Summary
Background
Incarceration can offer the opportunity for HIV care engagement, but is associated with poor HIV treatment outcomes after release. This study comprehensively assesses post-release linkage to HIV care (LTC) and the impact of transitional case management services.
Methods
To create a retrospective cohort of all adults with HIV released from Connecticut jails and prisons (2007–2014), we linked administrative custody and pharmacy databases with mandatory HIV/AIDS surveillance monitoring and case management data. We examined time to LTC (defined as first post-release HIV-1 RNA level) and viral suppression at time of LTC. Generalized estimating equations identified predictors of LTC within 14 and 30 days post-release.
Findings
Among 3,302 incarceration periods for 1,350 individuals, 21.1% (n=672/3181) and 34.0% (n=1042/3064) had LTC within 14 and 30 days post-release, respectively, and 28.9% (n=301/1042) had detectable viral levels at LTC. Factors positively associated with 14-day LTC included intermediate incarceration duration (31–364 days), year of release, transitional case management (reported in 34.2% [n=1128/3302] of releases), receipt of within-prison antiretroviral medications, and higher medical co-morbidity. Re-incarceration and conditional release were negatively associated with LTC. Race/ethnicity, bonded release, and psychiatric co-morbidity were additionally associated with 30-day LTC, but re-incarceration was not.
Interpretation
LTC post-release is suboptimal but improves when inmates’ medical, psychiatric, and case management needs are identified and addressed before release. Persons rapidly cycling through jail facilities are particularly vulnerable to missed linkage opportunities. Aligning justice and healthcare goals through integrated programming has great potential to improve long-term HIV treatment outcomes.
Funding
National Institutes of Health
Introduction
The United States has the highest incarceration rate globally (910 per 100,000 adults), concentrating people with both substance use disorders (SUDs) and HIV (1, 2). Annually, twelve million people transition from prisons and jails to communities, with one-sixth of the 1.2 million people living with HIV (PLH) passing through these settings (3). Criminal justice (CJ) settings are highly structured and can be positioned to diagnose, engage, and treat PLH with antiretroviral therapy (ART), which reduces within-prison morbidity and mortality (4, 5). Short-term detentions within and release from jails, however, are destabilizing and can undermine HIV treatment outcomes (6–8). Recidivism is often influenced by untreated SUDs and psychiatric disorders and can also negatively impact engagement in HIV care, resulting in suboptimal viral suppression (5–7, 9). Yet the longitudinal impact of incarceration and community reentry on continuity of HIV care remains poorly understood in part because prior observational studies have relied on either CJ or community data with limited ability to comprehensively link these two administrative sources.
Along the HIV care continuum towards viral suppression, linkage to community-based HIV care (LTC) is an early critical step (10). When transition is planned, PLH are often provided 10–30 days of ART, relying on community clinics to assume care thereafter. Medication refills typically require clinical assessment, including laboratory monitoring. Prior studies show that up to 80% of PLH released from prison fail to access ART medications within this timeframe, but it is unknown how this impacts LTC and viral suppression rates (11, 12). Moreover, none of these studies have evaluated enabling resources, such as case management, that may facilitate utilization of healthcare services during this transition. This study examines factors related to LTC post-release by evaluating a large cohort of PLH over an extended time period where viral loads (VL) drawn in both custodial and community settings are available and in a state where all healthcare delivery is integrated.
Methods
Study population
In 2013, the average daily census in the Connecticut Department of Correction (CTDOC) was 17,600 inmates (620 incarcerations per 100,000 adults) in 16 facilities, representing the highest incarceration rate in the Northeast (1). As one of six integrated CJ systems nationally, facilities house both sentenced and pre-trial detainees, and utilize a single healthcare provider, ensuring consistent delivery of medical treatment and other services.
All HIV testing in the CTDOC is voluntary, and HIV prevalence is 1.7%. After confirming HIV status, on-site HIV specialists prescribe guideline-concordant ART (10). Specialty nurses coordinate HIV care and referrals for assessment and treatment of psychiatric disorders. The CTDOC may also refer PLH for “transitional LTC” case management (TCM) services, initiated within 30 days before planned release and continued for up to 60 days post-release; they assist with transitional needs like housing, re-activation of medical insurance, and linkage to a community provider (13). Pre-trial detainees seldom receive TCM services. Upon release, PLH may receive a pharmacy voucher for 14 days of medications, including ART.
Individuals included in our final analysis met the following criteria (Figure 1): 1) adults ≥18 years old with confirmed HIV; 2) included in all administrative databases; 3) incarcerated in Connecticut at least once for ≥24 hours; and 4) released before the end of the 8-year observation period (January 1, 2007 – December 31, 2014).
Figure 1. Consort Diagram.
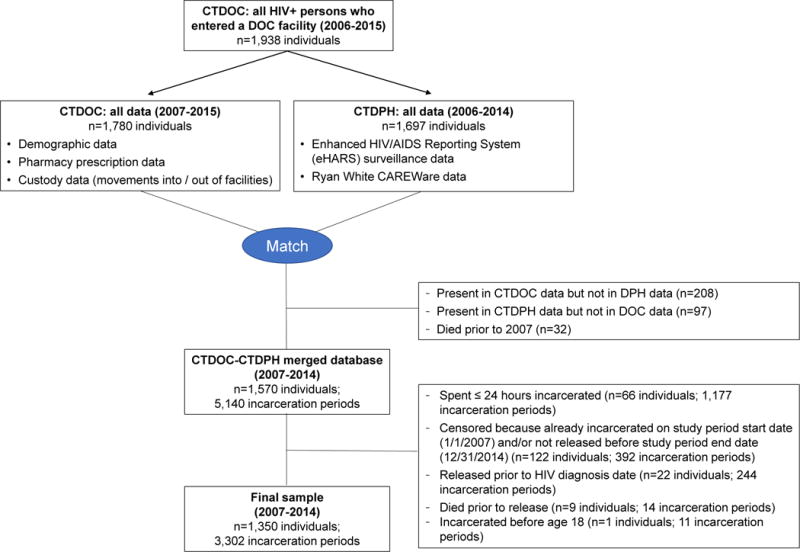
CTDOC: Connecticut Department of Correction; CTDPH: Connecticut Department of Public Health.
Data sources
We merged two CTDOC and two Connecticut Department of Public Health (CTDPH) data sources for analyses. CTDOC sources (2007–2015) included: 1) a custody database (demographics, entry and release dates, CJ conditions of release, health severity levels); and 2) a pharmacy database (all medications prescribed during incarceration, including ART). CTDPH data sources (2006–2014) included the: 1) enhanced HIV/AIDS Reporting System (eHARS) surveillance database that has mandatory laboratory reporting for HIV; and 2) CAREWARE TCM services database. With verified ≥95% completeness, Connecticut’s eHARS includes all longitudinal HIV-1 RNA monitoring data for all PLH from 2007 onwards, regardless of testing site (14). eHARS is cross-referenced with the National Death Index and Social Security databases for mortality and treatment outcomes outside Connecticut. AIDS service organizations use the CAREWare database, overseen by the CTDPH, to record any TCM provided according to required benchmarks; funding is based on services provided (15). Services provided through other community providers are not consistently available in CAREWare.
The CTDOC securely transferred their databases to the CTDPH, where on-site data managers used the Plus Link probabilistic record linkage program to match individuals using inmate number, name, and birthdate (16). The dataset was further restricted to PLH currently living in Connecticut. After merging, personal identifiers were removed and a de-identified database was provided for analysis. Institutional review boards at Yale University and CTDPH and the CTDOC Research Advisory Committee approved all procedures.
Study design
In this retrospective cohort study, we assessed LTC, defined as the first VL measured in the community, during the first year post-release. Recorded VLs serve as a proxy for routine HIV clinical care visits (both within the CTDOC and in the community) (17). After release, laboratory testing is often required before prescription refill because clinical data are not shared between CTDOC and community providers in real-time. CD4 data were not used because only AIDS-defining CD4 counts were reliably reported to CTDPH during the study observation period. As a secondary outcome, we defined viral suppression at the time of LTC as <400 copies/mL (4, 10). To avoid interruptions in ART, transitioning PLH must access care within 14–30 days. Thus, our primary outcomes were LTC within these two post-release timeframes (14 and 30 days).
We selected covariates based on the Behavioral Model for Vulnerable Populations adapted for CJ populations, which posits that an individual has predisposing, enabling or disabling, and need severity factors that impact healthcare utilization (18). Unlike most factors, enabling resources during community reintegration are most potentially amenable to intervention.
Predisposing factors
Predisposing factors included demographic information like age, sex, race/ethnicity, highest level of education attained, and marital status. Injection drug use (IDU) was based on the CTDPH original report for HIV exposure. Duration of HIV infection was calculated by subtracting HIV diagnosis dates from release dates.
Enabling/disabling factors
Using dates and types of movements into and out of facilities, we calculated length of incarceration as time spent in any CTDOC facility, and analyzed it continuously and categorically. In general, ≤30-day incarcerations took place in jail facilities, intermediate incarcerations of 31–364 days often included time spent in both jail and prison, and ≥365 day incarcerations included only sentenced prisoners. Recidivists (binary) were defined as those who were re-incarcerated at least once over the 8-year period. We categorized conditions of release as unsupervised, conditional release (to community-based supervision like parole or transitional housing), or bonded release. From CAREWare, we used dates of pre- and post-release encounters with case managers to create a dichotomous variable for receipt of TCM within 90 days pre-release and/or 14–30 days post-release.
Need factors
We defined viral suppression within 90 days before release as VL<400 copies/mL. Using pharmacy data, we assigned medications to the incarceration periods in which they were prescribed. Receipt of ART at any point during incarceration was coded dichotomously. We categorized additional medications into those for opioid use disorder (i.e., methadone, buprenorphine, or naltrexone, which were only available for brief supervised withdrawal), psychiatric disease (i.e., antipsychotics, antidepressants, or other neuropsychiatric medications), or other medical co-morbidities, which were all coded dichotomously. We further categorized medical co-morbidity by summing the number of medical conditions other than HIV requiring medication during the incarceration period, consistent with a co-morbidity index that reflects the burden of cumulative conditions in an individual.
Upon intake, CTDOC medical staff assign a psychiatric and addiction severity score (scale 1–5) to indicate level of service needs. These intake classification scores are used to determine the types of clinical care needed during incarceration and anticipated need for psychiatric and SUD treatment referrals upon release. We dichotomized psychiatric scores as 1–2 (low severity: no psychiatric history or a history of a currently inactive disorder not requiring treatment) vs. 3–5 (higher severity: mild, moderate, or severe disorder). A score of 4 indicated a need for special services and pharmacologic treatment and 5 indicated a crisis-level psychiatric disorder requiring close supervision or intensive support. Addiction scores of 3 indicated a moderate SUD requiring treatment, with scores 4–5 indicating a serious SUD requiring residential or intensive outpatient treatment. For individuals for whom severity scores were measured multiple times during a single incarceration period, we used the maximum score, representing the greatest overall severity for that incarceration period. Further information on psychiatric and SUD diagnoses was not available. To better reflect whether an inmate was identified as having a psychiatric issue and whether treatment was provided, the psychiatric severity score and treatment variables were combined to create a four-level categorical psychiatric need variable (1: low severity, untreated; 2: low severity, treated; 3: high severity, untreated; 4: high severity, treated).
Statistical analysis
The unit of analysis was a post-release period rather than an individual because most individuals had multiple incarcerations and post-release periods. We defined post-release periods as the time between the first day of (any type of) release from a CTDOC facility and the individual’s death, re-incarceration, or end of the observation period. For individuals with more than one incarceration, we examined each post-release period separately using generalized estimating equations (GEE) for binary outcomes to account for intra-subject correlation. Each post-release period included demographic characteristics of the individual and the characteristics of the incarceration and post-release period itself. We first described LTC within 14- and 30-day time periods following release. We then evaluated unadjusted predictors of having LTC within 14 and 30 days after release. Variables with bivariate associations of p<0.20 were included in the respective full multivariable model. Using backward selection, final parsimonious models included all variables with multivariable p-values <0.10. Because individuals on average were incarcerated fewer than 3 times during the observation period, we assumed the m-dependent correlation structure with m=3, which allowed for a correlation between the first three repeated measures and also minimized the quasi-likelihood under the independence model criterion (QIC). Findings were robust to changes in correlation structure. Sensitivity analyses exploring associations between addiction severity score, IDU risk, conditional release, re-incarceration, and incarceration duration found no significant collinearities or interactions that required inclusion. Age was initially modeled as continuous but ultimately dichotomized at the sample median for model fit. “Years since HIV diagnosis” was modeled both continuously and categorically based on quartiles and multiple clinically significant time points, but demonstrated a linear trend and was ultimately modeled as continuous. All analyses were performed using SAS version 9·4 (SAS Institute Inc.).
Role of the funding source
The funders played no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
Description of post-release periods
Of 3,302 eligible post-release periods, most were over 45 years old at the time of release (52.4% [n=1729]), male (77.6% [n=2562]), racial/ethnic minorities (82.7% [n=2731]), and unmarried (86.4% [n=2752/3184]). Most (70.2% [n=2317]) HIV infections were related to IDU and the median time since HIV diagnosis was 12.7 years (interquartile range [IQR]: 6.8–18.0).
On average, inmates were incarcerated 2.5 (± 2.0) times during the 8-year observation period. Median incarceration lengths and post-release periods were 73 (IQR: 25-201) and 296 (IQR: 104-817) days, respectively. There were 179 post-release periods during which the observed individual died. Most (60.7% [n=2003]) post-release periods were unsupervised. At least one TCM visit was recorded for 34.2% (n=1128) of periods, with 29.1% (n=962) having both pre- and post-release visits. During 66.4% (n=2191) and 39.0% (n=1287) of incarceration periods, respectively, inmates were prescribed ART and achieved pre-release viral suppression. Provision of TCM services, pre-release ART prescription, and viral suppression levels were higher for more contemporary releases (Supplementary Table 1).
In 55.1% (n=1818) of incarceration periods, individuals were solely being treated with ART, while 20.2% (n=668) had multiple medical co-morbidities. Almost half (45.3% [n=1497]) had low psychiatric severity scores on intake and never received any psychiatric medication, while 31.7% (n=1048) had high scores and received treatment. Addiction severity scores were high in 18.2% (n=591/3247) of periods, and 1.5% (n=50/3302) included medication prescriptions for an opioid use disorder.
Time to linkage to care and viral suppression
One-fifth of post-release periods involved LTC within 14 days after release, at which time 25.7% had a detectable VL (Figure 2). When the post-release time frame was extended to 30 days, an additional 12.9% had LTC (34.0% total), among which 33.5% had a detectable VL. By 90 days post-release, 60.7% of post-release periods involved LTC, but among the 26.5% of the sample that accessed care solely within the 31–90 day window, 38.9% had detectable virus. The proportion with detectable levels rose to 59.8% when the first VL assessed was between 6–12 months after release.
Figure 2.
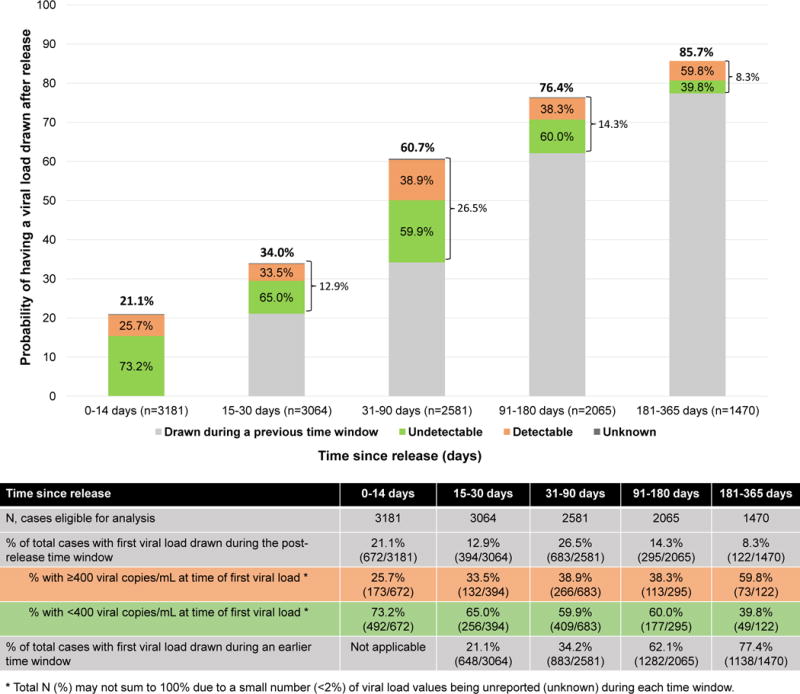
Time to linkage to care as measured by first HIV RNA viral load drawn after release from prison or jail
Factors associated with linkage to care within 14 days
In the 14-day multivariable model (Table 1), there were no significant predisposing factors that independently predicted LTC. Enabling resources that improved LTC included intermediate incarceration duration (31–364 days, but not longer) and receipt of TCM. Re-incarceration and conditional release were negatively associated with LTC. Two need factors were associated with LTC: receipt of ART during incarceration and having higher medical co-morbidity.
Table 1.
Binomial generalized estimating equations model assessing factors associated with having a viral load drawn within 14 days after release, adjusting for intra-subject correlation a
| Variable | n (%)b | Unadjusted Model OR (95% CI) | p-value | Full Adjusted Model OR (95% CI) | p-value | Parsimonious Adjusted Model OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Predisposing factors | |||||||
|
| |||||||
| Age at time of release | |||||||
| ≤ 45 years | 1516 (47.7%) | referent | referent | ||||
| > 45 years | 1665 (52.3%) | 1.35 (1.12–1.63) | 0.001 | 1.15 (0.93–1.42) | 0.19 | ||
|
| |||||||
| Sexc | |||||||
| Female | 717 (22.5%) | referent | |||||
| Male | 2464 (77.5%) | 0.93 (0.75–1.16) | 0.53 | ||||
|
| |||||||
| Race/Ethnicity | |||||||
| White | 553 (17.4%) | referent | |||||
| Black | 1326 (41.7%) | 0.94 (0.71–1.23) | 0.65 | ||||
| Hispanic | 1185 (37.3%) | 1.16 (0.88–1.53) | 0.29 | ||||
| Other | 117 (3.7%) | 0.89 (0.56–1.42) | 0.63 | ||||
|
| |||||||
| Education level | |||||||
| < High school | 1451 (45.6%) | referent | |||||
| ≥ High school | 1730 (54.4%) | 0.89 (0.74–1.07) | 0.22 | ||||
|
| |||||||
| Marital statusd | |||||||
| Not married | 2651 (86.5%) | referent | |||||
| Married | 413 (13.5%) | 0.93 (0.71–1.21) | 0.58 | ||||
|
| |||||||
| Injection drug use-related HIV risk | |||||||
| No | 955 (30.0%) | referent | referent | ||||
| Yes | 2226 (70.0%) | 1.28 (1.04–1.58) | 0.02 | 1.14 (0.91–1.42) | 0.27 | ||
|
| |||||||
| Years since HIV diagnosis (median, interquartile range) | 12.6 | ||||||
| Continuous (years) | (6.7–17.8) | 1.02 (1.00–1.03) | 0.02 | 1.00 (0.99–1.02) | 0.88 | ||
|
| |||||||
| Enabling or disabling factors | |||||||
|
| |||||||
| Length of incarceration | |||||||
| ≤ 30 days | 974 (30.6%) | referent | referent | referent | |||
| 31–364 days | 1798 (56.5%) | 2.11 (1.71–2.59) | <0.0001 | 1.52 (1.18–1.96) | 0 001 | 1.52 (1.19–1.95) | 0.001 |
| ≥ 365 days | 409 (12.9%) | 2.07 (1.55–2.76) | <0.0001 | 1.27 (0.89–1.81) | 0.18 | 1.25 (0.88–1.78) | 0.20 |
|
| |||||||
| Re-incarcerated | |||||||
| No | 582 (18.3%) | referent | referent | referent | |||
| Yes | 2599 (81.7%) | 0.78 (0.63–0.97) | 0.02 | 0.71 (0.57–0.89) | 0.003 | 0.70 (0.56–0.88) | 0.002 |
|
| |||||||
| Year of release | |||||||
| 2007–2008 | 818 (25.7%) | referent | referent | referent | |||
| 2009–2010 | 928 (29.2%) | 1.48 (1.18–1.87) | 0.0009 | 1.30 (1.01–1.66) | 0.04 | 1.28 (1.00–1.64) | 0.05 |
| 2011–2012 | 782 (24.6%) | 1.71 (1.34–2.18) | <0.0001 | 1.24 (0.94–1.62) | 0.13 | 1.22 (0.93–1.60) | 0.14 |
| 2013–2014 | 653 (20.5%) | 1.35 (1.03–1.76) | 0.03 | 0.90 (0.66–1.23) | 0.51 | 0.88 (0.65–1.18) | 0.39 |
|
| |||||||
| Conditions of release | |||||||
| Unsupervised | 1935 (60.8%) | referent | referent | referent | |||
| Conditional release | 836 (26.3%) | 0.79 (0.65–0.97) | 0.03 | 0.63 (0.50–0.78) | <0.0001 | 0.62 (0.50–0.78) | <0.0001 |
| Released on bond | 410 (12.9%) | 0.46 (0.34–0.62) | <0.0001 | 0.77 (0.54–1.09) | 0.14 | 0.75 (0.53–1.06) | 0.11 |
|
| |||||||
| Transitional case management received | |||||||
| Neither pre-nor post-release | 2109 (66.3%) | referent | referent | referent | |||
| Pre-release, post-release, or both | 1072 (33–7%) | 2.00 (1.69–2.37) | <0 0001 | 1.64 (1.36–1.99) | <0.0001 | 1.65 (1.36–1.99) | <0.0001 |
|
| |||||||
| Need factors | |||||||
|
| |||||||
| Prescribed ART during incarceration | |||||||
| No | 1063 (33.4%) | referent | referent | referent | |||
| Yes | 2118 (66.6%) | 2.02 (1.66–2.45) | <0.0001 | 1.35 (1.07–1.70) | 0.01 | 1.39 (1.11–1.74) | 0.004 |
|
| |||||||
| Virally suppressed prior to release | |||||||
| No/viral load value not reported | 1240 (39.0%) | referent | referent | ||||
| Yes | 1247 (39.2%) | 1.20 (1.00–1.44) | 0.06 | 0.95 (0.77–1.17) | 0.63 | ||
| Viral load not drawn prior to release | 694 (21.8%) | 0.74 (0.58–0.95) | 0.02 | 0.86 (0.67–1.12) | 0.27 | ||
|
| |||||||
| Number of medical co-morbidities other than HIV | |||||||
| 0 | 1751 (55.1%) | referent | referent | referent | |||
| 1 | 786 (24.7%) | 1.49 (1.21–1.82) | 0.0002 | 1.19 (0.95–1.49) | 0.13 | 1.23 (0.98–1.54) | 0.07 |
| ≥ 2 | 644 (20.3%) | 2.26 (1.85–2.78) | <0.0001 | 1.77 (1.39–2.25) | <0.0001 | 1.86 (1.48–2.36) | <0.0001 |
|
| |||||||
| Psychiatric need | |||||||
| Low severity score, untreated | 1446 (45.5%) | referent | referent | ||||
| Low severity score, treated | 201 (6.3%) | 1.52 (1.09–2.13) | 0.02 | 1.18 (0.82–1.71) | 0.38 | ||
| High severity score, untreated | 527 (16.6%) | 1.07 (0.82–1.40) | 0.60 | 1.14 (0.87–1.49) | 0.35 | ||
| High severity score, treated | 1007 (31.7%) | 1.52 (1.24–1.86) | <0.0001 | 1.15 (0.93–1.42) | 0.19 | ||
|
| |||||||
| Addiction severity scoree | |||||||
| 1–2 | 371 (11.9%) | referent | referent | referent | |||
| 3 | 2189 (70.0%) | 1.70 (1.25–2.30) | 0.0006 | 1.26 (0.92–1.73) | 0.15 | 1.32 (0.97–1.81) | 0.08 |
| 4–5 | 566 (18.1%) | 2.05 (1.45–2.91) | <0.0001 | 1.33 (0.92–1.92) | 0.13 | 1.41 (0.98–2.03) | 0.06 |
|
| |||||||
| Underwent supervised withdrawal for an opioid use disorder | |||||||
| No | 3135 (98.6%) | referent | |||||
| Yes | 46 (1.5%) | 1.04 (0.52–2.09) | 0.91 | ||||
Sample is restricted to individuals who spent at least 14 days in the community prior to re-incarceration or death. There were 3181 post-release periods (1347 individual-based clusters) eligible for analysis. In both the full model and final parsimonious model, there were 665/3126 (21.3%) post-release periods with a viral load drawn within 14 days.
Numbers listed are n (%) or, for variables modeled as continuous, median (interquartile range). Percentages may not sum to 100% due to rounding.
Incarceration periods for transgender males (n=6) and transgender females (n=5) have been included in the male and female categories, respectively.
Incarceration periods for individuals with missing/unreported marital status (n=117) were excluded from the bivariate analysis such that total n=3064.
Incarceration periods where the addiction severity score was never assessed (n=55) were excluded from the bivariate analysis such that total n=3126. Sensitivity analysis comparing full and final parsimonious models both including and excluding the addiction severity score variable did not significantly change the models.
Factors associated with linkage to care within 30 days
In Table 2, age >45 years and Hispanic ethnicity were predisposing factors that predicted LTC with borderline statistical significance. Enabling resources again included intermediate incarceration duration (31–364 days) and TCM. Conditional release (including bonded release) was negatively associated with 30-day LTC, but re-incarceration no longer remained significant. Releases in 2009–2010 had higher odds of 30-day LTC than 2007–2008 releases, but in a sensitivity analysis restricting the sample to 2009–2014 and excluding year of release as a covariate, findings were robust. Need factors, including pre-release treatment with ART, higher medical co-morbidity, and higher psychiatric severity, also predicted LTC.
Table 2.
Binomial generalized estimating equations model assessing factors associated with having a viral load drawn within 30 days after release, adjusting for intra-subject correlation a
| Variable | n (%)b | Unadjusted OR (95% CI) | p-value | Full Model Adjusted OR (95% CI) | p-value | Parsimonious Model Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Predisposing factors | |||||||
|
| |||||||
| Age at time of release | |||||||
| Age ≤ 45 years | 1465 (47.8%) | referent | referent | referent | |||
| Age > 45 years | 1599 (52.2%) | 1.26 (1.07–1.49) | 0.005 | 1.16 (0.96–1.40) | 0.12 | 1.18 (0.99–1.40) | 0.07 |
|
| |||||||
| Sexc | |||||||
| Female | 698 (22.8%) | referent | |||||
| Male | 2366 (77.2%) | 1.01 (0.82–1.23) | 0.96 | ||||
|
| |||||||
| Race/Ethnicity | |||||||
| White | 527 (17.2%) | referent | referent | referent | |||
| Black | 1266 (41.3%) | 1.01 (0.79–1.30) | 0.92 | 1.09 (0.84–1.42) | 0.52 | 1.08 (0.83–1.40) | 0.57 |
| Hispanic | 1158 (37.8%) | 1.20 (0.94–1.54) | 0.15 | 1.31 (1.00–1.70) | 0.05 | 1.31 (1.01–1.70) | 0.046 |
| Other | 113 (3.7%) | 1.10 (0.69–1.77) | 0.68 | 1.17 (0.74–1.87) | 0.51 | 1.17 (0.73–1.85) | 0.51 |
|
| |||||||
| Education level | |||||||
| < High school | 1401 (45.7%) | referent | |||||
| ≥ High school | 1663 (54.3%) | 0.93 (0.78–1.10) | 0.38 | ||||
|
| |||||||
| Marital statusd | |||||||
| Not married | 2552 (86.5%) | referent | |||||
| Married | 387 (13.5%) | 1.01 (0.80–1.29) | 0.91 | ||||
|
| |||||||
| Injection drug use-related HIV risk | |||||||
| No | 924 (30.2%) | referent | referent | ||||
| Yes | 2140 (69.8%) | 1.22 (1.02–1.47) | 0.03 | 1.04 (0.85–1.28) | 0.68 | ||
|
| |||||||
| Years since HIV diagnosis (median, interquartile range) | 125 | ||||||
| Continuous (years) | (6.7–17.8) | 1.02 (1.00–1.03) | 0.01 | 1.00 (0.99–1.02) | 0.90 | ||
|
| |||||||
| Enabling or disabling factors | |||||||
|
| |||||||
| Length of incarceration | |||||||
| ≤ 30 days | 926 (30.2%) | referent | referent | referent | |||
| 31–364 days | 1741 (56.8%) | 2.03 (1.71–2.42) | <0.0001 | 1.41 (1.14–1.74) | 0.002 | 1.43 (1.16–1.76) | 0.001 |
| ≥ 365 days | 397 (13.0%) | 2.02 (1.57–2.59) | <0.0001 | 1.18 (0.87–1.60) | 0.28 | 1.20 (0.89–1.63) | 0.23 |
|
| |||||||
| Re-incarcerated | |||||||
| No | 577 (18.8%) | referent | |||||
| Yes | 2487 (81.2%) | 0.96 (0.79–1.17) | 0.69 | ||||
|
| |||||||
| Year of release | |||||||
| 2007–2008 | 791 (25.8%) | referent | referent | referent | |||
| 2009–2010 | 892 (29.1%) | 1.52 (1.25–1.84) | <0.0001 | 1.29 (1.05–1.60) | 0.02 | 1.30 (1.06–1.61) | 0.01 |
| 2011–2012 | 752 (24.5%) | 1.46 (1.18–1.80) | 0.0005 | 1.00 (0.79–1.28) | 0.98 | 1.02 (0.80–1.29) | 0.87 |
| 2013–2014 | 629 (20.5%) | 1.51 (1.21–1.88) | 0.0002 | 1.01 (0–79–1.31) | 0.91 | 1.00 (0.78–1.28) | 1.00 |
|
| |||||||
| Conditions of release | |||||||
| Unsupervised | 1868 (61.0%) | referent | referent | referent | |||
| Conditional release | 805 (26.3%) | 0.97 (0.81–1.16) | 0.75 | 0.79 (0.65–0.96) | 0.02 | 0.79 (0.65–0.96) | 0.02 |
| Released on bond | 391 (12.8%) | 0.47 (0.36–0.60) | <0.0001 | 0.70 (0.53–0.94) | 0.02 | 0.75 (0.56–0.99) | 0.04 |
|
| |||||||
| Transitional case management received | |||||||
| Neither pre- nor post-release | 2013 (65.7%) | referent | referent | referent | |||
| Pre-release, post-release, or both | 1051 (34.3%) | 1.86 (1.60–2.17) | <0.0001 | 1.47 (1.24–1.74) | <0.0001 | 1.48 (1.25–1.76) | <0.0001 |
|
| |||||||
| Need factors | |||||||
|
| |||||||
| Prescribed ART during incarceration | |||||||
| No | 1029 (33.6%) | referent | referent | referent | |||
| Yes | 2035 (66.4%) | 2.10 (1.77–2.49) | <0.0001 | 1.46 (1.18–1.81) | 0.0006 | 1.53 (1.25–1.87) | <0.0001 |
|
| |||||||
| Virally suppressed prior to release | |||||||
| No/viral load value not reported | 1196 (39.0%) | referent | referent | ||||
| Yes | 1198 (39.1%) | 1.32 (1.12–1.56) | 0.001 | 0.99 (0.83–1.21) | 0.99 | ||
| Viral load not drawn prior to release | 670 (21.9%) | 0.87 (0.71–1.07) | 0.18 | 0.98 (0.79–1.22) | 0.87 | ||
|
| |||||||
| Number of medical co-morbidities other than HIV | |||||||
| 0 | 1684 (55.0%) | referent | referent | referent | |||
| 1 | 760 (24.8%) | 1.43 (1.20–1.71) | <0.0001 | 1.15 (0.94–1.39) | 0.18 | 1.13 (0.93–1.37) | 0.21 |
| ≥ 2 | 620 (20.2%) | 2.08 (1.71–2.53) | <0.0001 | 1.57 (1.25–1.97) | <0.0001 | 1.61 (1.29–2.02) | <0.0001 |
|
| |||||||
| Psychiatric need | |||||||
| Low severity score, untreated | 1391 (45.4%) | referent | referent | referent | |||
| Low severity score, treated | 193 (6.3%) | 1.75 (1.28–2.39) | 0.0004 | 1.37 (0.98–1.91) | 0.06 | 1.42 (1.03–1.98) | 0.03 |
| High severity score, untreated | 509 (16.6%) | 1.28 (1.03–1.61) | 0.03 | 1.39 (1.09–1.76) | 0.01 | 1.42 (1.12–1.80) | 0.003 |
| High severity score, treated | 971 (31.7%) | 1.61 (1.34–1.94) | <0.0001 | 1.26 (1.03–1.54) | 0.02 | 1.28 (1.05–1.56) | 0.02 |
|
| |||||||
| Addiction severity scoree | |||||||
| 1–2 | 363 (12.1%) | referent | referent | ||||
| 3 | 2119 (70.4%) | 1.53 (1.19–1.97) | 0.001 | 1.16 (0.89–1.52) | 0.27 | ||
| 4–5 | 529 (17.6%) | 1.91 (1.42–2.57) | <0.0001 | 1.28 (0.93–1.77) | 0.12 | ||
|
| |||||||
| Underwent supervised withdrawal for an opioid use disorder | |||||||
| No | 3020 (98.6%) | referent | |||||
| Yes | 44 (1.4%) | 1.02 (0.54–1.92) | 0.95 | ||||
Sample is restricted to individuals who spent at least 30 days in the community prior to re-incarceration or death. There were 3064 post-release periods (1342 individual-based clusters) eligible for analysis. For the full model and final parsimonious model, respectively, there were 1031/3011 (34.2%) and 1042/3064 (34.0%) post-release periods with a viral load drawn within 30 days.
Numbers listed are n (%) or, for variables modeled as continuous, median (interquartile range). Percentages may not sum to 100% due to rounding.
Incarceration periods for transgender males (n=6) and transgender females (n=5) have been included in the male and female categories, respectively.
Incarceration periods for individuals with missing/unreported marital status (n=115) were excluded from the bivariate analysis such that total n=2949.
Incarceration periods where the addiction severity score was never assessed (n=53) were excluded from the bivariate analysis such that total n=3011.
Discussion
To our knowledge, this is the largest and most complete cohort of PLH transitioning from prison or jail to communities. LTC post-release is extraordinarily low: 21% by 14 days and 34% by 30 days – critical times when PLH must access post-release care. By 6 months, 76% had at least one VL recorded, which suggests a gradual increase in LTC over time, but does not infer treatment retention after initial linkage. Incarceration experiences, including duration, conditions of release, and medical and psychiatric care, significantly influenced likelihood of LTC, as did TCM.
Using a validated healthcare utilization framework, integrated databases allowed us to examine the effect of factors that might influence LTC, especially TCM. PLH who are isolated from systems of structural and social support or unable to navigate resources are more likely to engage in high-risk HIV behaviors, relapse to substance use, be re-incarcerated, and default from ART (19, 20). TCM aims to mitigate some of these issues and better position PLH to engage in healthcare after release (21, 22). Jail-based longitudinal demonstration projects have found TCM to improve HIV treatment outcomes, but lacked a control group (21–24). While prospective trials show no benefit of TCM interventions on post-release outcomes, the control groups received pre-release discharge planning or “standard of care” TCM, which may have been sufficient (25–27).
These trials were also not designed to target high-risk PLH who are most likely to benefit from such services and were conducted in settings that may have lacked enabling resources (e.g., housing, addiction treatment) amenable to TCM intervention. Findings from this observational study in a real-world setting suggest that TCM is beneficial for LTC, but is not applied universally (only 34% of releases involved TCM, and unsentenced inmates were rarely eligible). Expanded TCM programming combined with an increase in CTDOC referrals could considerably improve LTC. When resources are limited, however, TCM should be targeted to those who need them most, especially those with short-term detentions and conditional releases.
We found that the duration and frequency of detention in prison/jail impacts continuity of HIV care. Short-term detentions (≤30 days) are especially destabilizing, likely due to the social and legal ramifications of CJ-involvement combined with an interruption in HIV care. Incarceration, especially within the first year after starting ART, predicts ART non-adherence and virologic failure (6, 28). Incarceration also has a dose-dependent negative effect on ART adherence (9). Once incarcerated, however, and when appropriate resources are applied, PLH detained for longer are more likely to achieve viral suppression than those with shorter incarcerations (6, 28). Though modeling studies suggest that eliminating incarceration of high-risk individuals would reduce HIV incidence, in settings where policies still promote incarceration of such individuals, PLH with longer incarcerations can be stabilized on effective ART and provided sufficient time to have their post-release needs identified and addressed (2, 4). For PLH who are otherwise unable to access or navigate community-based resources, CJ facilities may inadvertently serve as “medical homes”, particularly during longer incarcerations where discharge planning can occur much like that provided in hospital settings (4). Incarcerations over one year, however, may compromise self-sufficiency, which creates additional barriers to community reintegration (29, 30). The majority of inmates eventually return to communities and, while incarceration may be an opportunity to engage PLH in care, frequent or brief re-incarcerations are disruptive and detrimental to LTC. PLH who frequently and rapidly cycle through CJ systems should be targeted for intensive medical and TCM services.
Contrary to previous findings showing a positive association between supervised release and filling an ART prescription, we found that conditional release was associated with poor LTC (12). PLH conditionally released on parole, bond, or to transitional housing may face numerous health and legal challenges that can undermine LTC (19, 20). Conditional release may be disabling in that it imposes legal obligations that limit autonomy, adds to reintegration responsibilities, and competes with healthcare priorities during the post-release period. PLH released under conditions, especially bond, are more likely to have shorter incarceration periods with fewer opportunities to be receive ART or TCM prior to release, though we controlled for these associations in our multivariable models. Although TCM is available through parole offices, it is not well integrated into the supervision plan. Thus, conditional release is an important but missed opportunity to align public health and safety by integrating TCM into supervision plans and connecting PLH to care (23).
Receiving ART while incarcerated predicted post-release LTC (31, 32). Although ART was prescribed in only 66% of all incarcerations, prescription increased during more contemporary incarcerations. Reasons for these temporal changes are multifactorial and include changes in guidelines favoring treatment and simplified and more tolerable ART regimens. Moreover, PLH incarcerated for shorter periods, even if prescribed ART, may not have achieved viral suppression before release. In addition to ART, PLH who were treated for medical or psychiatric co-morbidities were also more likely to have LTC post-release, perhaps because of their flagged need for continuity of care and potentially more comprehensive discharge planning (22, 33). Psychiatric care may also serve as a conduit to general health and HIV care. In the United States, CJ systems are often disjointed and managed by various jurisdictions. While some CJ facilities may already have effective strategies to identify vulnerable PLH and connect them to services in the community, there needs to be more consistency in identifying healthcare and social needs immediately on CJ intake, followed by effective TCM targeted to people with greatest need (21). In the setting of national healthcare reform, TCM could help enroll inmates in expanded Medicaid/Medicare programs prior to release.
While multiple combined datasets allowed a comprehensive assessment of LTC, including viral suppression rates, clinical and treatment data, and potential explanatory factors, we acknowledge some limitations inherent to this secondary data analysis. Because CTDOC data were collected for custodial purposes, they missed granularity for some patient-level factors, such as housing status, medical insurance coverage, and substance use; addiction severity scores were our best proxy for current SUDs. The use of VL as the primary indicator of LTC may underrepresent LTC, especially if laboratory testing was provided just prior to release. That 60% of the released inmates who linked to care 91–180 days post-release were virally suppressed suggests that some people continued to receive ART and/or clinical care without reported VL monitoring. For example, some PLH with short incarceration periods may have prior active prescriptions and refills that were not discontinued during their incarceration, allowing them to delay seeking medical care after release. Previous studies using clinic data, however, relied on databases that do not include all PLH in the state and have documented inconsistencies in the accuracy and completeness of data (15, 31, 34). In contrast, our use of reliably reported biological data allowed for a direct and verifiable analysis of post-release LTC and viral suppression. Moreover, we included data on short-term detentions, which are not available in most studies of prisoners because most jail and prison systems are not integrated. More complete databases, reliable subject matching, and CTDPH database managers with extensive experience merging data mitigated challenges common in database linkage studies. While these findings are not generalizable to all CJ settings, they can inform directions for intervention in settings with similar syndemics including HIV, incarceration, SUDs, and psychiatric disorders.
As the first comprehensive, statewide, longitudinal study assessing LTC and viral suppression following release from prison or jail, this study informs health policy targeting at-risk, CJ-involved PLH. It lays groundwork for future studies that use “big data” to assess and improve HIV treatment outcomes, including data-to-care strategies that position health authorities to intervene when patients are out-of-care (35). Our findings indicate that CJ systems can provide highly effective healthcare resources to PLH during and following incarceration to achieve UNAIDS targets for optimizing care and ending the global HIV pandemic. Yet the potential long-term benefit of these resources is limited by the fragmented nature of CJ systems and segregation between penal and healthcare priorities after release. Comprehensive discharge planning and TCM for PLH should begin immediately after intake into facilities and center on integration of public safety and health. The most cost-effective, ethical, and beneficial strategy for preserving HIV continuity of care, however, is likely to avoid incarceration altogether.
Supplementary Material
Panel: Research in Context.
Evidence before this study
We searched PubMed for original research articles published between Jan 1, 2000, and August 25, 2017, using the following MeSH terms: [“prison”, “jail”, or “incarceration”] AND [“HIV”] AND [“treatment”, “outcomes”, “linkage to care”, or “retention in care”]. We identified 19 North American studies that examined outcomes for people living with HIV (PLH) released from criminal justice (CJ) settings. Findings show that a history of CJ involvement, recidivism, and detention for short periods are associated with poor HIV treatment outcomes (e.g., failure to engage in care, medication non-adherence, or virologic failure) in the community. Antiretroviral therapy (ART) provided during incarceration results in high viral suppression rates but studies of recidivists show that benefits do not persist after release. Three studies assessed post-release linkage to HIV care (LTC) by matching multiple pre-existing databases; ≤20% visited an HIV clinic or filled ART prescriptions within the 30-day post-release window necessary to avoid treatment interruption, and <50% had a clinic visit within 90 days. Major limitations included incomplete reporting of clinic visits and inability to assess viral suppression. A supplementary search by adding the MeSH term “case management” identified 14 publications. Jail-based demonstration projects showed that case management provided during community re-entry can improve post-release HIV treatment outcomes, yet three prison-based randomized controlled trials did not demonstrate a clear benefit.
Added value of this study
This study innovatively combines administrative statewide CJ, pharmacy, clinical, and objective HIV surveillance data in a state where custody data includes both prisoners and jail detainees and delivery of care is integrated. Additionally, this study uses a validated healthcare utilization framework, the Behavioral Model for Vulnerable Populations, to better assess factors associated with LTC. Prior studies have failed to explain how complex interactions with the CJ system (e.g., conditional release, short-term detentions) influence HIV treatment outcomes. This study is not limited by incomplete databases, loss to follow-up, recall or social desirability bias, or sample restrictions to recidivists, PLH actively taking ART, or other sub-populations of inmates (e.g., jail detainees vs. sentenced prisoners). Here, viral load provides an objective surrogate for LTC, verified through mandatory reporting from all certified laboratories in the entire state over an observation period of eight years. This is the first comprehensive, statewide assessment of post-release LTC and viral suppression for all CJ-involved PLH. It is also the first to objectively assess, in a real-world CJ setting, the impact of transitional case management services on HIV treatment outcomes.
Implications of all the available evidence
Previous studies show that CJ-involved PLH can achieve viral suppression during incarceration but, for reasons yet unclear, demonstrate poor HIV-related health outcomes after release. There has also been conflicting evidence on the effectiveness of transitional case management services in improving longitudinal HIV treatment outcomes. By comprehensively assessing post-release LTC for all CJ-involved PLH in Connecticut, USA using multiple administrative databases, this study confirms that LTC is suboptimal, but identifies salient targets for intervention. We show that the consistent targeted provision of transitional case management and integration of healthcare and CJ services are key to improving HIV treatment outcomes during and following the transition to community settings.
Acknowledgments
The National Institute on Drug Abuse (NIDA) provided funding for this project F30DA041247 (Loeliger) and career development support K24DA017072 (Altice) and K23DA033858 (Meyer). The project was also supported by the Yale University Medical Scientist Training Program under the National Institute of General Medical Sciences (NIGMS) T32GM007205 and the Yale Center for Interdisciplinary Research in AIDS (CIRA) under the National Institute of Mental Health (NIMH) P30MH062294. Funding sources played no role in data analysis or interpretation or the decision to submit the manuscript for publication. This research was conducted in collaboration with the Connecticut Departments of Correction and Public Health. We thank Kathleen Maurer, Patrick Hines, Cheryl Cepelak, and Heidi Jenkins for assisting with study design, guiding the interpretation of our findings, and fostering the inter-institutional collaborations that made this study possible. We also thank Kristen Shea, Suzanne Speers, Michael Ostapoff, and Melanie Alvarez for their invaluable assistance with data collection, extraction, and linkage; no compensation was received for these contributions. Finally, we sincerely thank Paula Dellamura for her crucial administrative support.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
KBL obtained funding for the project and designed the study, with guidance from FLA and JPM. KBL conducted all data analyses with guidance from and data interpretation by FLA, MMD, MMC, CG, and JPM. KBL drafted primary versions of the manuscript, with input from FLA, MMD, MMC, CG, and JPM. All authors have contributed significantly to the work and reviewed and approved the final version for submission.
Declaration of interests
We declare that we have no conflicts of interest.
Contributor Information
Kelsey B. Loeliger, Yale School of Medicine, Section of Infectious Diseases, Yale AIDS Program, 135 College St, Suite 323, New Haven, CT, USA 06510-2283; Yale School of Public Health, Department of Epidemiology of Microbial Diseases, New Haven, CT, USA.
Frederick L. Altice, Yale School of Medicine, Section of Infectious Diseases, Yale AIDS Program, 135 College St, Suite 323, New Haven, CT, USA 06510-2283; Yale School of Public Health, Department of Epidemiology of Microbial Diseases, New Haven, CT, USA; University of Malaya, Centre of Excellence in Research in AIDS, Kuala Lumpur, Malaysia.
Mayur M. Desai, Yale School of Public Health, Department of Chronic Disease Epidemiology, Yale School of Public Health 60 College Street, PO Box 208034, New Haven, CT, USA 06520-8034.
Maria M. Ciarleglio, Yale School of Public Health, Department of Biostatistics, Yale School of Public Health 60 College Street, PO Box 208034, New Haven, CT, USA 06520-8034.
Colleen Gallagher, Connecticut Department of Correction, Health and Addiction Services Quality Improvement Program, 24 Wolcott Hill Rd., Wethersfield, CT, USA 06109.
Jaimie P. Meyer, Yale School of Medicine, Section of Infectious Diseases, Yale AIDS Program, 135 College St, Suite 323, New Haven, CT, USA 06510-2283.
References
- 1.Glaze LE, Kaeble D. Bureau of Justice Statistics. U.S. Department of Justice - Office of Justice Programs; 2014. Correctional Populations in the United States, 2013. Accessed on March 10, 2017 at: https://www.bjs.gov/content/pub/pdf/cpus13.pdf. [Google Scholar]
- 2.Dolan K, Wirtz AL, Moazen B, Ndeffo-Mbah M, Galvani A, Kinner SA, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet. 2016;388(10049):1089–102. doi: 10.1016/S0140-6736(16)30466-4. [DOI] [PubMed] [Google Scholar]
- 3.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PloS one. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer JP, Cepeda J, Wu J, Trestman RL, Altice FL, Springer SA. Optimization of human immunodeficiency virus treatment during incarceration: viral suppression at the prison gate. JAMA internal medicine. 2014;174(5):721–9. doi: 10.1001/jamainternmed.2014.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milloy MJ, Montaner JS, Wood E. Incarceration of people living with HIV/AIDS: implications for treatment-as-prevention. Current HIV/AIDS reports. 2014;11(3):308–16. doi: 10.1007/s11904-014-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westergaard RP, Kirk GD, Richesson DR, Galai N, Mehta SH. Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(7):725–31. doi: 10.1093/cid/cir491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S, Harris TG, Nash D, Lennon MC, Thorpe LE. All-cause, drug-related, and HIV-related mortality risk by trajectories of jail incarceration and homelessness among adults in New York City. American journal of epidemiology. 2015;181(4):261–70. doi: 10.1093/aje/kwu313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai NP, Estes M, Moodie EE, Reingold AL, Tulsky JP. The impact of antiretroviral therapy in a cohort of HIV infected patients going in and out of the San Francisco county jail. PloS one. 2009;4(9):e7115. doi: 10.1371/journal.pone.0007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milloy MJ, Kerr T, Buxton J, Rhodes T, Guillemi S, Hogg R, et al. Dose-response effect of incarceration events on nonadherence to HIV antiretroviral therapy among injection drug users. The Journal of infectious diseases. 2011;203(9):1215–21. doi: 10.1093/infdis/jir032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services. AIDSinfo Clinical Guidelines Portal: Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Accessed on February 28, 2015 at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/458/plasma-hiv-1-rna--viral-load--and-cd4-count-monitoring2015 [updated May 1, 2014]
- 11.Baillargeon JG, Giordano TP, Harzke AJ, Baillargeon G, Rich JD, Paar DP. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public health reports. 2010;125(Suppl 1):64–71. doi: 10.1177/00333549101250S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, et al. Accessing antiretroviral therapy following release from prison. Jama. 2009;301(8):848–57. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagan E. Medicaid Suspension Policies for Incarcerated People: 50-State Map. 2016 Accessed on August 6, 2017 at: http://familiesusa.org/product/medicaid-suspension-policiesincarcerated-people-50-state-map. [updated July 12, 2017]
- 14.Centers for Disease Control and Prevention. Funding Opportunity Announcement: PS13-1302 National HIV Surveillance System (NHSS) 2013 Accessed on August 25, 2017 at: https://www.cdc.gov/hiv/funding/announcements/ps13-1302/index.html. [updated January 2013]
- 15.Health Resources & Services Administration (HRSA) Ryan White & Global HIV/AIDS Programs. Program & Grants Management- CAREWare. 2017 Accessed on March 29, 2017 at: https://hab.hrsa.gov/program-grants-management/careware. [updated March.
- 16.Centers for Disease Control and Prevention. National Program of Cancer Registries. Registry Plus Link Plus. Accessed on August 31, 2015 at: http://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm [updated January 13, 2015]
- 17.Hu YW, Kinsler JJ, Sheng Z, Kang T, Bingham T, Frye DM. Using laboratory surveillance data to estimate engagement in care among persons living with HIV in Los Angeles County, 2009. AIDS Patient Care STDS. 2012;26(8):471–8. doi: 10.1089/apc.2011.0371. [DOI] [PubMed] [Google Scholar]
- 18.Gelberg L, Andersen RM, Leake BD. The Behavioral Model for Vulnerable Populations: application to medical care use and outcomes for homeless people. Health services research. 2000;34(6):1273–302. [PMC free article] [PubMed] [Google Scholar]
- 19.Luther JB, Reichert ES, Holloway ED, Roth AM, Aalsma MC. An exploration of community reentry needs and services for prisoners: a focus on care to limit return to high-risk behavior. AIDS patient care and STDs. 2011;25(8):475–81. doi: 10.1089/apc.2010.0372. [DOI] [PubMed] [Google Scholar]
- 20.Haley DF, Golin CE, Farel CE, Wohl DA, Scheyett AM, Garrett JJ, et al. Multilevel challenges to engagement in HIV care after prison release: a theory-informed qualitative study comparing prisoners’ perspectives before and after community reentry. BMC public health. 2014;14:1253. doi: 10.1186/1471-2458-14-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spaulding AC, Messina LC, Kim BI, Chung KW, Lincoln T, Teixeira P, et al. Planning for success predicts virus suppressed: results of a non-controlled, observational study of factors associated with viral suppression among HIV-positive persons following jail release. AIDS and behavior. 2013;17(Suppl 2):S203–11. doi: 10.1007/s10461-012-0341-8. [DOI] [PubMed] [Google Scholar]
- 22.Althoff AL, Zelenev A, Meyer JP, Fu J, Brown SE, Vagenas P, et al. Correlates of retention in HIV care after release from jail: results from a multi-site study. AIDS Behav. 2013;17(Suppl 2):S156–70. doi: 10.1007/s10461-012-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draine J, Ahuja D, Altice FL, Arriola KJ, Avery AK, Beckwith CG, et al. Strategies to enhance linkages between care for HIV/AIDS in jail and community settings. AIDS care. 2011;23(3):366–77. doi: 10.1080/09540121.2010.507738. [DOI] [PubMed] [Google Scholar]
- 24.Zaller ND, Holmes L, Dyl AC, Mitty JA, Beckwith CG, Flanigan TP, et al. Linkage to treatment and supportive services among HIV-positive ex-offenders in Project Bridge. J Health Care Poor Underserved. 2008;19(2):522–31. doi: 10.1353/hpu.0.0030. [DOI] [PubMed] [Google Scholar]
- 25.MacGowan RJ, Lifshay J, Mizuno Y, Johnson WD, McCormick L, Zack B. Positive Transitions (POST): Evaluation of an HIV Prevention Intervention for HIV-Positive Persons Releasing from Correctional Facilities. AIDS and behavior. 2015;19(6):1061–9. doi: 10.1007/s10461-014-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohl DA, Scheyett A, Golin CE, White B, Matuszewski J, Bowling M, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS and behavior. 2011;15(2):356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohl DA, Golin CE, Knight K, Gould M, Carda-Auten J, Groves JS, et al. Randomized Controlled Trial of an Intervention to Maintain Suppression of HIV Viremia After Prison Release: The imPACT Trial. Journal of acquired immune deficiency syndromes. 2017;75(1):81–90. doi: 10.1097/QAI.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palepu A, Tyndall MW, Chan K, Wood E, Montaner JS, Hogg RS. Initiating highly active antiretroviral therapy and continuity of HIV care: the impact of incarceration and prison release on adherence and HIV treatment outcomes. Antiviral therapy. 2004;9(5):713–9. [PubMed] [Google Scholar]
- 29.Liem M, Kunst M. Is there a recognizable post-incarceration syndrome among released “lifers”? Int J Law Psychiatry. 2013;36(3–4):333–7. doi: 10.1016/j.ijlp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Haney C. The Pyschological Impact of Incarceration: Implications for Post-Prison Adjustment. U.S Department of Health and Human Services: Office of the Assistant Secretary for Planning and Evaluation; 2001. Accessed on August 27, 2017 at: https://aspe.hhs.gov/basicreport/psychological-impact-incarceration-implications-post-prison-adjustment-II. [updated January 30–31, 2002: Working papers prepared for the “From Prison to Home” Conference] [Google Scholar]
- 31.Montague BT, Rosen DL, Sammartino C, Costa M, Gutman R, Solomon L, et al. Systematic Assessment of Linkage to Care for Persons with HIV Released from Corrections Facilities Using Existing Datasets. AIDS patient care and STDs. 2016;30(2):84–91. doi: 10.1089/apc.2015.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booker CA, Flygare CT, Solomon L, Ball SW, Pustell MR, Bazerman LB, et al. Linkage to HIV care for jail detainees: findings from detention to the first 30 days after release. AIDS Behav. 2013;17(Suppl 2):S128–36. doi: 10.1007/s10461-012-0354-3. [DOI] [PubMed] [Google Scholar]
- 33.Meyer JP, Cepeda J, Springer SA, Wu J, Trestman RL, Altice FL. HIV in people reincarcerated in Connecticut prisons and jails: an observational cohort study. The lancet HIV. 2014;1(2):e77–e84. doi: 10.1016/S2352-3018(14)70022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Committee on Review Data Systems for Monitoring HIV Care- Institute of Medicine. Monitoring HIV Care in the United States: Indicators and Data Systems - Barriers to the Collection of HIV Care Data. Washington, DC: National Academies Press (US): National Academy of Sciences; 2012. Accessed on March 10, 2017 at https://www.ncbi.nlm.nih.gov/books/NBK201369/ [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Data to Care: Using HIV Surveillance Data to Support the HIV Care Continuum. Vol. 2017. Atlanta, GA: 2017. Effective Interventions. Accessed on at: https://effectiveinterventions.cdc.gov/en/HighImpactPrevention/PublicHealthStrategies/DatatoCare.aspx. [updated 2017] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


