Scholar]
- 2.Osterman MJK, Martin JA. Trends in low-risk cesarean delivery in the United States, 1990–2013. Natl Vital Stat Reports. 2014;63(6):1–16. [PubMed] [Google Scholar]
- 3.Creanga AA, Bateman BT, Butwick AJ, et al. Morbidity associated with cesarean delivery in the United States: Is placenta accreta an increasingly important contributor? Am J Obstet Gynecol. 2015;213(3):384.e1–384e11. doi: 10.1016/j.ajog.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Newlin C, Kuehl TJ, Pickrel A, Cawyer CR, Jones RO. Cesarean section incision complications and associated risk factors: A quality assurance project. Open J Obstet Gynecol. 2015;(November):789–794. [Google Scholar]
- 5.Conner S, Verticchio J, Tuuli M, Odibo A, Macones G, Cahill A. Maternal obesity and risk of postcesarean wound complications. Am J Perinatol. 2013;31(4):299–304. doi: 10.1055/s-0033-1348402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tipton AM, Cohen SA, Chelmow D. Wound infection in the obese pregnant woman. Semin Perinatol. 2011;35(6):345–349. doi: 10.1053/j.semperi.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Ayres-de-Campos D. Obesity and the challenges of caesarean delivery: Prevention and management of wound complications. Best Pract Res Clin Obstet Gynaecol. 2015;29(3):406–414. doi: 10.1016/j.bpobgyn.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Sarsam SE, Elliott JP, Lam GK. Management of wound complications from cesarean delivery. [Accessed February 25, 2017];Obstet Gynecol Surv. 2005 60(7):462–473. doi: 10.1097/01.ogx.0000166603.43959.aa. http://www.ncbi.nlm.nih.gov/pubmed/15995563. [DOI] [PubMed] [Google Scholar]
- 9.Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103:477–486. doi: 10.1002/bjs.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of. 2008 doi: 10.1002/9780470712184. [DOI] [Google Scholar]
- 12.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs C, Orth T, Gerkovich M, Heitmann E, Parrish M, Lu G. Traditional dressing compared with an external negative pressure system in preventing wound complications. Obstet Gynecol. 2014;123:145S. doi: 10.1097/01.AOG.0000447128.16566.58. [DOI] [Google Scholar]
- 15.Villers MS, Hopkins MK, Harris BS, Brancazio LR, Grotegut CA, Phillips Heine R. Negative pressure wound therapy reduces cesarean delivery surgical site infections in morbidly obese women. Am J Obstet Gynecol. 2017;216(1):S207. doi: 10.1016/j.ajog.2016.11.599. [DOI] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Egger M, Smith GD, Hartung DM, Paynter R, Helfand M. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaboyer W, Anderson V, Webster J, Sneddon A, Thalib L, Gillespie BM. Negative pressure wound therapy on surgical site infections in women undergoing elective Caesarean sections: A pilot RCT. Healthc (Basel, Switzerland) 2014;2(4):417–428. doi: 10.3390/healthcare2040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruhstaller K, Downes K, Chandrasekaran S, Srinivas S, Durnwald C. Prophylactic Wound Vacuum Therapy after Cesarean Section to Prevent Wound Complications in the Obese Population: A Randomized Controlled Trial (the ProVac Study) Am J Perinatol. 2017;34(11):1125–1130. doi: 10.1055/s-0037-1604161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuuli MG, Martin S, Stout MJ, et al. Pilot randomized trial of prophylactic negative pressure wound therapy in obese women after cesarean delivery. Am J Obstet Gynecol. 2017;216(1):S245. doi: 10.1016/j.ajog.2016.11.670. [DOI] [Google Scholar]
- 21.Mark KS, Alger L, Terplan M. Incisional negative pressure therapy to prevent wound complications following cesarean section in morbidly obese women: a pilot study. Surg Innov. 2014;21(4):345–349. doi: 10.1177/1553350613503736. [DOI] [PubMed] [Google Scholar]
- 22.Swift SH, Zimmerman MB, Hardy-Fairbanks AJ. Effect of Single-Use Negative Pressure Wound Therapy on Postcesarean Infections and Wound Complications for High-Risk Patients. [Accessed February 25, 2017];J Reprod Med. 2015 60(5–6):211–218. http://www.ncbi.nlm.nih.gov/pubmed/26126306. [PubMed] [Google Scholar]
- 23.Gunatilake RP, Swamy GK, Brancazio LR, et al. Closed-Incision Negative-Pressure Therapy in Obese Patients Undergoing Cesarean Delivery: A Randomized Controlled Trial. Am J Perinatol Reports. 2017;7(3):e151–e157. doi: 10.1055/s-0037-1603956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Feb 29, Identifier NCT00654641, Prevention of wound complications after cesarean delivery in obese women utilizing negative pressure wound therapy; 2008 April 2 [cited 2017 Sept 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT00654641 https://clinicaltrials.gov/ct2/show/NCT00654641. [Google Scholar]
- 25.Hyldig N, Vinter C, Kruse K, et al. Prophylactic incisional negative pressure wound therapy significantly reduces the risk of wound infection in obese women after caesarean section: the Happy Belly Study, a multicentre randomised controlled trial. 2016 [Google Scholar]
- 26.Swanson EW, Cheng H, Susarla SM, Mph DMD, Lough DM, Kumar AR. Does negative pressure wound therapy applied to closed incisions following ventral hernia repair prevent wound complications and hernia recurrence? A systematic review and meta-analysis. Plast Surg. 2016;24(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 27.Han S-K. Innovations and Advances in Wound Healing. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016. Negative-Pressure Wound Therapy; pp. 183–200. [DOI] [Google Scholar]
- 28.Mcnulty AK, Schmidt M, Feeley T, Kieswetter K. Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. 2007 doi: 10.1111/j.1524-475X.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 29.Karlakki S, Brem M, Giannini S, Khanduja V, Stannard J, Martin R. Negative pressure wound therapy for management of the surgical incision in orthopaedic surgery. [Accessed July 6, 2017];Bone Jt Res. 2013 2(12) doi: 10.1302/2046-3758.212.2000190. http://bjr.boneandjoint.org.uk.beckerproxy.wustl.edu/content/2/12/276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echebiri NC, McDoom MM, Aalto MM, Fauntleroy J, Nagappan N, Barnabei VM. Prophylactic use of negative pressure wound therapy after Cesarean delivery. Obstet Gynecol. 2015;125(2):299–307. doi: 10.1097/AOG.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 31.Heard C, Chaboyer W, Anderson V, Gillespie BM, Whitty JA. Cost-effectiveness analysis alongside a pilot study of prophylactic negative pressure wound therapy. J Tissue Viability. 2017;26(1):79–84. doi: 10.1016/j.jtv.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Tuffaha HW, Gillespie BM, Chaboyer W, Gordon LG, Scuffham PA. Cost-utility analysis of negative pressure wound therapy in high-risk cesarean section wounds. J Surg Res. 2015;195(2):612–622. doi: 10.1016/j.jss.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Feb 29, Identifier NCT03009110, Preventing adverse incisional outcomes at cesarean multicenter trial (Prevena-C); 2016 Dec 28 [cited 2017 Sept 2]. Available from https://clinicaltrials.gov/ct2/show/NCT03009110. [Google Scholar]
- 34.Australian New Zealand Clinical Trials Registry [Internet] Sydney (NSW): NHMRC Clinical Trials Centre, University of Sydney (Australia); 2005. Identifier ACTRN12615000286549. Adding negative pRESSure to improve healING (the DRESSING trial); 2015 Mar 2016 [cited 2017 Sept 2]. Available from https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368069. [Google Scholar]