Abstract
Although singly ablating Fabp1 or Scp2/Scpx genes may exacerbate the impact of high fat diet (HFD) on whole body phenotype and non-alcoholic fatty liver disease (NAFLD), concomitant upregulation of the non-ablated gene, preference for ad libitum fed HFD, and sex differences complicate interpretation. Therefore, these issues were addressed in male and female mice ablated in both genes (Fabp1/Scp2/Scpx null or TKO) and pair-fed HFD. Wild-type (WT) males gained more body weight as fat tissue mass (FTM) and exhibited higher hepatic lipid accumulation than WT females. The greater hepatic lipid accumulation in WT males was associated with higher hepatic expression of enzymes in glyceride synthesis, higher hepatic bile acids, and upregulation of transporters involved in hepatic reuptake of serum bile acids. While TKO had little effect on whole body phenotype and hepatic bile acid accumulation in either sex, TKO increased hepatic accumulation of lipids in both, specifically phospholipid and cholesteryl esters in males and females and free cholesterol in females. TKO-induced increases in glycerides were attributed not only to complete loss of FABP1, SCP2 and SCPx, but also in part to sex-dependent upregulation of hepatic lipogenic enzymes. These data with WT and TKO mice pair-fed HFD indicate that: i) Sex significantly impacted the ability of HFD to increase body weight, induce hepatic lipid accumulation and increase hepatic bile acids; and ii) TKO exacerbated the HFD ability to induce hepatic lipid accumulation, regardless of sex, but did not significantly alter whole body phenotype in either sex.
Keywords: obesity, fatty acid, liver, FABP1, SCP-2, SCP-x
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common of all liver disorders, occurring in 10–58% of the US population [33,55,95,115,120]. High fat diets (HFD) induce obesity and NAFLD [33,53,55,115,120,124]. However, despite the fact that NAFLD is more prevalent in women [23,24], most HFD-induced rodent models of NAFLD have focused only on males fed HFD ad libitum (rev. in [62]. Under ad libitum feeding conditions, such male rodents strongly prefer and eat more HFD as compared to normal chow (rev. in [62]. Thus, there is a need for controlled, pair-fed HFD studies not only of male but even more so female rodents to study sexual dimorphism of NAFLD independent of increased total intake of HFD.
While the biochemical basis for NAFLD is not completely understood, two potential liver cytosolic lipid binding/chaperone proteins have been identified as potential candidates: First is the hepatic fatty acid binding protein (FABP1). Genomic studies suggest that a very prevalent SNP in human FABP1 resulting in a T94A amino acid substitution is highly associated with NAFLD [77,96]. FABP1 is the single most prevalent liver cytosolic protein that binds, transports, and targets bound lipidic ligands such as long chain fatty acids and their CoA thioesters [46,74], endocannabinoids [45], bile acids [63], cholesterol [65], and lipidic xenobiotics [47,73,77] to intracellular organelles for storage, oxidation, receptor regulation, or gene regulation. FABP1 is upregulated in human NAFLD and in NAFLD animal models [10,17,38,124]. Gene targeting studies indicate that Fabp1 gene ablation decreases hepatic lipid accumulation in control-chow fed mice [18,65,91–93,123]. While Fabp1 silencing ameliorates hepatic steatosis, inflammation, and oxidative stress in HFD fed male mice with NAFLD [82], the impact of sex differences in the context of HFD has not been addressed. Second, protein sterol carrier protein-2 (SCP2, encoded by the Scp2/Scpx gene) also binds/chaperones such lipidic ligands. SCP2 binds, transports, and targets bound lipidic ligands such as long chain fatty acids and their CoA thioesters [19,27,28], endocannabinoids [61,70], bile acids [32], cholesterol [101,111], and phospholipids [20,31,105] to intracellular organelles for storage, oxidation, excretion, or receptor regulation. Although ablating the Scp2/Scpx gene decreases hepatic lipid accumulation in control rodent chow-fed male mice [56], the impact of sex differences especially in the context of HFD are unclear.
Finally, it is important to note that while studies with mice singly ablated in either the Fabp1 or Scp2/Scpx gene have proven useful in resolving the roles of these genes in determining whole body phenotype and hepatic lipid metabolism, simple interpretation of findings is complicated by compensatory upregulation of the non-ablated gene. For example, individually ablating Scp2/Scpx or Scpx elicits compensatory upregulation of FABP1 which may have obscured at least in part the impact of the loss of SCP2 [4,30,107]. Therefore, the current study examined the effect of ablating both the Fabp1 and Scp2/Scpx genes (i.e. Fabp1/Scp2/Scpx ablation or TKO) on whole body and liver lipid phenotype in both male and female mice pair-fed HFD.
Materials and Methods
Materials
Triacylglycerol (L-type Triglyceride M, TG), free cholesterol (free cholesterol, C), total cholesterol (cholesterol E, TC), phospholipid (phospholipid, PL) and non-esterified fatty acid (HR Series NEFA-HR, NEFA) diagnostic kits from Wako Chemicals (Richmond, VA) were used to determine levels of the respective lipids. β-hydroxybutyrate (β-Hydroxybutyrate LiquiColor, β-HOB) and high density lipoprotein cholesterol (Direct HDL-Cholesterol, HDL-C) diagnostic kits from Stanbio Laboratory (Boerne, TX) were used to determine levels of β-HOB and HDL-C. Apolipoprotein B (APOB) and apolipoprotein A-I (APOA1) levels were measured using diagnostic kits from Diazyme Labs (Poway, CA). The Bradford protein micro-assay (Cat # 500-0001, bovine gamma globulin) from Bio-Rad (Hercules, CA) was used to determine protein levels. All reagents and solvents used were of the highest grade available.
Mice
Wild-type (WT) C57BL/6NCr mice were purchased from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). Fabp1/Scp2/Scpx null (TKO) mice backcrossed >10 generations to the C57BL/6NCr background were generated by our laboratory as in [112]. The term TKO refers to loss of all three proteins even though they are encoded by only two genes (i.e. Fabp1 and Scp2/Scpx). Mice were housed in controlled conditions (T = 25 °C, H= 60–70% added humidity) and 12:12 h light/dark cycle. Mouse protocols were approved by the Texas A&M Institutional Animal Care and Use Committee in compliance with the Guide for the Care and Use of Laboratory Animals. Mice were monitored daily for injury or disease, sentinel monitored quarterly, and were shown free of all known rodent pathogens.
Dietary High Fat
Two groups of 16 each WT male, WT female, TKO male, and TKO female mice aged 7 weeks were individually housed in Tecniplast Sealsafe IVC cages with external water bottles and wire lid holders for food pellets. Mice were acclimated for 1 week on a defined, 10 kcal% fat control chow (#D12450B, Research Diets, New Brunswick, NJ) known to be free of phytol and phytoestrogen. Phytol and phytoestrogen were avoided because they may impact sex differences and FABP1 expression as described earlier [62]. In each group of 16 individually-housed mice, 8 mice were continued an additional 12 weeks on this same defined diet while the other 8 mice were pair-fed based upon food weight an isocaloric high fat diet (HFD, # D12451, Research Diets, New Brunswick, NJ). The high fat diet was formulated by modifying the defined control diet by increasing fat from 10 kcal% to 45 kcal% while decreasing carbohydrate from 70 kcal% to 35 kcal% while keeping protein constant as described [2].
Whole body phenotype by dual-energy x-ray absorptiometry (DEXA) to determine fat tissue mass (FTM) and lean tissue mass (LTM)
Mice were anesthetized at the beginning (day 0) and end (day 84) of the dietary study using a ketamine/xylazine mixture (0.01 mL/g body weight; 10 mg ketamine/mL and 1 mg xylazine/mL in 0.9% saline solution) as previously described [3]. Dual-energy X-ray absorptiometry (DEXA) images of each mouse were obtained using a Lunar PIXImus densitometer (Lunar Corp., Madison, WI) as previously described [8] after calibration using a phantom mouse with known bone mineral density and fat tissue mass as described [8,88]. Whole body fat tissue mass (FTM) and bone-free lean tissue mass (LTM) were obtained by exposing the entire mouse, minus the head region, to sequential beams of high- and low-energy X-rays and taking X-ray images on a luminescent panel. Soft tissue mass was differentiated from bone mass by measuring the ratios of attenuation at different energies followed by separating soft tissue mass into FTM and LTM as previously described [8].
Tissue collection
At the end of the dietary study and after overnight fast, mice were anesthetized as above, blood was collected via cardiac puncture followed by cervical dislocation as the secondary form of euthanasia according to the AVMA Guidelines for the Euthanasia of Animals. The blood was coagulated overnight at 4°C, followed by centrifugation at 14,000 rpm for 20 min at 4°C; the serum fraction was removed for storage at 80 °C for subsequent lipid and protein analysis. Likewise, livers were collected, flash frozen, and stored at −80°C for subsequent analysis of lipids, western blotting, and/or qRT-PCR as described in the following sections.
Liver homogenization and protein analysis
Liver samples (~0.1 gram) were minced extensively followed by the addition of 0.5 mL PBS (pH 7.4) and homogenization with a motor-driven pestle (Tekmar Co, Cincinnati, OH) at 2000 rpm. The Bradford protein micro-assay (Bio-rad, Hercules, CA) was used to determine protein levels in the liver homogenates according to the manufacturer’s instructions. Protein levels were determined on aliquots of homogenates in Costar 96-well assay plates (Corning, Corning, NY) and read using a BioTek Synergy 2 micro-plate reader (BioTek Instruments, Winooski, VT).
Liver and serum lipid analysis
Liver homogenate lipid classes (TG, free C, total C, PL, and NEFA) were measured using Wako diagnostic kits in accordance with the manufacturer’s instructions. Liver cholesteryl ester concentration (CE) was determined by subtracting free (non-esterified) cholesterol concentration from the total concentration. Quantitatively there are no significant differences in liver homogenate lipid levels measured with the above kits as compared to solvent extracting lipids from liver homogenate and analyzing extracts along with appropriate lipid standards (C, CE, TG, NEFA, and PL) by thin layer chromatography [71].
Serum lipids (free C, total C), HDL-cholesterol (HDL-C)], APOA1 and APOB levels were determined using commercially available diagnostic kits in accordance with the manufacturer’s instructions (see above). The assays were modified to support the use of 96-well plates and micro-plate reader as described above. Serum cholesteryl ester (CE) concentrations were calculated by subtraction of serum free C from serum total C. Serum non-HDL-C was calculated by subtracting serum HDL-C from serum total C.
Western Blotting
The following antibodies were used in western blotting to determine protein levels in liver homogenates as described in [56]: Rabbit and goat polyclonal antibody to mouse APOA1 (SC-23606), farnesoid x receptor (FXR), (SC-13063), multidrug resistance protein (MDR) (SC-8313), LDL-Receptor (LDL-R) (SC-11826), liver x receptor (LXR) (SC-1201), retinoid x receptor α (RXRα) (SC-553), sterol regulatory element-binding protein 1 (SREBP1) (SC-367), microsomal triglyceride transfer protein (MTP) (SC-33116), ATP-binding cassette sub-family G members 1, 4, 5 and 8 (ABCG1, 4, 5 and 8) (SC-11150, SC-33825, SC-25796, SC-30010), short heterodimer partner and small heterodimer partner (SHP) (SC-15283), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (SC-27578), cytoplasmic 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS1) (SC-33829), hormone sensitive lipase (HSL) (SC-25843), multidrug resistance associated protein 2 (MRP2) (SC-5770) and phosphatidylcholine transfer protein (PCTP) (SC-23672) were obtained from Santa Cruz Biotechnology (Dallas, TX). Rabbit and mouse polyclonal antibody to mouse APOB (ab31992) was purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibody to mouse FABP1 (11294), SCP2 (12249), and SCPx (11306) were prepared as described [8,9]. Goat polyclonal antibody to mouse glutathione s-transferase (GST) (27457701V) was obtained from GE Life Sciences (Pittsburgh, PA). Bacterial polyclonal antibody to mouse 3alpha-hydroxysteroid reductase (3αHSD) (H9117-01) was from US Biological (Peabody, MA). Rabbit polyclonal antibody to mouse PPARα (PA1-822A) was obtained from Pierce Antibody (Rockford, IL) and to mouse scavenger receptor class B member 1 (SR-B1) (NB400-104), ATP-binding cassette transporter member 1 (ABCA1) (NB400-105) from Novus Biological (Littleton, CO). Alkaline phosphatase-conjugated goat polyclonal antibody to rabbit IgG (product # A3687) and rabbit polyclonal antibody to goat IgG (product # A4187) were from Sigma-Aldrich (St. Louis, MO). Alkaline phosphatase-conjugated rabbit polyclonal antibody to mouse IgG (product # ab6729-I) was from Abcam (Cambridge, MA). Antibodies to housekeeper proteins were obtained as follows: Mouse monoclonal antibody to mouse GAPDH (MAB374) from Millipore (Billerica, MA); rabbit polyclonal antibody to mouse cytochrome c oxidase subunit IV (COX4) (ab16056) from Abcam (Cambridge, MA) and mouse polyclonal antibody to mouse Beta Actin (BA) (SC-4778) from Santa Cruz Biotechnology (Dallas, TX).
Since the proteins of interest were differentiated by size from COX4, BA or GAPDH on the tricene gels, the membrane blots were cut into two, and each was probed with antisera against the protein of choice and against COX4, BA or GADPH housekeeper proteins to ensure uniform protein loading. Alkaline-phosphatase conjugated goat anti-rabbit or mouse IgG were used to visualize protein bands. Blot images were then obtained with a using an Epson Perfection V700 Photo scanner (Long Beach, CA)and image proteins (mean 8-bit grayscale density) quantitated by densitometric analysis using NIH Image (available by anonymous FTP). Levels of each protein were then normalized to the mean level of the respective housekeeper protein (COX4, BA or GAPDH). Proteins with no source of pure protein were expressed as relative fold differences between samples as described earlier [4].
Real-time qRT-PCR
The following probes were obtained from Applied Biosystems (Foster City, CA) for quantitating gene specific mRNA levels in mouse liver homogenates as follows: glycerol-3-phosphate acyltransferase (GPAT) (Gpam; Mm00833328_m1), 1-acylglycerol-3-phosphate-O-acyltransferase (AGPAT) (Agpat2; Mm00458880_m1), Lipin 2 (Lpin2; Mm00522390_m1), diacylglycerol acyltransferase (DGAT) (Dgat2; Mm00499536_m1), acetyl-coA carboxylase (ACC1) (Acaca; Mm01304285_m1) , fatty acid synthase (FASN) (Fasn; Mm00662319_m1), organic anion-transporting polypeptide 1 (OATP1) (Slco1; Mm01267414_m1), organic anion-transporting polypeptide 2 (OATP2) (Slc22a7; Mm00460672_m1), carnitine palmitoyltransferase 1a (CPT1A) (Cpt1a; Mm00550438_m1), carnitine palmitoyltransferase 2 (CPT2) ( Cpt2; Mm00487202_m1), acyl-CoA oxidase-1 (ACOX1) (Acox1; Mm00443579_m1), acetyl-CoA acetyltransferase (ACAT2) (Soat2; Mm00448823_m1), bile salt export pump (BSEP) (Abcb11; Mm00445168_m1), sterol regulatory element-binding protein 2 (SREBP2) (Srebf2; Mm01306289_m1) and cytochrome P7A1, CYP7A1 (Cyp7a1, Mm00484152_m1).
Briefly, total mRNA was isolated from livers and purified using an RNeasy minikit (Quiagen, Valencia, CA) as per the manufacturer’s protocol. Concentrations and quality of mRNA were measured using the ND-1000 method (Nanodrop Technologies, Inc., Wilmington, DE) where a 260/280 ratio of 1.9–2.1 was accepted as mRNA of good quality. For qRT-PCR, expression patterns were assessed using TaqMan® One-Step PCR Master Mix reagent kit, gene-specific TaqMan PCR probes, and primers. An ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) and thermal cycling conditions of 48 degrees C for 30 minutes, 95 degrees C for 10 minutes before the first cycle, 95 degrees C for 15 seconds and 60 degrees C for 1 minute, repeated 40 times, were used for quantitating mRNA expression. Prior to amplification, total mRNA was first reverse transcribed in the first step of the thermal cycler protocol (48 degrees C for 10 minutes) using TaqMan one step chemistry. mRNA expression was normalized to 18S RNA (a housekeeping gene) as in [59] and made relative to the control mouse group (male WT mice on high fat diet) set to one for final calculations.
Statistics
Data were expressed as averages ± standard error of the mean (SEM). Statistical analysis was performed by one-way ANOVA followed by Newman-Keuls post hoc analysis (GraphPad Prism, San Diego, CA, San Jose, CA and Sigma Plot, Systat, San Jose, CA). Statistical differences of p< 0.05 were considered significant as follows: *, p< 0.05 for TKO vs WT; #, p < 0.05 for female vs male.
Results
Whole Body Phenotype: Impact of Sex and TKO in High Fat Fed Mice
Singly ablating Fabp1 increases weight gain [2,3,76] while singly ablating Scp2/Scpx decreases weight gain [56] in a sexually dimorphic manner. Since singly ablating these genes concomitantly upregulates expression of the non-ablated gene in a sex-dependent manner, it was important to determine the impact of ablating both genes on whole body phenotype in both male and female mice on a high fat diet (HFD).
Although wild-type (WT) male mice ate more food than WT females, TKO did not significantly impact total food consumed by either males or females (Table 1). Consistent with greater food intake, WT male mice gained more total body weight than WT female mice, but TKO had no effect on either males or females. Furthermore when total body weight was calculated on the basis of total high fat food consumed, WT males converted more of this increased high fat food intake to weight gain than WT females. But again TKO did not alter total weight gain/total high fat food consumed (Table 1). Male WT mice gained approximately 2-fold more than female WT mice on both HFD and control diet when measuring total body weight gain per high fat diet consumed in kcal. While the male TKO mice body weight gain/total HFD consumed was much higher than the female TKO, both the male and female TKO mice body weight gain/total HFD consumed was comparable to that of their WT mice counterparts. Also, the male and female TKO mice on control diet had similar increases in total body weight gain/total control diet consumed. Unlike the male TKO mice, the female TKO mice exhibited no increase resulting from HFD as compared to control diet.
Table 1. Effect of Fabp1/Scp2/Scpx gene ablation and sex on food consumption and body weight in mice fed a high fat diet.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Total food consumed, average daily food consumption, total body weight (BW) gain, and total body weight (BW) gain/total food consumed were obtained as described in Methods. Whole body fat tissue mass (FTM) and lean tissue mass (LTM) were determined by Lunar PIXImus dual-energy x-ray absorptiometry (DEXA) as described in Methods. WT: wild type; TKO: triple knock out; CO: control diet, HF: high fat diet.
| Male WT | Male TKO | Female WT | Female TKO | |||||
|---|---|---|---|---|---|---|---|---|
| CO | HF | CO | HF | CO | HF | CO | HF | |
| Total Food Consumed (kcal) | 1039 ± 27 | 1143 ± 19^ | 1021 ± 28 | 1174 ± 11^ | 943 ± 15# | 1056 ± 17^# | 999 ± 26 | 1085 ± 12^# |
| Average Daily Food Consumption (kcal/day) | 11.8 ± 0.31 | 13.0 ± 0.22^ | 11.6 ± 0.32 | 13.3 ± 0.12^ | 10.7 ±0.17# | 11.4 ±0.29^# | 12.0 ±0.19* | 12.3 ±0.13# |
| Total Food Consumed (%BW) | 11.3 ±0.188 | 9.3 ±0.204^ | 11.6 ±0.156 | 9.4 ±0.147^ | 14.0 ±0.197* | 12.3 ±0.353*^ | 14.1 ±0.331* | 11.8 ±0.382*^ |
| Total BW Gain (g) | 11.0 ±0.854 | 15.6 ±1.351^ | 8.1 ±1.407 | 14.6 ±1.001^ | 4.8 ±0.428# | 6.5 ±0.586# | 7.4 ±0.749 | 8.3 ±0.527# |
| Total BW Gain /Total Food Consumed (g/kcal) | 0.0106 ±0.0006 | 0.0137 ±0.001^ | 0.0078 ±0.0012* | 0.0125± 0.0009^ | 0.0052 ±0.0005# | 0.0063 ±0.0006# | 0.0074 ±0.0007 | 0.0077 ±0.0005# |
| Total LTM Change (g) | 3.5 ± 0.6 | 3.9 ± 0.4 | 2.4 ± 0.9 | 2.9 ±0.8 | 3.0 ± 0.3 | 3.3 ± 0.3 | 3.0 ± 0.4 | 3.9 ± 0.4 |
| Total FTM Change (g) | 6.3 ± 0.5 | 11 ± 1^ | 4 ± 1* | 10.3 ±0.6^ | 0.6 ±0.2# | 3.3 ±0.7^# | 1.3 ±0.4# | 2.1 ±0.7# |
Values represent the mean ± SEM, n=6–8.
p <0.05 for TKO vs. WT within the same diet and sex;
p<0.5 for control diet vs high fat diet within the same genotype and sex;
p <0.05 for male vs. female within the same genotype and diet.
PIXImus dual-energy x-ray absorptiometry (DEXA) scans were performed to determine if any difference in weight gain was due to selective loss of lean tissue mass (LTM) or fat tissue mass (FTM) as described in Methods. Both male and female WT mice on HFD gained significantly more FTM as compared to their counterparts on control diet. The male TKO mice also gained significantly more on HFD vs control diet but not so for the female TKO mice. WT males had greater weight gain as FTM than their female WT counterparts (Table 1). TKO on HFD did not alter the relative proportion of LTM vs FTM in either male or female mice.
Final liver weight (grams wet weight) of WT male mice was significantly greater than that of WT females, but was not associated with greater protein content of the liver (Table 2). TKO did not significantly alter total liver weight or liver protein content of either males or females. Finally, when data were expressed as liver weight/body weight, the parameter was not significantly altered by either sex or TKO (Table 2).
Table 2. Effect of Fabp1/Scp2/Scpx gene ablation and sex on liver weight in mice fed a high fat diet.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Final liver weight, liver weight/body weight (BW) and total liver protein were obtained as described in Methods. WT: wild type; TKO: triple knock out; CO: control diet, HF: high fat diet.
| Male WT | Male TKO | Female WT | Female TKO | |||||
|---|---|---|---|---|---|---|---|---|
| CO | HF | CO | HF | CO | HF | CO | HF | |
| Final Liver Weight (g) | 1.30 ± 0.09 | 1.16 ± 0.08 | 1.19 ± 0.1 | 1.24 ± 0.07 | 0.9 ± 0.02# | 0.76 ± 0.02# | 1.01 ± 0.06 | 0.81 ± 0.02# |
| Final Liver Weight/BW (g/g) | 0.041 ±0.001 | 0.031 ±0.001^ | 0.041 ±0.002 | 0.034 ±0.001^ | 0.042 ±0.001^ | 0.033 ±0.001 | 0.044 ±0.001 | 0.034 ±0.002^ |
| Final Total Liver Protein (mg/g) | 192 ± 14 | 165 ± 15 | 147 ± 12 | 207 ±15*^ | 154 ± 7 | 151 ± 7 | 143 ± 8 | 154 ±14# |
Values represent the mean ± SEM, n=6–8.
p <0.05 for TKO vs. WT within the same diet;
p<0.5 for control diet vs high fat diet within the same genotype;
p <0.05 for male vs. female within the same genotype.
Effect of Sex and TKO on Clinical and Morphological Hepatic Pathology
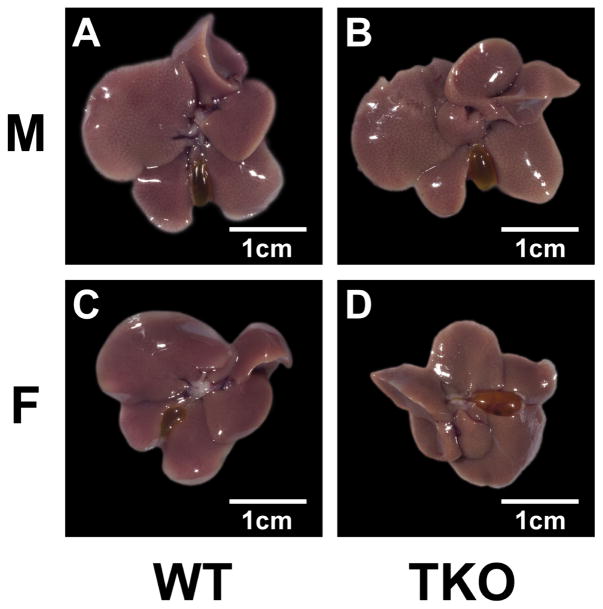
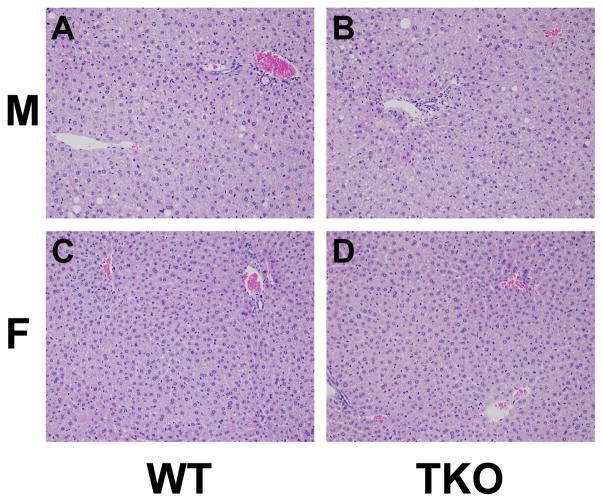
Both WT and TKO male mice had higher levels of lipid accumulation in the liver both grossly and histologically where females did not show evidence of lipid accumulation (Fig. 1 and 2). TKO did not affect Kupffer cell density or size and there was no other histologic evidence of diffuse inflammation in the liver of WT or TKO mice. In both WT and KO mice there were a few very small scattered foci of inflammation which are a common incidental background finding in the liver of mice of all ages [116]. These foci were slightly more frequent in TKO mice which may explain the slightly higher ALT and AST in TKO mice (Table 3). Although AST and ALT were slightly increased in TKO mice the levels were still in the normal range for mice (less than 150 and 400 units/l, respectively (blood chemistry and hematology in 8 inbred strains of mice, MPD:Eumorphia. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, Maine USA. http://phenome.jax.org [Cited 29 Oct, 2014]) (Table 3).
Figure 1. Effect of Fabp1/Scp2/Scpx gene ablation and sex on gross liver pathology in high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Livers were collected and processed as described in Methods.
Figure 2. Effects of Fabp1/Scp2/Scpx gene ablation and sex on histological liver pathology in high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Livers were collected and processed as described in Methods.
Table 3. Effect of Fabp1/Scp2/Scpx gene ablation and sex on AST and ALT in mice fed a high fat diet.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Final serum AST and ALT were obtained as described in Methods. WT: wild type; TKO: triple knock out.
| Male WT | Male TKO | Female WT | Female TKO | |
|---|---|---|---|---|
| AST IU/L | 31 ± 4 | 118 ± 9* | 42 ± 6 | 79 ± 5#* |
| ALT IU/L | 35 ± 4 | 104 ± 7* | 27 ± 3 | 100 ± 10* |
Values represent the mean ± SEM, n=7.
p <0.05 for TKO vs. WT;
p <0.05 for Male vs. Female.
Effect of Sex and TKO on Hepatic Accumulation of Glyceride Lipids in High Fat Fed Mice
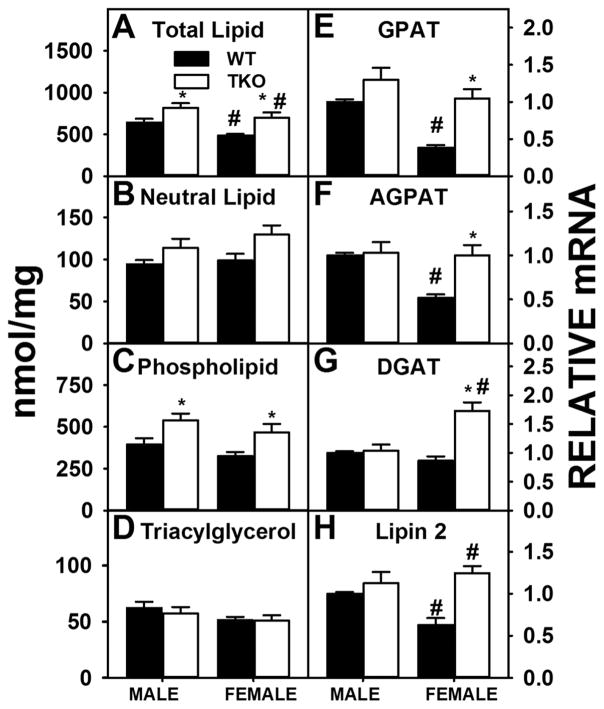
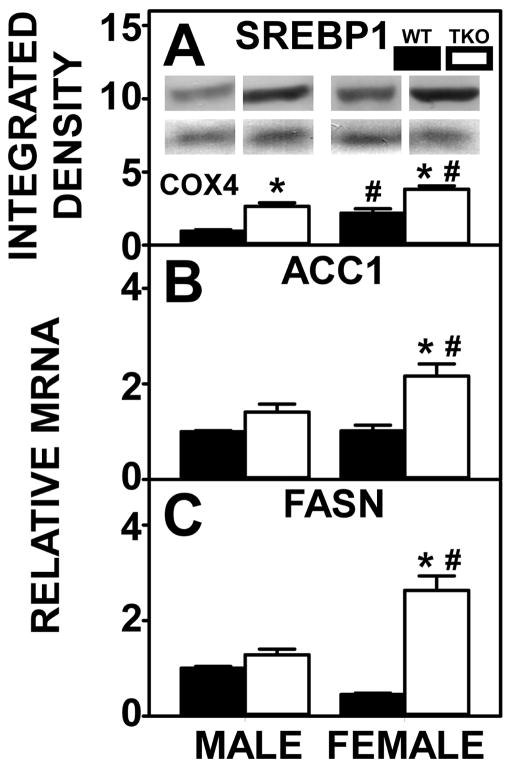
Total liver lipid levels were higher in HFD-fed WT male than WT female mice (Fig. 3A) which was associated with increased hepatic lipid accumulation both grossly and histologically and higher levels of phospholipid in WT males than WT females, albeit not significantly (Fig. 3C). WT males also showed a two-fold increase in expression of the key enzymes in the synthesis of phosphatidic acid (precursor of both phospholipids and triacylglycerols), i.e. GPAT (Fig 3E) and AGPAT (Fig 3F). Expression of Lipin 2, a key enzyme in converting phosphatidic acid to diacylglycerol for the synthesis of phospholipids (phosphatidylcholine, phosphatidylethanolamine) was higher in WT males than WT females (Fig. 3H) also. However, the lack of difference in expression of DGAT (which transacylates diglyceride to form triglyceride) between the WT male and WT female groups (Fig. 3G) was consistent with no significant difference in liver triacylglycerol levels between WT male and WT female mice (Fig. 3D). Finally, it is important to note that despite the fact that SREBP1 was actually lower in WT male than WT female livers (Fig. 4A), liver expression of SREBP1 target enzymes FASN (Fig. 4C) and ACC1, the rate limiting enzyme of de novo fatty acid synthesis, did not differ between the two sexes in WT mice (Fig. 4B).
Figure 3. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic accumulation of lipids in high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Levels of total hepatic lipid (A), hepatic neutral lipid (B), hepatic phospholipid (C) and hepatic triacylglycerol (D) were measured and qRT-PCR was performed to measure the expression of GPAT (Gpam gene) (E), AGPAT (Agpat2 gene) (F), DGAT (Dgat2 gene) (H) and Lipin 2 (Lpin2 gene) (H) as described in Methods. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
Figure 4. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic expression of key proteins in lipid synthesis in high fat fed mice.
Livers from male and female WT and TKO mice on a C57BL/6NCr background pair-fed a HFD were examined by western blot to measure relative hepatic protein levels of SREBP1 (A) as described in Methods. The housekeeping gene COX4 was used as a loading control to normalize protein expression of SREBP1. The inset in panel A shows representative western blots of relative protein expression in each mouse group. qRT-PCR was performed to determine relative transcription of the SREBP1 target genes ACC1 (Acaca gene) (B) and FASN (Fasn gene) (C) as described in Methods. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
TKO significantly increased hepatic total lipid levels in males and females (Fig. 3A), due primarily to higher levels of phospholipid (Fig. 3C) while levels of neutral lipids (Fig. 3B), especially triacylglycerol (Fig. 3D) were not altered in either sex. TKO induced increases in hepatic phospholipid in male mice was associated with increased expression of SREBP1 (Fig. 4A) despite no change in SREBP1 target enzymes ACC1 (Fig. 4B) and FASN (Fig. 4C). However, higher liver level of phospholipid in female TKO mice was attributed to increases in expression of enzymes in phospholipid synthesis [GPAT (Fig 3E), AGPAT (Fig 3F), Lipin 2 (Fig. 3H)] as well as increased SREBP1 (Fig. 4A) and its target enzymes in de novo fatty acid synthesis, i.e., ACC1 (Fig. 4B) and FASN (Fig. 4C).
Taken together, these findings indicate the presence of significant sexual dimorphism in hepatic accumulation of lipids in HFD fed mice. Nevertheless, TKO induced hepatic accumulation of phospholipid in both sexes with increases in phospholipid synthetic enzymes in female high fat fed mice.
Effect of Sex and TKO on Hepatic Fatty Acid Oxidation in High Fat Fed Mice
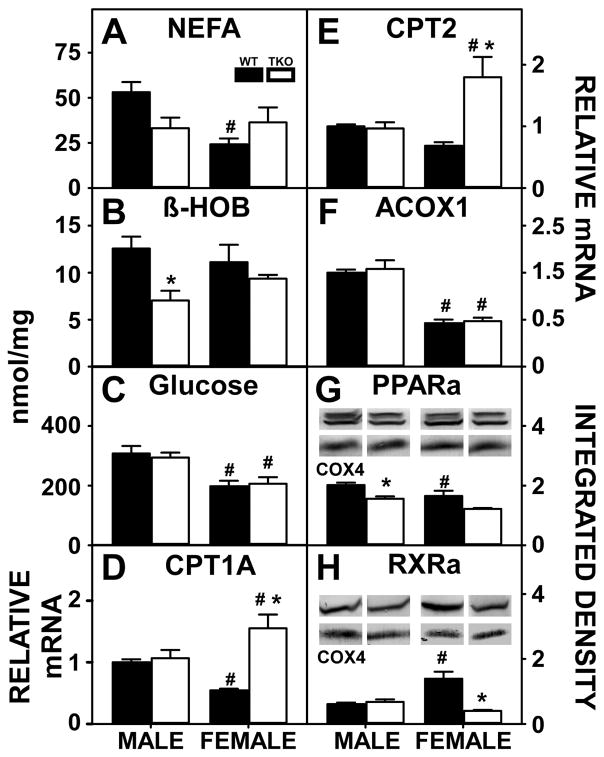
Decreased hepatic fatty acid oxidation may also contribute to hepatic accumulation of lipids. The gene products of both the Fabp1 [2,5] and Scp2/Scpx [56,84,106] genes facilitate fatty acid uptake/oxidation. Therefore, it was important to consider potential contributions from loss of both FABP1 as well as SCP2/SCPx that may have reduced hepatic fatty acid oxidation to thereby contribute to hepatic accumulation of lipids. This possibility was tested by examining serum levels of non-esterified fatty acids and β-hydroxybutyrate (β-HOB), a physiological measure of fatty acid oxidation, as well as determining hepatic expression of key receptors and enzymes involved in fatty acid oxidation in HFD fed mice.
Although serum levels of NEFA (Fig. 5A) were higher in WT males than females, serum levels of β-HOB (Fig. 5B) did not differ between the WT male and WT female mice. Similarly serum glucose (Fig. 5C), another oxidative substrate was higher in males than females. This was despite the fact that WT males had higher hepatic expression of fatty acid oxidative enzymes, i.e. CPT1A (Fig. 5D), CPT2 (Fig. 5E), and ACOX1 (Fig. 5F). Nuclear receptors involved in fatty acid oxidation were also measured where PPARα (Fig. 5G) was higher in WT males than females but RXRα was lower in WT males than WT females (Fig. 5H).
Figure 5. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic fatty acid oxidation high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. NEFA (A), βHOB (B) and glucose (C) were measured as described in Methods. qRT-PCR was performed to determine the relative transcription of the lipid oxidative enzymes Cpt1a (D), Cpt2 (E) and Acox1 (F) as described in Methods. The nuclear receptors PPARα (G) and RXR (H) were examined by western blot to measure relative hepatic protein levels as described in Methods. The housekeeping gene COX4 was used as a loading control to normalize protein expression of PPARα and RXR. The inset in panels G-H show representative western blots of relative protein expression in each mouse group. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
TKO significantly reduced serum β-HOB (Fig. 5B) in males while TKO had no effect on serum glucose (Fig. 5C) in neither males nor females. Decreases in serum β-HOB in TKO males were associated with loss of FABP1 and SCP2/SCPx and reduced expression of the FABP1 target nuclear receptor PPARα (Fig. 5G) with no effect on the FABP1 target proteins CPT1A (Fig. 5D), CPT2 (Fig. 5E) or ACOX1 (Fig. 5F). For TKO females the unaltered NEFA (Fig. 5A) and β-HOB (Fig. 5B) was associated with potentially offsetting increases in expression of several FABP1 target proteins [CPT1A (Fig. 5D) and CPT2 (Fig. 5E)], while RXRα (Fig. 5H) was decreased compared to WT females.
Taken together, these data suggested that the greater lipid accumulation in HFD fed WT males than WT females was not associated with reduced fatty acid oxidation. However, TKO motivated hepatic lipid accumulation through downregulation of the FABP1 targeted nuclear receptors involved in the regulation of peroxisomal beta-oxidation, PPARα and RXRα, in males and females, respectively.
Impact of Sex and TKO on Hepatic Cholesterol Accumulation in High Fat Fed Mice
Both Fabp1 [60,63,65] and Scp2/Scpx [72,112,114,121] gene products bind, enhance uptake, facilitate metabolism, and function in removal of cholesterol from the liver into bile. Therefore, the impact of sex and TKO on the hepatic accumulation of cholesterol was determined in HFD fed mice.
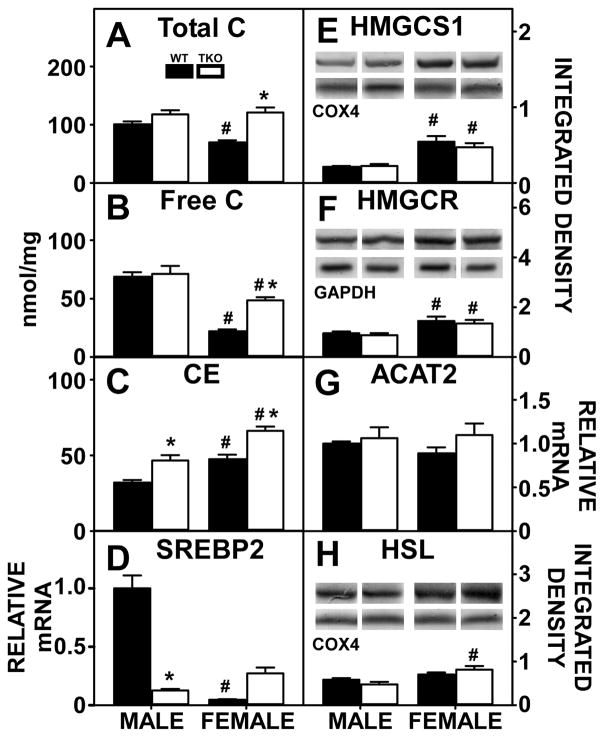
Hepatic cholesterol levels were markedly sex-dependent and impacted by TKO. Hepatic levels of total cholesterol was significantly higher in WT male than WT female mice (Fig. 6A), due primarily to increased free cholesterol (Fig. 6B) at the expense of cholesteryl ester (Fig. 6C). The higher liver cholesterol level in WT males was associated with higher levels of SREBP2 (Fig. 6D) despite WT females expressing higher levels of SREBP2 target proteins in de novo cholesterol synthesis, i.e. HMGCS1 (Fig. 6E) and HMGCR (Fig. 6F). Hepatic expression of enzymes in cholesterol esterification, i.e. ACAT2 (Fig. 6G) and hydrolysis, i.e. HSL (Fig. 6H) did not account for the hepatic cholesterol accumulation in WT males.
Figure 6. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic cholesterol accumulation in high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Total C (A), free C (B) and cholesteryl ester (C) were measured as described in Methods. qRT-PCR was performed to determine the relative transcription of SREBP2 (Srebf2 gene) (D) and ACAT2 (Soat2 gene) (G) as described in Methods. Western blots were performed to measure relative hepatic protein levels of HMGCS1 (E), HMGCR (F) and HSL (H) as described in Methods. The housekeeping gene COX4 was used as a loading control to normalize protein expression of HMGCS1 and HSL and GAPDH was used to normalize HMGCR. The inset in panels E, F and H show representative western blots of relative protein expression in each mouse group. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
TKO markedly increased hepatic total cholesterol accumulation in females but not males (Fig. 6A). Increased total cholesterol in TKO females was attributed to increases in both hepatic free cholesterol (Fig. 6B) and cholesteryl ester (Fig. 6C). Though males did not experience an increase in total cholesterol, TKO significantly increased cholesteryl ester accumulation (Fig. 6C). Despite increases in hepatic cholesterol in females, there was no significant change in hepatic level of SREBP2 (Fig. 6D) as well as no change in expression of HMGCS1 (Fig. 6E) and HMGCR (Fig. 6F). Again, altered hepatic expression of enzymes in cholesterol esterification, i.e. ACAT2 (Fig. 6G) and hydrolysis, i.e. HSL (Fig. 6H) in TKO mice did not account for the hepatic cholesterol accumulation. In contrast, expression of HSL was higher in TKO females than WT females.
In general, these data indicate that loss of Fabp1 and Scp2/Scpx gene products, rather than major upregulation of de novo cholesterol synthesis and esterification contributed to increased hepatic cholesterol levels in HFD fed TKO mice.
Impact of Sex and TKO on Hepatic Expression of Proteins involved in Hepatic Uptake and Efflux/Secretion of Cholesterol in High Fat Fed Mice
FABP1 enhances the uptake of HDL-cholesterol into the cytosol [114], while SCP2 facilitates both uptake and efflux of lipoprotein-derived cholesterol into/from the cytosol [80,112,114]. Therefore, it was essential to determine potential contributions of compensatory alterations in other proteins mediating lipoprotein cholesterol uptake/secretion/excretion in male and female Fabp1/Scp2/Scpx gene ablated mice fed HFD.
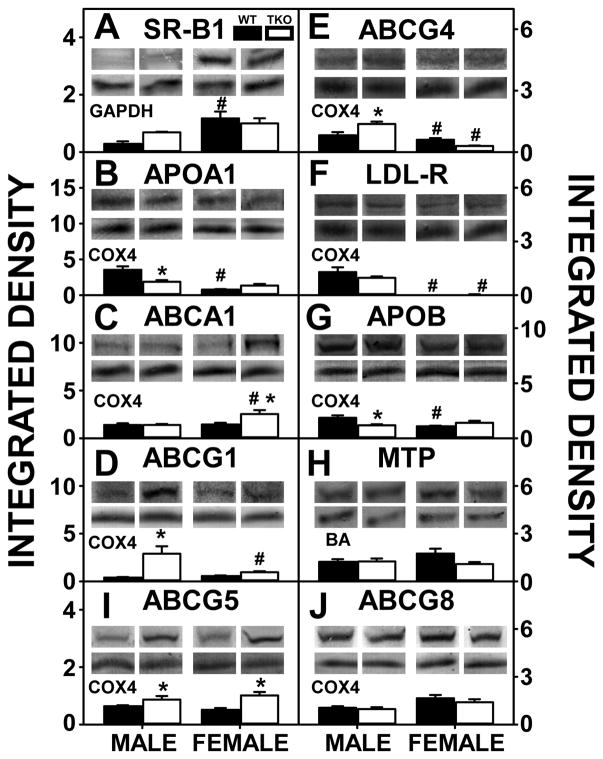
Hepatic expression of SR-B1, the receptor for uptake of HDL-cholesterol, was significantly lower in WT male than WT female mice (Fig. 7A). This decrease was offset in part by WT males expressing higher hepatic levels of proteins in hepatic HDL secretion, i.e. APOA1 (Fig. 7B) and ABCG4 (Fig. 7E). Concomitantly, ABCG1 (Fig. 7D), another protein involved in nascent cholesterol secretion, did not differ between the sexes. Liver levels of LDL-R, the receptor for the uptake of LDL-cholesterol, were also markedly higher in WT male than WT female mice (Fig. 7F). However, again, this increase was offset in part by higher levels of a key hepatic protein involved in secretion of cholesterol as VLDL (precursor of LDL), i.e. APOB (Fig. 7G). MTP, another protein involved in secretion of cholesterol as VLDL, was unaffected by sex. (Fig. 7H). Despite sexual dimorphism in hepatic cholesterol accumulation, there was no difference in the major liver canalicular proteins involved in translocating cholesterol into bile, i.e. ABCG5 (Fig. 7I) and ABCG8 (Fig. 7J), between males and females.
Figure 7. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic expression of key proteins in hepatic uptake and efflux/secretion of cholesterol in high fat fed mice.
Livers from male and female WT and TKO mice on a C57BL/6NCr background pair-fed a HFD were examined by western blot to measure relative hepatic protein levels of SR-B1 (A), APOA1 (B), ABCA1 (C), ABCG1 (D), ABCG5 (I), ABCG4 (E), LDL-R (F), APOB (G), MTP (H) and ABCG8 (J) as described in Methods. The housekeeping gene GAPDH was used as a loading control to normalize protein expression of SR-B1, COX4 was used to normalize APOA1, ABCA1, ABCG1, ABCG5, ABCG4, LDL-R, APOB and ABCG8 and BA was used to normalize MTP. The insets in panels A-J show representative western blots of relative protein expression in each mouse group. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
TKO did not affect hepatic level of SR-B1 in either sex (Fig. 7A) but did increase hepatic level of several proteins involved in nascent HDL secretion including ABCG1 (Fig. 7D) and ABCG4 (Fig. 7E) while significantly decreasing APOA1 (Fig. 7B) in males. Although TKO had no effect on SR-B1 in females (Fig. 7A), it differentially impacted proteins involved in nascent HDL formation by increasing ABCA1 (Fig. 7C), and ABCG1 (Fig. 7D) and decreasing ABCG4 (Fig. 7E). TKO increased hepatic expression of ABCG5 (Fig. 7I) in both males in females while having no impact on ABCG8 (Fig. 7J).
These data indicated that the higher hepatic cholesterol level in WT males was associated with higher levels of proteins in the non-HDL-cholesterol uptake pathway (LDL-R) despite the higher level of the key protein in nascent LDL secretion, APOB. The TKO-induced increase in hepatic cholesterol in males was associated at least in part with concomitant downregulation of the key protein in nascent HDL secretion (APOA1). In contrast, the TKO-induced increase in hepatic cholesterol in females did not consistently correlate with concomitant upregulation or downregulation of liver proteins in HDL-cholesterol uptake/efflux, LDL-cholesterol uptake, or VLDL-cholesterol secretion.
Effect of Sex and TKO on Hepatic Expression of Intracellular Proteins involved in Cytosolic Transport of Cholesterol in High Fat Fed Mice
FABP1 facilitates cytosolic transport of cholesterol to the plasma membrane for secretion of cholesterol as lipoprotein [2,89], to the endoplasmic reticulum for esterification by ACAT2 [49], or to bile canaliculi for biliary cholesterol excretion [36,63,121]. Similarly, SCP2 also facilitates cholesterol transfer from the plasma membrane to the endoplasmic reticulum [29,102] for esterification by ACAT2 [34,86], to mitochondria and peroxisomes for oxidation to steroids [13,14] or bile acids [30,54,81], and to bile canaliculi for biliary excretion [36,54,121]. Thus, it was important to determine the effect of sex and TKO on hepatic expression of cytosolic cholesterol binding/transfer proteins in HFD fed mice.
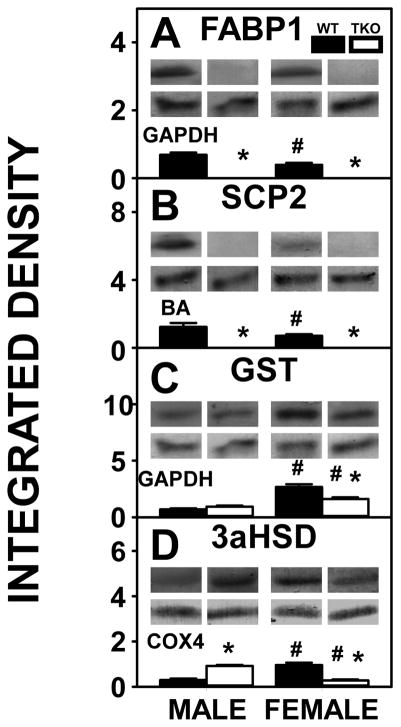
The higher hepatic cholesterol level in WT male mice was associated with WT males having higher levels of both FABP1, the most prevalent intracellular cholesterol binding/transport protein (Fig. 8A) as well as SCP2 (Fig 6B). However, hepatic levels of other less prevalent intracellular cholesterol binding proteins, GST (Fig. 8C) and 3αHSD (Fig. 8D) were lower in WT males than WT females.
Figure 8. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic expression of intracellular proteins involved in cytosolic transport of cholesterol in high fat fed mice.
Livers from male and female WT and TKO mice on a C57BL/6NCr background pair-fed a HFD were examined by western blot to measure relative hepatic protein levels of FABP1 (A), SCP2 (B), GST (C) and 3αHSD (D) as described in Methods. The housekeeping gene GAPDH was used as a loading control to normalize protein expression of FABP1 and GST, BA was used to normalize SCP2 and COX4 was used to normalize 3αHSD. The insets in panels A-D show representative western blots of relative protein expression in each mouse group. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
TKO resulted in complete loss of FABP1 (Fig. 8A) and SCP2 (Fig. 8B) in both males and females. This resulted in potential partial compensatory upregulation of 3αHSD in males (Fig. 8D) while neither GST (Fig. 8C) nor 3αHSD (Fig. 8D) were upregulated in females.
Taken together, the finding of higher hepatic cholesterol levels in WT males than WT females was associated with higher levels of FABP1 and SCP2. In TKO mice, the absence of FABP1 and SCP2 was compensated for only in part by increased level of one less common cholesterol binding protein, i.e. 3αHSD, and not GST (Fig. 8C, D), in males, while both 3αHSD (Fig. 8D) and GST (Fig. 8C) were decreased in females.
Effect of Sex and TKO on Serum Lipoproteins and Apoproteins in High Fat Fed Mice
The findings in the preceding sections suggest potential contributions of sex and TKO to altering serum apoprotein and lipoprotein profile in HFD fed mice. This possibility was examined as described in Methods. In addition, serum apolipoprotein bound cholesterol has been shown to be affected by FABP1 expression, where FABP1 ablation significantly reduces serum HDL cholesterol levels in mice fed a standard chow. Therefore, it was essential to examine the effects of ablation in mice fed a HFD diet.
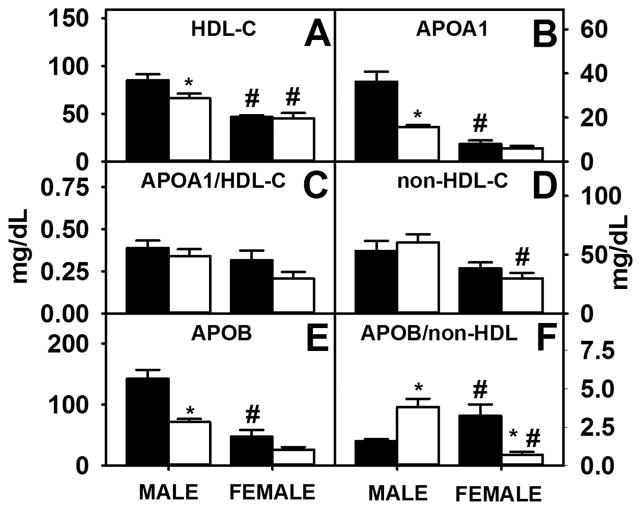
FABP1 upregulation in male WT mice contributed to higher serum levels of HDL-cholesterol (Fig. 9A) and APOA1 (Fig. 9B), although the ratio of APOA1/HDL-cholesterol did not significantly differ between the sexes (Fig. 9C). Higher levels of HDL-C in WT males also were associated with lower SR-B1, the hepatic receptor for HDL-C. Despite increased expression of LDL-R, the hepatic receptor for LDL-C, the serum level of non-HDL-cholesterol (Fig. 9D) and APOB (Fig. 9E) were higher in WT males, but the ratio of non-HDL-cholesterol/APOB was not significantly different (Fig. 9F). In addition, TKO males had higher levels of serum non-HDL-C (Fig. 9A), APOB (Fig. 9E) and APOB/non-HDL-C ratio (Fig. 9F) than TKO females.
Figure 9. Effects of Fabp1/Scp2/Scpx gene ablation and sex on serum lipoproteins and apolipoproteins in high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Levels of serum HDL-C (A), apoA1 (B) and non-HDL-C (D) were measured and ratios of apoA1/HDL-C (C) and apoB/non-HDL-C (F) were calculated as described in Methods. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
In male mice, TKO decreased serum levels of HDL-cholesterol (Fig. 9A) and APOA1 (Fig. 9B) without altering the ratio of APOA1/HDL-cholesterol (Fig. 9C). While TKO decreased APOB levels in both males and females (Fig. 9E), the level of non-HDL-cholesterol was not significantly altered (Fig. 9D). Though the ratio of APOB/non-HDL-C was increased in TKO males, decreased APOB levels in TKO females, albeit not significantly, contributed to a decreased APOB/non-HDL-C ratio (Fig. 9F).
Overall, sex and TKO affected serum lipoprotein and apoprotein levels in males and females. Further, higher serum HDL-C in WT males was associated with higher hepatic FABP1 and lower hepatic SR-B1. Though LDL-R levels were increased in both WT and TKO male groups compared to both female groups, non-HDL-C levels only differed between TKO males and females. These data confirm that Fabp1/Scp2/Scpx gene ablation yields a sexually dimorphic response in serum apolipoproteins involved in serum cholesterol transport.
Impact of Sex and TKO on Hepatic Proteins involved in Biliary Bile Formation in High Fat Fed Mice
Bile acid production and canalicular excretion drives co-secretion of cholesterol into bile to regulate hepatic cholesterol level. FABP1 binds bile acids [60,63,66,67] and enhances transfer/targeting of HDL-derived cholesterol (and cholesteryl ester after hydrolysis) to the bile canaliculus for biliary excretion [36,63,121]. SCP2 enhances cholesterol transfer to mitochondria and peroxisomes for oxidation to bile acids [30,54,81] and to the bile canaliculus for cholesterol biliary excretion [36,54,121]. Therefore, the impact of sex and TKO on hepatic expression of proteins involved in bile acid production and dynamics was determined in HFD fed mice.
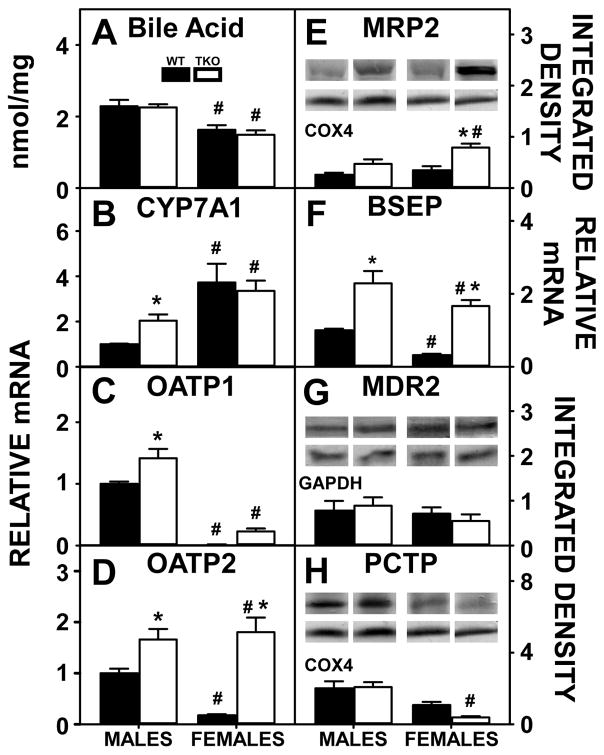
Hepatic levels of bile acids were significantly higher in WT males than WT females (Fig. 10A) which was associated with decreased levels in the expression of the rate limiting enzyme in hepatic bile acid synthesis, i.e. CYP7A1 (Fig. 10B), as bile acids repress the transcription of CYP7A1. Higher hepatic bile acids also was associated with higher levels of hepatic proteins involved in bile acid re-uptake from the blood, i.e. OATP1 (Fig. 10C) or OATP2 (Fig. 10D). However, BSEP (Fig. 10F), which codes for a protein involved in canalicular bile acid secretion, was also higher in WT males than females. Another hepatic protein in canalicular bile acid secretion into bile, MDR2 (Fig. 10G) along with hepatic proteins in cytosolic phosphatidylcholine transport and canalicular secretion into bile, i.e. MRP2 (Fig. 10E) and PCTP (Fig. 10H) remained unchanged between WT males and females.
Figure 10. Effects of Fabp1/Scp2/Scpx gene ablation and sex on biliary bile formation in high fat fed mice.
Male and female WT and TKO mice on a C57BL/6NCr background were pair-fed a HFD. Hepatic Bile acid (A) was measured as described in Methods. qRT-PCR was performed to determine the relative transcription of CYPA1 (Cyp7a1 gene) (B) and OATP1 (Slco1a1 gene) (C), OATP2 (Slc227a7 gene) (D) and BSEP (Abca11 gene) (F) as described in Methods. Western blots were performed to measure relative hepatic protein levels of MRP2 (E) MDR2 (G) and PCTP (H) as described in Methods. The housekeeping gene COX4 was used as a loading control to normalize protein expression of MRP2 and PCTP and GAPDH was used to normalize MDR2. The insets in panels E, G and H show representative western blots of relative protein expression in each mouse group. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
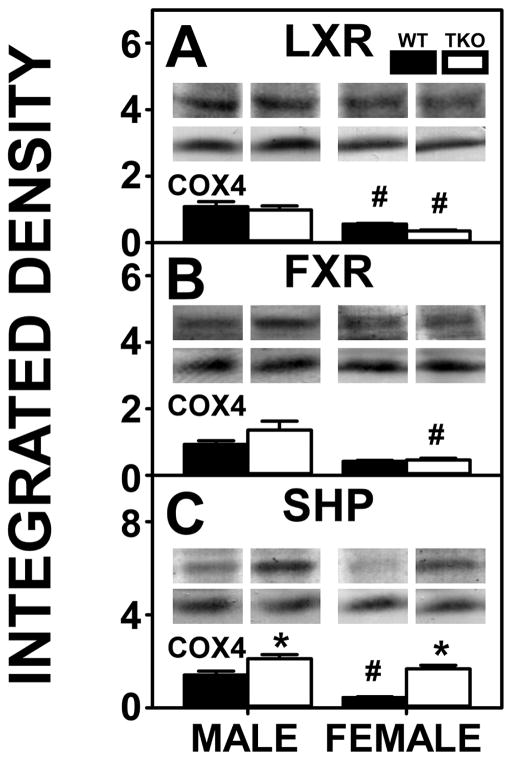
Though expression of CYP7A1 in WT males was less than females, levels of the nuclear regulatory protein that contributes to hepatic cholesterol homeostasis by upregulating CYP7A1 in the presence of high cholesterol, i.e. LXR (Fig. 11A), was higher in WT males than females. This also held true for TKO males compared to TKO females. WT males also expressed higher levels of a nuclear regulatory protein involved in downregulating the import and synthesis of bile acids, i.e. SHP (Fig. 11C). FXR, which induces SHP [21] was not affected by sex in WT mice (Fig 9. B).
Figure 11. Effects of Fabp1/Scp2/Scpx gene ablation and sex on hepatic expression of nuclear receptors nuclear receptors regulating transcription of proteins in bile acid synthesis in high fat fed mice.
Livers from male and female WT and TKO mice on a C57BL/6NCr background pair-fed a HFD were examined by western blot to measure relative hepatic protein levels of LXR (A), FXR (B) and SHP (C) as described in Methods. The housekeeping gene COX4 was used as a loading control to normalize protein expression of these proteins. The insets in panels A-D show representative western blots of relative protein expression in each mouse group. Values represent the mean ± SEM, n=8. *p <0.05 for TKO vs. WT. #p <0.05 for Male vs. Female.
TKO had little effect on hepatic bile acid levels in either male or female mice (Fig. 10A). While complete loss of key proteins involved in bile acid formation, binding, and cytosolic transport (FABP1, SCP2, SCPx), was expected to lower liver bile acid levels, concomitant upregulation of the key synthetic enzyme (CYP7A1, Fig. 10B) in males, and apical proteins in bile acid reuptake from serum, i.e. OATP1 (Fig. 10C), OATP2 (Fig. 10D) and MRP2 (Fig. 10F) in both sexes, apparently compensated to maintain liver bile acid levels. This was in spite of a TKO induced upregulation of a major canalicular bile acid export pump (BSEP, Fig. 10E) while another canalicular bile acid pump was not affected (MDR, Fig. 10H). TKO induced upregulation of SHP in both males and females compared to their WT counterparts (Fig. 11C) but did not impact hepatic expression of other nuclear receptors regulating transcription of proteins in bile acid synthesis in either males or females (Fig. 11A–B).
Taken together, these findings suggested that HFD fed WT males’ higher liver bile acid level was associated with greater reuptake despite increased BSEP, involved in canalicular bile acid secretion. TKO did not alter hepatic bile acid levels largely due to compensatory upregulation of the rate limiting bile acid synthetic enzyme in males and of apical transporters for bile acid reuptake in both sexes.
Discussion
Although singly ablating Fabp1 or Scp2/Scpx genes may exacerbate the impact of HFD on whole body phenotype and hepatic lipid metabolism, dietary regimen (mice prefer and consume more ad libitum fed HFD), sex-differences, and/or concomitant upregulation of the non-ablated gene complicate interpretation of such findings. This is especially evident in mice singly ablated in Scp2/Scpx or Scpx wherein hepatic levels of FABP1 are concomitantly upregulated [4,30,107]. To further resolve the impact of Scp2/Scpx gene ablation on whole body and hepatic phenotype independent of FABP1 upregulation, the current study was undertaken by examining the impact of also ablating Fabp1 in Scp2/Scpx null mice (i.e. Fabp1/Scp2/Scpx ablation or TKO). In order to preclude potential complications of data interpretation, these studies were performed by: i) pair-feeding high fat diet (HFD) independent of increased preference and intake of HFD (rev. in [62] and ii) phytoestrogen-free and phytol-free HFD independent of potential estrogenic [117,118] or PPARα [22,37,122] induction effects of these lipophilic molecules. The data provided the following new insights on the impact of these genes on whole body and liver lipid phenotype in both male and female mice pair-fed HFD.
First, female WT mice consumed more total high fat diet as percent of body weight than their male WT counterparts but the WT male mice gained significantly more body weight than female WT mice. The study herein continued from 8 weeks to 20 weeks. Studies involving C57BL6 mice examining various high fat diets, demonstrate various weight gains for male vs female mice. It is difficult to compare each study due to the different starting ages, substrains, duration, diet compositions, and microbiota. In one particular study wherein C57BL/6N mice were put on a HFD at 4 weeks of age, females gained similar weight to males until about after 16 weeks duration and overtook the males in body weight after about 30 week duration [78]. However another HFD study involving C56BL/6J mice beginning at 3 weeks (after weaning) and lasting until the mice were 9 months, showed the male WT on HFD continuing to gain weight at a similar or slightly higher rate [48]. Further, while the male mice outdistanced their counterparts on normal diet throughout, the female mice on HFD were similar to their counterparts on normal diet up to about 4 months of age [48]. A different study involving C57BL/6J mice revealed that the fatty acid profile of HFD was linked to sex differences in weight gain [1]. Further complicating direct comparisons, the C57BL/6J substrain is more sensitive to high fat diet-induced obesity than the C57BL/6N substrain [94].
Herein, when comparing total body weight gain per high fat diet consumed in kcal, the male WT mice gained approximately 2-fold more than female WT mice. The 2-fold increase was similar to the WT male vs female gain on control diet. In contrast, the TKO male vs female mice on control diet did not differ in total body weight gain per total food consumed on control diet. While the female TKO mice exhibited no increase as compared to control diet, the male TKO mice significantly gained more than the male WT mice on the control diet—comparable to that of the WT male mice on HFD.
The body weight gain exhibited by the male WT vs female WT mice was associated with increased hepatic levels of the two major cytosolic long chain fatty acid (LCFA) and LCFA-CoA binding proteins, i.e. FABP1 and SCP2. Even in the context of control-chow fed diets, hepatic protein levels of FABP1, SCP2 and SCPx are also higher in livers of male than female mice [7,8,45,56].
Second, male WT mice exhibited greater hepatic lipid accumulation than their female counterparts fed the same HFD. While FABP1 stimulates both anabolic and catabolic aspects of LCFA metabolism, in the context of HFD the net effect of the anabolic processes appeared greater in WT male than WT female mice. The WT male mice’s greater hepatic lipid accumulation was associated directly with: i) Higher hepatic levels of FABP1 and SCP2. FABP1 is known to enhance uptake [5,71,83,85,99] and cytosolic transport of exogenous LCFA [5,74,83] for anabolic processes such as esterification in the endoplasmic reticulum [50–52] and lipoprotein budding from endoplasmic reticulum for secretion [89,108]. Likewise, SCP2 also enhances uptake of LCFA and cholesterol [80,84], LCFA cytosolic transport [84], and esterification in the endoplasmic reticulum [15,16,87,110]; ii) Higher hepatic expression of several key enzymes in the synthesis of phospholipids (GPAT, AGPAT, and Lipin 2). In contrast, over all, LCFA catabolism was not higher in HFD-fed WT males than females. Consistent with the higher hepatic expression of FABP1 and SCP2 in HFD-fed WT males than females, hepatic expression of LCFA oxidative enzymes was higher in HFD-fed WT males than females (CPT1A, CPT2, and ACOX1). FABP1 facilitates LCFA/LCFA-CoA targeting for oxidation in mitochondria and peroxisomes [5,43] as well as targeting to nuclei for LCFA and LCFA-CoA activating PPARα transcription of oxidative enzymes [42,44]. SCP2 also enhances LCFA oxidation [6]. Nevertheless, the HFD-fed WT male and female mice did not differ in LCFA oxidation as indicated by unaltered serum β-HOB levels. This suggested that in the context of HFD, the higher levels of FABP1 and SCP2 in WT males than females resulted in preferential targeting of LCFA towards anabolic metabolism (i.e. lipid synthesis and storage). Consistent with this possibility, the greater body weight gain of HFD-fed WT males was associated with 5-fold greater gain as FTM as well as LTM as compared to females.
Third, while TKO male mice had less weight gain than WT mice on control diet, the TKO had little effect on whole body phenotype in either male or female HFD-fed mice as compared to their WT counterparts. Singly ablating the Fabp1 gene increases whole body weight gain in females and less so in males [2,3,68,69]. Conversely, singly ablating the Scp2/Scpx gene decreases body weight, especially in females [56,107].Taken together, these findings suggested that the reduced whole body weight of Scp2/Scpx gene ablated mice was not attributable to concomitant upregulation of FABP1. The two genes appeared to impact oppositely the whole body weight gain in HFD fed mice.
Fourth, TKO significantly increased hepatic accumulation of lipid (phospholipid, cholesterol, cholesteryl ester) in both male and female HFD-fed mice. Despite the loss of both FABP1 and SCP2, three factors contributed to increased hepatic lipid accumulation. They include the following: i) Upregulation of other cytosolic lipid ligand (LCFA, cholesterol) ‘chaperone’ proteins (3αHSD) in TKO males but not females; ii) Upregulation of key proteins in phospholipid synthesis (GPAT, AGPAT, and/or Lipin 2) in females and de novo LCFA synthesis (SREBP1 in males and females; ACC1 and FASN in females). Singly ablating Fabp1 decreased hepatic triacylglycerol in males while increasing phospholipid, cholesterol and cholesteryl ester in females [65]. Despite concomitant upregulation of FABP1, singly ablating Scp2/Scpx increased hepatic accumulation of lipid (phospholipid, triacylglycerol, cholesteryl ester) in both male and female mice [56]. Taken together, these data indicated that FABP1 and SCP2/SCPx had a greater impact on hepatic triacylglycerol and cholesterol, respectively, but in a sex dependent manner; iii) Decreased LCFA oxidation as indicated by decreased serum β-HOB in male mice. Despite downregulation of hepatic expression of nuclear receptors regulating expression of LCFA oxidative enzymes in both males and females (PPARα and RXRα, respectively), expression of several LCFA oxidative enzymes (CPT1A, CPT2) was upregulated in TKO females but not males. Singly ablating Fabp1 reduced LCFA oxidation as well as ligand-induced PPARα transcription of LCFA oxidative enzymes [2,5,47,71,97,98]. Despite concomitant upregulation of FABP1, singly ablating Scp2/Scpx also decreased LCFA oxidation [107]. Again, these findings suggested that SCP2/SCPx may have a greater impact on LCFA oxidation than FABP1.
Fifth, sex, more than Fabp1/Scp2/Scpx gene ablation, played a significant role in hepatic bile acid synthesis, reuptake and secretion. WT male mice exhibited higher levels of hepatic bile acid with concomitant downregulation of CYP7A1 expression compared to WT females. WT males also exhibited increased proteins involved in the reuptake of bile acid from serum (OATP1 and OATP2) despite increases in BSEP and SHP which are involved in the secretion and decreased synthesis of bile acids, respectively. FABP1 and SCP2/SCPx have been shown to play distinct, but complimentary roles in hepatic bile acid homeostasis where FABP1 is involved in the retention of bile acids and SCP2/SCPx functions in the formation and biliary secretion of bile acids. These findings suggest that the increase in hepatic bile acid concentration in WT mice was impacted by upregulation of FABP1 and SCP2 and specifically, their bile acid retention and formation functions. Again, TKO had no effect on hepatic bile acid levels. This was due to the concomitant upregulation of bile acid synthetic and reuptake proteins (CYP7A1, OATP1, OATP2) and contrasting upregulation of proteins involved in inhibiting the synthesis and promoting the canalicular secretion of bile acids (SHP, MRP2 and BSEP).
Sixth and finally, ablation of Scp2/Scpx in Fabp1 null mice (TKO) impacted hepatic phospholipid mass to a greater extent than ablating Fabp1 alone—especially in the context of HFD. For example, in HFD-fed mice TKO significantly increased liver phospholipid mass in both males and females (shown herein)—in marked contrast to LKO alone which did not alter hepatic phospholipid level in either sex [64]. However, in control-fed mice, TKO selectively increased hepatic phospholipid in females but not males (not shown)—similarly as shown for LKO in control-fed females vs males [64,66,67,71]. In contrast, ablating only the Scp-2/Scp-x gene (DKO) alone had no effect on hepatic phospholipid content in either male or female control-fed mice [56]. Taken together, these findings suggest that the impact of Scp2/Scpx and Fabp1 gene products on hepatic phospholipid phenotype is complex, highly dependent on the expression of one or both these genes, and is significantly dependent on the context of sex and dietary fat. Both direct and indirect mechanism(s) may be involved. For example, both FABP1 and SCP-2 are known to bind fatty acyl-CoA and directly stimulate microsomal glycerol-3-phosphate acyltransferase (GPAT)—the rate limiting step in synthesis of phosphatidic acid from which other phospholipid subclasses are derived [11,50,52,110]. Whether FABP1 and/or SCP-2 may also and possibly differentially impact other fatty acyl-CoA transacylation enzymes in the formation of phospholipid classes vs triacylglycerols is not known. Alternately, FABP1 (but not SCP-2) is known to transfer bound ligands (fatty acids, fatty acyl-CoAs, xenobiotics) through the cytosol, into nuclei wherein it binds to nuclear receptors (e.g. PPARα) to facilitate ligand transfer and activate transcription of numerous genes in fatty acid metabolism (rev. in [2,44,104,109,119]. While the absence of FABP1 and SCP-2 (i.e. TKO) would thereby normally be expected to reduce PPARα transcriptional activity, inhibition of phytol metabolism elicits the opposite response since in phytol-fed mice [79]. TKO markedly inhibits phytol metabolism in phytol-fed mice and induces accumulation of high levels of phytol metabolites (phytanic acid and pristanic acid) that are potent PPARα activators [79]. These metabolites in turn induce transcription of key enzymes regulating the synthesis of phosphatidic acid (Gpam, Agpat) as well as synthesis/hydrolysis of triacylglycerol (Lipin, Dgat) [79]. Resolving whether FABP1 also either directly impacts enzyme activities and/or transcription of mRNAs encoding enzymes in phospholipid synthetic steps downstream from Gpat (e.g. Pcyt1a, Pemt, Pcyt2, Ptdss1 and 2) is beyond the timeframe and scope of this manuscript.
In summary, both Fabp1 and Scp2/Scpx gene products play important roles in regulating whole body phenotype and hepatic lipid accumulation in mice. Hepatic FABP1 protein level is greatly upregulated in human NAFLD [17,35,77] and in animal models of NAFLD [10,124]. Furthermore, a highly prevalent SNP in the coding region of the human Fabp1 gene results in a T94A amino acid substitution [12,26,77,103] that is associated with NAFLD [77,96]. Three additional promising fields of research on potential roles of FABP1 (and potentially SCP2) in the development of NAFLD are: i) The role of microbiome alteration in NAFLD pathogenesis. Studies have shown that gut dysbiosis not only leads to human obesity, but also that many microbial cell components resulting from gut dysbiosis contribute to hepatic inflammation and steatosis [125]. It has also been shown that fecal transplantation from mice with NAFLD into healthy wild-type mice results in NAFLD in those healthy mice [100]. Environmental modifiers such as differences in gut microbiome have been suggested to contribute to the phenotype of Fabp1 gene ablated mice [90]. ii) The impact of FABP1 on the hepatic endocannabinoid system in the development of NAFLD. Recent discoveries with Fabp1 gene ablated mice have resolved a link between FABP1, the endocannabinoid (EC) system and NAFLD. FABP1 is the most prevalent endocannabinoid and cannabinoid binding/’chaperone’ protein present in hepatic cytosol of mice [45,103]. Ablation of the Fabp1 gene increased hepatic levels of AEA and 2-AG, endogenous agonists of the cannabinoid receptor CB1, which have been associated with NAFLD [45]. Although SCP-2 also binds AEA and 2-AG with high affinity [39,45,58], Scp-2/Scp-x gene ablation decreases brain levels of endocannabinoids but concomitantly increases CB1 levels [40]. However, nothing is known regarding the impact of ablating the Scp-2/Scp-x gene or SNPs therein on the hepatic endocannabinoid system and/or NAFLD. iii) TKO results in different metabolic patterns between male and female mice due to sex differences in FABP1 and SCP-2/SCP-x expression as well as to Fabp1 and Scp2/Scp-x having different metabolic functions in health and disease. For example, phytol (a normal dietary constituent derived from the side chain of chlorophyll) markedly decreases body weight gain and increases weight loss more so in female than male WT mice [8,57,79]. This difference has been attributed to the lower expression of the protein products of the Fabp1 and/or Scp2/Scp-x genes, both of which are involved in phytol metabolism [5,8,75,107,113]. With regards to human disease, increased FABP1 level and human genetic variation in the Fabp1 gene have been associated with development of NAFLD as indicated above. While Scp-2/Scp-x gene ablation in mice is associated with altered phytol, lipid metabolism, and neurotoxicity [5,8,75,107,113], human genetic variation in Scp-2/Scp-x also results in neurotoxity exhibited as neurodegeneration, brain iron accumulation, leukencephalopathy, dystonia, and motor neuropathy [25,41].
Overall in the setting of health as compared to excessive adiposity, our study supports that endogenous (sex) and phenotypic (WT vs TKO) factors impact the metabolic functions of the genes, Fabp1 and Scp-2/Scp-x wherein lipid accumulation in mice fed a high fat diet highlights the role of Fabp1 and/or Scp2/Scpx genes as potential targets for therapeutic intervention in the treatment of NAFLD.
Highlights.
Ablating Fabp1 or Scp2 may exacerbate ad lib fed high-fat diet (HFD) induced NAFLD.
Confounded by upregulated non-ablated gene, preference for HFD, and sex difference.
Pair-fed HFD differentially impacts body weight and hepatic lipid in male vs female,
Ablating both genes (TKO) exacerbates hepatic lipid, but not weight gain in either sex.
TKO selectively increased liver phospholipid & cholesteryl ester, but not triglyceride.
Acknowledgments
This work was supported in part by the US Public Health Service/National Institutes of Health grants RO1 DK41402 (FS, ABK), R25 OD016574 (SM, ABK), and T35 OD010991 (SM, ABK).
Abbreviations
- 3αHSD
3alpha-hydroxysteroid reductase
- ABCA1
ATP-binding cassette sub-family A member 1
- ABCG1, 4 , 5 or 8
ATP-binding cassette sub-family G member 1,4, 5 or 8
- ACAT2
acetyl-CoA acetyltransferase (Soat2 gene)
- ACC1
Acetyl-CoA carboxylase (Acaca gene)
- ACOX1
acyl-CoA oxidase-1 (Acox1 gene)
- AGPAT
1-acylglycerol-3-phosphate-O-acyltransferase (Agpat2 gene)
- APOA1
apolipoprotein A-I
- APOB
apolipoprotein B
- BA
Beta-Actin
- β-HOB
β-hydroxybutyrate
- BSEP
bile salt export pump (Abcb11 gene)
- C
free cholesterol
- CE
cholesteryl ester
- COX4
cytochrome c oxidase subunit IV
- CPT1a or 2
carnitine palmitoyltransferase 1a or 2 (Cpt1a or 2 gene)
- CYP7A1
cytochrome P7A1 (Cyp7a1 gene)
- DEXA
dual-energy X-ray absorptiometry
- DGAT
diacylglycerol acyltransferase (Dgat2 gene)
- FABP1/L-FABP
liver fatty acid binding protein
- FASN
fatty acid synthase (Fasn gene)
- FTM
fat tissue mass
- FXR
farnesoid x receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPAT
glycerol-3-phosphate acyltransferase (Gpam gene)
- GST
glutathione s-transferase
- HDL-C
high density lipoprotein cholesterol
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase)
- HFD
high fat diet
- HMGCS1
cytosolic 3-hydroxy-3-methylglutaryl-CoA synthase
- HSL/CEH
hormone sensitive lipase/cholesteryl ester hydrolase
- LDL-R
low density lipoprotein receptor
- Lipin 2
phosphatidate phosphatase (Lpin2 gene)
- LTM
lean tissue mass
- LXR
liver x receptor
- MDR
multidrug resistance protein
- MRP2
multidrug resistance associated protein
- MTP
microsomal triglyceride transfer protein
- NEFA
non-esterified fatty acid
- non-HDL-C
non-HDL cholesterol
- OATP1 or 2
organic anion-transporting polypeptide 1 or 2 (Slco1a1 or Slc22a7 genes, respectively)
- PCTP
phosphatidylcholine transfer protein
- PL
phospholipid
- PPARα
peroxisome proliferator activated receptor alpha
- qRT-PCR
quantitative real-time polymerase chain reaction
- RXRα
retinoid x receptor α
- SCP2
sterol carrier protein 2, SCPx, sterol carrier protein x
- SEM
standard error of the mean
- SHP
short heterodimer partner
- SR-B1
scavenger receptor class B member 1
- SREBP1 and 2 (Srebf2 gene for SREBP2)
sterol regulatory element-binding protein 1 and 2
- TC
total cholesterol
- TG
triglyceride/triacylglycerol
- TKO
fatty acid binding protein/sterol carrier protein- 2/sterol carrier protein-x null mouse on C57BL/6NCr background (Fabp1/Scp2/Scpx null or gene ablated)
- WT
wild-type C57BL/6NCr mouse.
Footnotes
Conflict of interest
The authors declare that no conflicts of interest with contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akoum SE, Lamontagne V, Cloutier I, Tanguay J-F. Nature of fatty acids in high fat diets differentially delineates obesity-linked metabolic syndrome components in male and female C57BL/6J mice. Diabetology and Metabolic Syndrome. 2011;3 doi: 10.1186/1758-5996-3-34. http:/www.dmsjournal.com/content3-1/34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and Dietary Obesity. Journal of Nutritional Biochemisty. 2010;21:1015–1032. doi: 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atshaves BP, McIntosh AL, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene ablated mice. Lipids. 2010;45:97–110. doi: 10.1007/s11745-009-3379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atshaves BP, McIntosh AL, Landrock D, Payne HR, Mackie J, Maeda N, Ball JM, Schroeder F, Kier AB. Effect of SCP-x gene ablation on branched-chain fatty acid metabolism. Am J Physiol. 2007;292:939–951. doi: 10.1152/ajpgi.00308.2006. [DOI] [PubMed] [Google Scholar]
- 5.Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem. 2004;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- 6.Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock K, Maeda N, Kier AB, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res. 2007;48:2193–2211. doi: 10.1194/jlr.M700102-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol. 2005;288:C543–C558. doi: 10.1152/ajpcell.00359.2004. [DOI] [PubMed] [Google Scholar]
- 8.Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Atshaves BP, Petrescu A, Starodub O, Roths J, Kier AB, Schroeder F. Expression and Intracellular Processing of the 58 kDa Sterol Carrier Protein 2/3-Oxoacyl-CoA Thiolase in Transfected Mouse L-cell Fibroblasts. J Lipid Res. 1999;40:610–622. [PubMed] [Google Scholar]
- 10.Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest and Liver Phys. 2007;294:G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 11.Bordewick U, Heese M, Borchers T, Robenek H, Spener F. Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol Chem Hoppe-Seyler. 1989;370:229–238. doi: 10.1515/bchm3.1989.370.1.229. [DOI] [PubMed] [Google Scholar]
- 12.Brouillette C, Bose Y, Perusse L, Gaudet D, Vohl M-C. Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate. J Hum Gen. 2004;49:424–432. doi: 10.1007/s10038-004-0171-2. [DOI] [PubMed] [Google Scholar]
- 13.Chanderbhan R, Kharroubi A, Noland BJ, Scallen TJ, Vahouny GV. Sterol carrier protein 2: Further evidence for its role in adrenol steroidogenesis. Endocrine Res. 1986;12:351–370. doi: 10.3109/07435808609035445. [DOI] [PubMed] [Google Scholar]
- 14.Chanderbhan RF, Kharroubi A, Pastuszyn A, Gallo LL, Scallen T. Direct Evidence for Sterol Carrier Protein-2 (SCP-2) Participation in ACTH Stimulated Steroidogenesis in Isolated Adrenal Cells. In: Chang TY, Freeman DA, editors. Intracellular Cholesterol Trafficking. Kluwer Academic Publishers; Boston: 1998. pp. 197–212. [Google Scholar]
- 15.Chao H, Billheimer JT, Kier AB, Schroeder F. Microsomal long chain fatty acyl CoA transacylation: differential effect of SCP-2. Biochim Biophys Acta. 1999;1439:371–383. doi: 10.1016/s1388-1981(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 16.Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res. 2003;44:72–83. doi: 10.1194/jlr.m200191-jlr200. [DOI] [PubMed] [Google Scholar]
- 17.Charlton M, Viker K, Krishnan A, Sanderson S, Veldt B, Kaalsbeek AJ, Kendrick M, Thompson G, Que F, Swain J, Sarr M. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–1384. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A, Tang Y, Davis V, Hsu F-F, Kennedy SM, Song H, Turk J, Brunt EM, Newberry EP, Davidson NO. L-FABP modulates murine stellate cell activation and diet induced nonalcoholic fatty liver disease. Hepatology. 2013;57:2202–2212. doi: 10.1002/hep.26318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dansen TB, Westerman J, Wouters F, Wanders RJ, van Hoek A, Gadella TW, Wirtz KW. High affinity binding of very long chain fatty acyl CoA esters to the peroxisomal non-specific lipid transfer protein (sterol carrier protein-2) Biochem J. 1999;339:193–199. [PMC free article] [PubMed] [Google Scholar]
- 20.Demel RA, Kalsbeek R, Wirtz KW, Van Deenen LM. The protein-mediated net transfer of phosphatidylinositol in model systems. Biochim Biophys Acta. 1977;466:10–22. doi: 10.1016/0005-2736(77)90204-8. [DOI] [PubMed] [Google Scholar]
- 21.Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, SHP, mediates bile acid-induced inhibition of the rat bile acid transporter, NTCP. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 22.Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein-2-/sterol carrier protein x-deficient mice. J Biol Chem. 1999;274:2766–2772. doi: 10.1074/jbc.274.5.2766. [DOI] [PubMed] [Google Scholar]
- 23.Fengler VHI, Macheiner T, Kessler SM, Czepukojc B, Gemperlein K, Muller R, Kiemer AK, Magnes C, Haybaeck J, Lackner C, Sargsyan K. Susceptibility of different mouse wild-type strains to develop diet induced NAFLD/AFLD associated liver disease. PLoS ONE. 2016;11:pe0155163. doi: 10.1371/journal.pone.0155163. doi:101371/journalpone0155163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fengler VHI, Macheiner T, Sargsyan K. Manifestation of NAFLD/NASH in different dietary mouse models. Hepatology. 2016;4:94–102. [Google Scholar]
- 25.Ferdinandusse S, Kostopoulos P, Denis S, Rusch R, Overmars H, Dillman U, Reith W, Haas D, Wanders RJA, Duran M, Marziniak M. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukencephalopathy with dystonia and motor neuropathy. Am J Hum Genet. 2006;78:1046–1052. doi: 10.1086/503921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher E, Weikert C, Klapper M, Lindner I, Mohlig M, Spranger J, Boeing H, Schrezenmeir J, Doring F. L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol Gen and Metab. 2007;91:278–284. doi: 10.1016/j.ymgme.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem. 1996;271:31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- 28.Frolov A, Miller K, Billheimer JT, Cho T-C, Schroeder F. Lipid specificity and location of the sterol carrier protein-2 fatty acid binding site: A fluorescence displacement and energy transfer study. Lipids. 1997;32:1201–1209. doi: 10.1007/s11745-997-0154-5. [DOI] [PubMed] [Google Scholar]
- 29.Frolov AA, Woodford JK, Murphy EJ, Billheimer JT, Schroeder F. Fibroblast membrane sterol kinetic domains: modulation by sterol carrier protein 2 and liver fatty acid binding protein. J Lipid Res. 1996;37:1862–1874. [PubMed] [Google Scholar]
- 30.Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J Biol Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- 31.Gadella TW, Wirtz KW. Phospholipid binding and transfer by non-specific lipid transfer protein (SCP-2): a kinetic model. Eur J Biochem. 1994;220:1019–1028. doi: 10.1111/j.1432-1033.1994.tb18707.x. [DOI] [PubMed] [Google Scholar]
- 32.Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh A, Martin G, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40:498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 33.Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic liver disease and insulin resistance: from bench to bedside. Diabetes and Metabolism. 2013;39:16–26. doi: 10.1016/j.diabet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Gavey KL, Noland BJ, Scallen TJ. The participation of sterol carrier protein2 in the conversion of cholesterol to cholesterol ester by rat liver microsomes. J Biol Chem. 1981;256:2993–2999. [PubMed] [Google Scholar]
- 35.Guzman C, Benet M, Pisonero-Vaquero S, Moya M, Garcia-Mediavilla MV, Martinez-Chantar ML, Gonzalez-Gallego J, Castell JV, Sanchez-Campos S, Jover R. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARa; and repressed by C/EBPa: implicaiton in FABP1 down-regulation in nonalcoholic liver disease. Biochim Biophys Acta. 2013;1831:803–818. doi: 10.1016/j.bbalip.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Hafer A, Katzberg N, Muench C, Scheibner J, Stange EF, Seedorf U, Fuchs M. Studies With Sterol Carrier Protein-2 (SCP-2) Gene Knockout Mice Identify Liver Fatty Acid Binding Protein (FABP1) As Intracellular Cholesterol Transporter Contributing to Biliary Cholesterol Hypersecretion and Gallstone Formation. 2000. p. 135. [Google Scholar]
- 37.Hanhoff T, Benjamin S, Borchers T, Spener F. Branched-chain fatty acids as activators of peroxisome proliferators. Eur J Lip Sci Technol. 2005;107:716–729. [Google Scholar]
- 38.Higuchi N, Kato M, Tanaka M, et al. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP, and L-FABP in non-alcoholic fatty liver disease. Exp and Ther Med. 2011;2:1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillard CJ, Huang H, Vogt CD, Rodrigues BE, Neumann TS, Sem DS, Schroeder F, Cunningham CW. Endocannabinoid Transport Proteins: Discovery of Tools to Study Sterol Carrier Protein-2. In: Patricia VE, Reggio H, editors. Methods In Enzymology: Endocannabinoid Transport Proteins, Methods in Enzymology. Academic Press imprint of Elsevier; Cambridge, MA, USA: 2017. pp. 99–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillard CJ, Liedhegner E, Tinklenberg J, Stuhr K, Doncheck E, Du L, Cunningham C. Genetic deletion of SCP-2 selectivelly enhances amygdalar endocannabinoid signaling. FASEB J. 2015;29 p.Supplement 770.9. [Google Scholar]
- 41.Horvath R, Lewis-Smith D, Douroudis K, Duff J, et al. SCP2 mutations and neurodegeneration with brain iron accumulation. Neurology. 20165;85:1909–1911. doi: 10.1212/WNL.0000000000002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. Glucose regulates fatty acid binding protein interaction with lipids and PPARa. J Lipid Res. 2010;51:3103–3116. doi: 10.1194/jlr.M005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hostetler HA, Lupas D, Tan Y, Dai J, Kelzer MS, Martin GG, Woldegiorgis G, Kier AB, Schroeder F. Acyl-CoA binding proteins interact with the acyl-CoA binding domain of mitochondrial carnitine palmitoyltransferase I. Mol Cell Biochem. 2011;355:135–148. doi: 10.1007/s11010-011-0847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, Schroeder F. Liver type Fatty Acid Binding Protein (L-FABP) interacts with peroxisome proliferator activated receptor-a in cultured primary hepatocytes. J Lipid Res. 2009;50:1663–1675. doi: 10.1194/jlr.M900058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, McIntosh AL, Martin GG, Landrock D, Chung S, Landrock KK, Dangott LJ, Li S, Kier AB, Schroeder F. FABP1: a novel hepatic endocannabinoid and cannabinoid binding protein. Biochemistry. 2016;55:5243–5255. doi: 10.1021/acs.biochem.6b00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, McIntosh AL, Martin GG, Landrock K, Landrock D, Gupta S, Atshaves BP, Kier AB, Schroeder F. Structural and functional interaction of fatty acids with human liver fatty acid binding protein (L-FABP) T94A variant. FEBS J. 2014;281:2266–2283. doi: 10.1111/febs.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang H, McIntosh AL, Martin GG, Petrescu AD, Landrock K, Landrock D, Kier AB, Schroeder F. Inhibitors of fatty acid synthesis induce PPARa-regulated fatty acid b-oxidative enzymes: synergistic roles of L-FABP and glucose. PPAR Research. 2013;2013:1–22. doi: 10.1155/2013/865604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang L-L, Wang C-H, Li T-L, Chang S-D, Lin L-C, Chen C-P, Chen C-T, Liang K-C, Ho I-K, Yang W-S, Chiou L-C. Sex differences in high fat diet induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity. 2010;18:463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- 49.Jefferson JR, Slotte JP, Nemecz G, Pastuszyn A, Scallen TJ, Schroeder F. Intracellular sterol distribution in transfected mouse L–cell fibroblasts expressing rat liver fatty acid binding protein. J Biol Chem. 1991;266:5486–5496. [PubMed] [Google Scholar]
- 50.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: Stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys. 1997;341:112–121. doi: 10.1006/abbi.1997.9957. [DOI] [PubMed] [Google Scholar]
- 51.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys. 1997;341:112–121. doi: 10.1006/abbi.1997.9957. [DOI] [PubMed] [Google Scholar]
- 52.Jolly CA, Wilton DA, Schroeder F. Microsomal fatty acyl CoA transacylation and hydrolysis: fatty acyl CoA species dependent modulation by liver fatty acyl CoA binding proteins. Biochim Biophys Acta. 2000;1483:185–197. doi: 10.1016/s1388-1981(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 53.Kamath S, Chavez AO, Gestaldelli A, et al. Coordinated defects in hepatic long chain fatty acid metabolism and triglyceride accumulation contribute to insulin resistance in non-human primates. PLoS ONE. 2011;6:e27617. doi: 10.1371/journal.pone.0027617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kannenberg F, Ellinghaus P, Assmann G, Seedorf U. Aberrant oxidation of the cholesterol side chain in bile acid synthesis of sterol carrier protein-2/sterol carrier protein-x knockout mice. J Biol Chem. 1999;274:35455–35460. doi: 10.1074/jbc.274.50.35455. [DOI] [PubMed] [Google Scholar]
- 55.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic liver disease. J Gastroenterology. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klipsic D, Landrock D, Martin GG, McIntosh AL, Landrock KK, Mackie JT, Schroeder F, Kier AB. Impact of SCP-2/SCP-x gene ablation and dietary cholesterol on hepatic lipid accumulation. Am J Physiol Gastrointest and Liver Phys. 2015;309:G387–G399. doi: 10.1152/ajpgi.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landrock D, Milligan S, Martin GG, McIntosh AL, Landrock K, Schroeder F, Kier AB. Effect of Fabp1/Scp-2/Scp-x ablation on whole body and hepatic phenotype of phytol-fed male mice. Lipids. 2017;52:385–397. doi: 10.1007/s11745-017-4249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liedhegner ES, Vogt CD, Sem DS, Cunninham CW, Hillard CJ. Sterol carrier protein-2: binding protein for endocannabinoids. Mol Neurobiol. 2014;50:149–158. doi: 10.1007/s12035-014-8651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Martin GG, Atshaves BP, Landrock KK, Landrock D, Storey SM, Howles PN, Kier AB, Schroeder F. Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion. Am J Physiol Gastrointest and Liver Phys. 2014;307:G1130–G1143. doi: 10.1152/ajpgi.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin GG, Chung S, Landrock D, Landrock K, Dangott LJ, Peng X, Kaczocha M, Murphy EJ, Kier AB, Schroeder F. Female mice are resistant to Fabp1 gene ablation induced alterations in brain endocannabinoid levels. Lipids. 2016;51:1007–1020. doi: 10.1007/s11745-016-4175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin GG, Landrock D, Chung S, Dangott LJ, Seeger DR, Murphy EJ, Golovko MY, Kier AB, Schroeder F. Fabp1 gene ablation inhibits high fat diet-induced increase in brain endocannabinoids. J Neurochem. 2017;140:294–306. doi: 10.1111/jnc.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin GG, Landrock D, Landrock KK, Howles PN, Atshaves BP, Kier AB, Schroeder F. Relative contributions of L-FABP, SCP-2/SCP-x, or both to hepatic biliary phenotype of female mice. Arch Biochem Biophys. 2015;588:25–32. doi: 10.1016/j.abb.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin GG, Landrock D, Chung S, Dangott LJ, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Loss of fatty acid binding protein-1 alters the hepatic endocannabinoid system response to a high fat diet. J Lip Res. 2017;58:2114–2126. doi: 10.1194/jlr.M077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai P-J, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol. 2009;297:G1053–G1065. doi: 10.1152/ajpgi.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J. 2005;391:549–560. doi: 10.1042/BJ20050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol. 2006;290:G36–G48. doi: 10.1152/ajpgi.00510.2004. [DOI] [PubMed] [Google Scholar]
- 68.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver Fatty Acid Binding Protein GeneAblated Female Mice Exhibit Increased AgeDependent Obesity. J Nutr. 2008;138:1859–1865. doi: 10.1093/jn/138.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem. 2009;324:101–115. doi: 10.1007/s11010-008-9989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin GG, Chung S, Landrock D, Landrock KK, Huang H, Dangott LJ, Peng X, Kaczocha M, Seeger DR, Murphy EJ, Golovko MY, Kier AB, Schroeder F. FABP1 gene ablation impacts brain endocannabinoid system in male mice. J Neurochem. 2016;138:407–422. doi: 10.1111/jnc.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 72.Martin GG, Hostetler HA, McIntosh AL, Tichy SE, Williams BJ, Russell DH, Berg JM, Spencer TA, Ball JA, Kier AB, Schroeder F. Structure and function of the sterol carrier protein-2 (SCP-2) N-terminal pre-sequence. Biochem. 2008;47:5915–5934. doi: 10.1021/bi800251e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin GG, McIntosh AL, Huang H, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human liver fatty acid binding protein (L-FABP) T94A variant alters structure, stability, and interaction with fibrates. Biochemistry. 2013;52:9347–9357. doi: 10.1021/bi401014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 75.McIntosh AL, Storey SM, Huang H, Kier AB, Schroeder F. Sex-dependent impact of Scp-2/Scp-x gene ablation on hepatic phytol metabolism. Arch Biochem Biophys. 2017;635:17–26. doi: 10.1016/j.abb.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McIntosh AL, Atshaves BP, Landrock D, Landrock KK, Martin GG, Storey SM, Kier AB, Schroeder F. Liver fatty acid binding protein gene-ablation exacerbates weight gain in high fat fed female mice. Lipids. 2013;48:435–448. doi: 10.1007/s11745-013-3777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McIntosh AL, Huang H, Storey SM, Landrock K, Landrock D, Petrescu AD, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human FABP1 T94A variant impacts fatty acid metabolism and PPARa activation in cultured human female hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2014;307:G164–G176. doi: 10.1152/ajpgi.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obesity. 2012;36:262–272. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- 79.Milligan S, Martin GG, Landrock D, McIntosh AL, Mackie JT, Schroeder F, Kier AB. Impact of dietary phytol on lipid metabolism in SCP2/SCPx/L-FABP null mice. Biochim Biophys Acta - Mol Cell Biol Lip. 2017;1862:291–304. doi: 10.1016/j.bbalip.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moncecchi DM, Murphy EJ, Prows DR, Schroeder F. Sterol carrier protein-2 expression in mouse L-cell fibroblasts alters cholesterol uptake. Biochim Biophys Acta. 1996;1302:110–116. doi: 10.1016/0005-2760(96)00044-6. [DOI] [PubMed] [Google Scholar]
- 81.Muench C, Hafer A, Katzberg N, Scheibner J, Stange EF, Seedorf U, Fuchs M. Relevance of the Sterol Carrier Protein-2 Gene for Bile Acid Synthesis and Gallstone Formation in Genetically Susceptible Mice. 2000. p. 1167. [Google Scholar]
- 82.Mukai T, Egawa M, Takeuchi T, Yamashita H, Kusudo T. Silencing of FABP1 ameliorates hepatic steastosis, inflammation, and oxidative stress in mice with NAFLD. FEBS Open Bio. 2017:1–8. doi: 10.1002/2211-5463.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am J Physiol. 1998;275:G244–G249. doi: 10.1152/ajpgi.1998.275.2.G244. [DOI] [PubMed] [Google Scholar]
- 84.Murphy EJ. Sterol carrier protein-2 expression increases fatty acid uptake and cytoplasmic diffusion in L-cell fibroblasts. Am J Physiol. 1998;275:G237–G243. doi: 10.1152/ajpgi.1998.275.2.G237. [DOI] [PubMed] [Google Scholar]
- 85.Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta. 1996;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- 86.Murphy EJ, Schroeder F. Sterol carrier protein-2 mediated cholesterol esterification in transfected L-cell fibroblasts. Biochimica Et Biophysica Acta. 1997;1345:283–292. doi: 10.1016/s0005-2760(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 87.Murphy EJ, Schroeder F. Sterol carrier protein-2 mediated cholesterol esterification in transfected L-cell fibroblasts. Biochim Biophys Acta. 1997;1345:283–292. doi: 10.1016/s0005-2760(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 88.Nagy TR, Clair A-L. Precision and accuracy of dual-energy X-ray absorptiometry for in vivo body composition in mice. Obesity Research. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 89.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM. Liver fatty acid binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem. 2007;282:17974–17984. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 90.Newberry EP, Kennedy S, Xie Y, Luo J, Jiang H, Ory DS, Davidson NO. Phenotypic divergence in two lines of L-FABP(−/−) mice reflects substrain differences and environmental modifiers. Am J Physiol Gastrointest and Liver Phys. 2015;309:G648–G661. doi: 10.1152/ajpgi.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, Crooke RM, Graham MJ, Davidson NO. Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple muring genetic models. Hepatology. 2008;48:1097–1105. doi: 10.1002/hep.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-FABP−/− mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest and Liver Phys. 2008;294:G307–G314. doi: 10.1152/ajpgi.00377.2007. [DOI] [PubMed] [Google Scholar]
- 93.Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 94.Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, Leiter EH. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity. 2010;18:1902–1905. doi: 10.1038/oby.2009.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paz-Filho G, Mastronardi CA, Parker BJ, Khan A, Inserra A, Matthaei KI, Erhart-Bornstein M, Bornstein S, Wong M-L, Licinio J. Molecular pathways involved in the improvement of nonalcoholic fatty liver disease. J Mol Endocrinol. 2013;51:167–179. doi: 10.1530/JME-13-0072. [DOI] [PubMed] [Google Scholar]
- 96.X-Peng E, Wu YL, Lu Q-Q, Ju Z-J, Lin X. Two genetic variants in FABP1 and susceptibility to non-alcoholic fatty liver disease in a Chinese population. Gene. 2012;500:54–58. doi: 10.1016/j.gene.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 97.Petrescu AD, Huang H, Martin GG, McIntosh AL, Storey SM, Landrock D, Kier AB, Schroeder F. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARa regulated b-oxidative enzymes. Am J Physiol Gastrointest and Liver Phys. 2013;304:G241–G256. doi: 10.1152/ajpgi.00334.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petrescu AD, McIntosh AL, Storey SM, Huang H, Martin GG, Landrock D, Kier AB, Schroeder F. High glucose potentiates liver fatty acid binding protein (L-FABP) mediated fibrate induction of PPARa in mouse hepatocytes. Biochim Biophys Acta. 2013;1831:1412–1425. doi: 10.1016/j.bbalip.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-Cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- 100.Roy TL, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Gerard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2012;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 101.Schroeder F, Frolov A, Schoer J, Gallegos A, Atshaves BP, Stolowich NJ, Scott AI, Kier AB. Intracellular Sterol Binding Proteins, Cholesterol Transport and Membrane Domains. In: Chang TY, Freeman DA, editors. Intracellular Cholesterol Trafficking. Kluwer Academic Publishers; Boston: 1998. pp. 213–234. [Google Scholar]
- 102.Schroeder F, Frolov AA, Murphy EJ, Atshaves BP, Jefferson JR, Pu L, Wood WG, Foxworth WB, Kier AB. Recent advances in membrane cholesterol domain dynamics and intracellular cholesterol trafficking. Proc Soc Exp Biol Med. 1996;213:150–177. doi: 10.3181/00379727-213-44047. [DOI] [PubMed] [Google Scholar]
- 103.Schroeder F, McIntosh AL, Martin GG, Huang H, Landrock D, Chung S, Landrock KK, Dangott LJ, Li S, Kaczocha M, Murphy EJ, Atshaves BP, Kier AB. Fatty acid binding protein-1 (FABP1) and the human FABP1 T94A variant: Roles in the endocannabinoid system and dyslipidemias. Lipids. 2016;51:655–676. doi: 10.1007/s11745-016-4155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 105.Schroeder F, Zhou M, Swaggerty CL, Atshaves BP, Petrescu AD, Storey S, Martin GG, Huang H, Helmkamp GM, Ball JM. Sterol carrier protein-2 functions in phosphatidylinositol transfer and signaling. Biochemistry. 2003;42:3189–3202. doi: 10.1021/bi026904+. [DOI] [PubMed] [Google Scholar]
- 106.Seedorf U, Ellinghaus P, Nofer JR. Sterol carrier protein-2. Biochim Biophys Acta. 2000;1486:45–54. doi: 10.1016/s1388-1981(00)00047-0. [DOI] [PubMed] [Google Scholar]
- 107.Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes and Development. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siddiqi SA, Mansbach CM. Phosphorylation of Sar1b releases the liver fatty acid binding protein from a multiprotein complex in the intestinal cytosol enabling it to bind to ER and bud the pre-chylomicron transport vesicle. J Biol Chem. 2012;287:10178–10188. doi: 10.1074/jbc.M111.327247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smathers RL, Galligan JJ, Shearn CT, Fritz KS, Mercer K, Ronis M, Orlicky DJ, Davidson NO, Petersen DR. Susceptibility of L-FABP −/− mice to oxidative stress in early-stage alcoholic liver. J Lipid Res. 2013;54:1335–1345. doi: 10.1194/jlr.M034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Starodub O, Jolly CA, Atshaves BP, Roths JB, Murphy EJ, Kier AB, Schroeder F. Sterol carrier protein-2 immunolocalization in endoplasmic reticulum and stimulation of phospholipid formation. Am J Physiol. 2000;279:C1259–C1269. doi: 10.1152/ajpcell.2000.279.4.C1259. [DOI] [PubMed] [Google Scholar]
- 111.Stolowich NJ, Frolov A, Petrescu AD, Scott AI, Billheimer JT, Schroeder F. Holo-sterol carrier protein-2: 13C-NMR investigation of cholesterol and fatty acid binding sites. J Biol Chem. 1999;274:35425–35433. doi: 10.1074/jbc.274.50.35425. [DOI] [PubMed] [Google Scholar]
- 112.Storey SM, Atshaves BP, McIntosh AL, Landrock KK, Martin GG, Huang H, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from primary cultured mouse hepatocytes. Am J Physiol. 2010;299:244–254. doi: 10.1152/ajpgi.00446.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Storey SM, Huang H, McIntosh AL, Martin GG, Kier AB, Schroeder F. Impact of Fabp1/SCP-2/SCP-x gene ablation (TKO) on hepatic phytol metabolism. J Lipid Res. 2017;58:1153–1165. doi: 10.1194/jlr.M075457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012;302:G824–G839. doi: 10.1152/ajpgi.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tailleux A, Wouters K, Staels B. Role of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 116.Taylor I. Mouse. In: McInnes EF, editor. Background Lesions in Laboratory Animals. Saunders Elsevier; Edinburgh: 2012. p. 55. [Google Scholar]
- 117.Thigpen JE, Setchell KD, Ahlmark KB, Kocklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab An Science. 1999;49:530–536. [PubMed] [Google Scholar]
- 118.Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Envron Health Persp. 1999;107:A182–A183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Velkov T. Interactions between human liver fatty acid binding protein and peroxisome proliferator activated receptor drugs. PPAR Research. 2013;2013:1–14. doi: 10.1155/2013/938401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vluggens A, Reddy JK. Nuclear receptors and transcription factors in the development of fatty liver disease. Curr Drug Metabolism. 2012;13:1422–1435. doi: 10.2174/138920012803762710. [DOI] [PubMed] [Google Scholar]
- 121.Wang J, Bie J, Ghosh S. Intracellular cholesterol transport protein enhance hydrolysis of HDL-CEs and facilitate elimination of cholesterol into bile. J Lipid Res. 2016;57:1712–2719. doi: 10.1194/jlr.M069682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J Lipid Res. 1999;40:708–714. [PubMed] [Google Scholar]
- 123.Xie Y, Newberry EP, Kennedy SM, Luo J, Davidson NO. Increased susceptibility to diet-induced gallstones in liver fatty acid binding protein knockout mice. J Lipid Res. 2009;50:977–987. doi: 10.1194/jlr.M800645-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
- 125.Zhu L, Baker RD, Baker SS. Gut microbiome and nonalcoholic fatty liver diseases. Pediat Res. 2014;77:245–251. doi: 10.1038/pr.2014.157. [DOI] [PubMed] [Google Scholar]