Abstract
Previous research suggests a pivotal role of the prefrontal cortex (PFC) in word selection during tasks of confrontation naming (CN) and verb generation (VG), both of which feature varying degrees of competition between candidate responses. However, discrepancies in prefrontal activity have also been reported between the two tasks, in particular more widespread and intense activation in VG extending into (left) ventrolateral PFC, the functional significance of which remains unclear. We propose that these variations reflect differences in competition resolution processes tied to distinct underlying lexico‐semantic operations: Although CN involves selecting lexical entries out of limited sets of alternatives, VG requires exploration of possible semantic relations not readily evident from the object itself, requiring prefrontal areas previously shown to be recruited in top‐down retrieval of information from lexico‐semantic memory. We tested this hypothesis through combined independent component analysis of functional imaging data and information‐theoretic measurements of variations in selection competition associated with participants’ performance in overt CN and VG tasks. Selection competition during CN engaged the anterior insula and surrounding opercular tissue, while competition during VG recruited additional activity of left ventrolateral PFC. These patterns remained after controlling for participants’ speech onset latencies indicative of possible task differences in mental effort. These findings have implications for understanding the neural–computational dynamics of cognitive control in language production and how it relates to the functional architecture of adaptive behavior.
Keywords: language production, cognitive control, information theory, functional imaging
1. INTRODUCTION
In contrast to externally specified tasks of word reading (WR) or repetition, naming objects or generating actions associated with these objects require the capacity to select words and meanings from competing alternatives stored in lexico‐semantic knowledge (Fraisse, 1969; Indefrey & Levelt, 2004). Although controlled access to lexico‐semantic memory is central to fluent language production, its underlying neural–computational substrates remain poorly understood. Evidence has accumulated that the prefrontal cortex (PFC), in particular Broca's area (B[rodmann] A[rea] 9/44/45), plays a pivotal role in resolving competition during tasks of confrontation naming (CN) (Kan and Thompson‐Schill, 2004) and verb generation (VG) (Novick, Trueswell, & Thompson‐Schill, 2010; Thompson‐Schill, D'esposito, Aguirre, & Farah, 1997). However, differences in PFC involvement have also been reported between the two tasks, including more intense and broadly distributed frontal activity in VG compared with confrontation naming (Bourguignon, 2014; Edwards et al., 2010; Etard et al., 1999; Herholz et al., 1997; Indefrey & Levelt 2004). Clinical reports of deficits following frontal injury have also noted that more diffuse and anterior PFC damage can impair language generation (e.g., verbal fluency, story generation, sentence construction from single words, etc.) despite relatively preserved naming capacities (Costello & Warrington, 1989; Esmonde, Giles, Xuereb, & Hodges, 1996; Luria & Tsvetkova, 1968; Robinson, Blair, & Cipolotti, 1998). To date, however, a neural–computational account of these discrepancies has been elusive, notably with regard to the functional significance of anterior PFC activity in generation compared with naming performance.
To elucidate this issue, we propose that variations in PFC involvement between CN and VG reflect differences in these tasks' level of competition tied to their underlying processes of lexico‐semantic analysis. More specifically, CN involves bottom‐up recognition of objects and selection of words out of relatively limited sets of competitors. In contrast, VG recruits top‐down retrieval and selection of many possible semantic relations not readily evident from the object itself, recruiting greater processing resources for lexico‐semantic search compared with CN (Edwards et al., 2010; Kurland, Reber, & Stokes, 2014). The latter operation known as conceptual expansion—that is, the enlargement of one's knowledge of concepts beyond their initial definition—has been associated with activity in the left ventrolateral PFC (VLPFC, BA45/46/47) and is assumed to imply “cognitive control processes that modulate the selection of competing alternatives that are retrieved from one's own semantic store” (Abraham et al., 2012, p. 1913). Consistent with this perspective, activity in left VLPFC has been shown to depend on the level of competition during word generation tasks, with significantly larger BOLD signal changes in less versus more constrained generation conditions (e.g., naming a flower versus naming a red flower, cf. Tremblay & Gracco, 2006). Furthermore, anterior and middle regions of VLPFC exhibit strong BOLD signal changes in tasks involving controlled lexico‐semantic retrieval and selection, respectively (Badre, Poldrack, Paré‐Blagoev, Insler, & Wagner, 2005)—a finding in line with an extensive literature supporting the importance of this brain sector in the cognitive control of semantic memory (Badre & Wagner 2007; Lau, Phillips, & Poeppel, 2008). These observations suggest that task‐related increases in the demands for competition resolution in CN versus VG may recruit a gradient of PFC activity from posterior to anterior regions reflecting the transition from lexical selection to conceptual expansion.
We tested this hypothesis in a fMRI study using an information‐theoretic approach (Bourguignon, 2014; Lachman, 1973). In this framework, the primary computational metric of selection competition is the statistical concept of entropy (H)
| (1) |
summing the inverse log‐proportion of words i…N produced across speakers confronted with a production cue. Entropy is inversely related to the level of inter‐speaker agreement regarding the word(s) most likely to express a given concept. Accordingly, concepts eliciting the same word across speakers have high inter‐speaker agreement and comparably low entropy. As the number and relative distribution of words produced for the same concept vary, entropy increases accordingly, and with it the need to explore larger amounts of competing alternatives. Relative to more basic measures of response frequency or ratio, entropy covers more accurate information on the distribution of all responses associated with a given cue (Snodgrass & Vanderwart, 1980) and figures amongst the strongest predictors of naming latency (Alario et al., 2004; Lachman, 1973; Severens, Van Lommel, Ratinckx, & Hartsuiker, 2005). Furthermore, previous imaging evidence for the explanatory range of entropy in accounting for PFC involvement across different types of adaptive behavior (Koechlin, Ody, & Kouneiher, 2003, Koechlin & Summerfield, 2007; Yoshida & Ishii, 2006) illustrates its potential for the neural–computational study of competition resolution during language processing.
Combining the neural–computational advantages of entropy with preliminary evidence for task‐related gradients in speech‐related PFC activity (Bourguignon, 2014; Indefrey & Levelt, 2004), we therefore predict that cue‐related entropy variations in CN should covary with BOLD signal changes in anterior insula (Ant. Ins.) and surrounding opercular tissue, while entropy variations in VG should explain additional activity in (left) anterior and middle VLPFC. Other regions potentially involved in these processes include the presupplementary motor area and anterior cingulate cortex (ACC) (BA24/32). In contrast, primary motor–premotor areas (M1, BA4/6) supporting speech articulation should exhibit no reliable sensitivity to variations in entropy, since competition resolution processes are typically assumed to precede articulation (Bourguignon, 2014; Indefrey & Levelt, 2004).
Importantly, the requirement for conceptual expansion and related processes of competition resolution in VG compared with CN possibly entails sensitive differences in these tasks' inherent difficulty, which might in turn explain the activation patterns predicted above as the result of task‐related variations in mental effort rather than competition resolution. Another challenge faced in fMRI research on language production relates to data contamination by speech‐related movements and hemodynamic artifacts that are notoriously difficult to eliminate (Gracco, Tremblay, & Pike, 2005). We addressed these methodological points by combing independent component analysis of fMRI data (ICA)—an advanced multivariate technique that has proven effective in separating meaningful brain signal from noise originating from orofacial movements or respiratory functions‐related hemodynamic variations (Geranmayeh et al., 2012, Geranmayeh, Wise, Mehta, & Leech, 2014; van de Ven, Esposito, & Christoffels, 2009, see also Section 2 for detail)—and sparse‐sampling MR acquisition (Gracco et al., 2005) permitting detailed examination of the predictive effect of cue‐related entropy on PFC activation while factoring out participants' speech onset latencies indicative of mental effort. Findings from this investigation may have important implications in clarifying the neural–computational processes underlying competition resolution during language production and how they relate to general adaptive mechanisms deployed during complex action.
2. METHODS
2.1. Participants
Sixteen right‐handed (Oldfield, 1971) native speakers of English (eight male/eight female, age: 19–29) with normal or corrected‐to‐normal vision and no reported history of speech, language and/or hearing disorders took part in the study under informed consent and in return for monetary compensation. The experiment was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and with the ethics requirements of the Faculty of Medicine at McGill University.
2.2. Tasks and materials
Ninety speech cues were selected from Snodgrass and Vanderwart's (1980) standardized word and picture materials. Pictures were used as cues for CN and VG, while written versions of target names were used for a purely motor word reading (WR) task in which verbal responses are entirely specified by the cue itself (i.e., no selection competition). To guard against any risk of MR signal attenuation associated with habituation or learning (Raichle et al., 1994), cues were randomly distributed across three experimental lists in such a way that a cue used for one task never re‐occurred in the other tasks for the same participant. In total, each participant thus saw thirty original and unique cues per task (WR, CN, and VG). Participants across lists were matched for age, verbal IQ (Wechsler, 1999), performance IQ (Raven, Raven, & Court, 2003) and handedness (Oldfield, 1971, cf. Supporting Information Table S1). Cues were presented in three blocks of ten trials to avoid task‐switching confounds and were interspersed with shorter periods of rest (30 rest trials in total per list). Each block began with task‐specific instructions (WR: READ; PN: NAME; VG: VERB, cf. Figure 1 for illustration). The order of task blocks was pseudo‐randomized and counterbalanced across lists. Participants were screened, briefed and trained with an abbreviated version of the task a few days before scanning, then once again minutes before scanning. The pictures and words used in the training phase were different from those used in the experimental phase.
Figure 1.
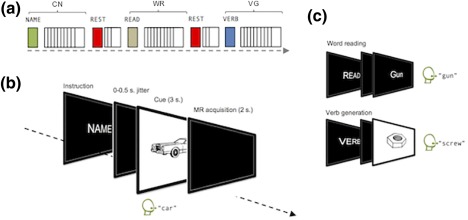
Summary of the study protocol. (a) Illustrative sequence of one CN block, one WR block and one VG block. Each task or rest block began with task‐related instructions matched for letter length (CN, NAME; WR, READ; VG, VERB; REST, REST) and contained ten trials. For each task, participants went through 3 blocks of 10 trials, for a total of 30 trials per task. Task‐blocks were pseudo‐randomized and counterbalanced across lists. Between task blocks, smaller blocks of rest trials (30 rest trials per scanning session) were inserted, during which the projector screen remained black and participants were required to remain still with their eyes open. (b) Detailed time course of a given trial during a CN block. (c) Illustration of instructions, cue type and responses during WR and VG blocks [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. Measures of selection competition: entropy and inter‐speaker agreement
Shannon entropy (H, cf. Equation (1) in the Supporting Information “Introduction” and for additional information) constituted the primary predictor of competition‐related variations in speech onset latency and BOLD signal change during CN and VG. A second measure of competition, “response agreement” (henceforth: AG), reflecting the percentage of speakers producing the same word in response to a picture, was also included for two reasons. First, AG is inversely related to H. This opposite relationship between H and AG helped us guard against invalid relationships in the data because positive (negative) correlations between speech onset/BOLD signal change and AG (H) would likely signal the presence of spurious correlations. Second, although AG has been used to measure selection competition in previous studies of language production (Barry, Morrison, & Ellis, 1997; Kan & Thompson‐Schill, 2004), it is also conceptually similar to measures designed to assess memory retrieval (rather than selection) between cues and targets (Moss & Older, 1996; Nelson, Schreiber, & Xu, 1998). According to these approaches, the frequency with which a word is produced in response to a cue determines the strength of semantic association between the word and the cue and, consequently, the level of effort required to retrieve the word upon cue presentation (weaker associates entail greater retrieval effort). From the perspective of a possible functional–anatomic dissociation between semantic retrieval and selection processes in anterior and middle VLPFC, respectively (Badre & Wagner, 2007), it is possible that AG and H may have different predictive effects on BOLD signal change in these distinct sub‐sections of VLPFC (see section 2.6.2 for detail).
Cue‐related H and AG values were taken from the norms of Snodgrass and Vanderwart (1980) and Kurland et al. (2014) for CN and VG, respectively. The norming sample of participants consisted of 42 healthy English‐speaking volunteers for CN (cf. Snodgrass and Vanderwart, 1980) and fifty healthy English‐speaking volunteers for VG (cf. Kurland et al., 2014, cf. Supporting information Table S2 for summary statistics). As observed previously in Kurland et al. (2014), VG was naturally more competitive than CN, as reflected in generally higher mean entropy and lower mean agreement values. One‐way analyses of variance were conducted between lists to ensure that no list was more competitive than the others in either CN or VG (all Fs < 1.5, ps > .24). Correlation analyses also excluded significant relationships between competition in CN and competition in VG for the same cues (Rs < 0.15, p > .15). Experimental lists were also matched based on Snodgrass and Vanderwart's (1980) normed values of image agreement, visual complexity and concept familiarity. Finally, cues between CN and VG were matched for age of acquisition (Kuperman, Stadthagen‐Gonzalez, & Brysbaert, 2012) and frequency of the object name (Brysbaert & New, 2009, cf. Supporting information Table S3 for summary statistics and additional information on these measures).
2.4. Data acquisition
All experimental sessions took place at the Montreal Neurological Institute (Quebec, Canada) using the protocol illustrated in Figure 1. Concurrent behavioral and neurophysiological data acquisition was performed on a 3T Siemens Magnetom TrioTim scanner. Anatomical images were first acquired with a T1‐weighted 3D MPRAGE sequence [TR = 2.2 s; TE = 2.98 s; slice thickness 1.00 mm; voxel‐size = 1 × 1 × 1 mm; flip angle = 9°, FOV = 256 × 256 mm). Thereafter, functional images were acquired using a T2*‐weighted EPI sequence functional scan (33 interleaved axial slices; slice thickness = 4 mm; in‐plane resolution 64 × 64, TR = 6.5 s; delay in TR = 4.5 s; TE = 30 ms; flip angle 90°; FOV = 256 × 256 mm). Participants lay supine in the scanner bore with their head immobilized with a polystyrene‐filled vacuum bag and a forehead restraining device. A sparse sampling acquisition protocol was used (Gracco et al., 2005): For each trial, speech cues were presented on an MR‐compatible projector screen for 3 s, during which time participants were required to produce a unique word as quickly and as clearly as possible or to say “I don't know” in case they could not find a response within the 3‐s window. A 0–500 ms random time‐jitter preceded cue presentation. The projector screen then turned black for an additional ∼1.5‐s lag (adjusted for the preceding time‐jitter) prior to a 2‐s MR acquisition period (i.e., gradients turned on). One functional volume was acquired per cue for each task for a total of 30 volumes per task per participant. Participants' overt responses were recorded through an MR‐compatible microphone (Optoacoustics FOMRI‐III, Or Yehuda, Israel) mounted on the scanner head coil. In total, an experimental session lasted about 30 min. Individual datasets were inspected for motion artifacts using the MCFLIRT tool implemented in the FSL package (FMRIB, Jenkinson, Bannister, Brady, & Smith, 2002). Inspection revealed a mean absolute displacement (displacement from each image relative to the reference image) of 0.4 mm (SD = 0.22) and a mean relative displacement (difference between an image at time point N and the image at time point N + 1) of 0.09 mm (SD = 0.04) indicating very little movement during scanning (below voxel size).
2.5. Behavioral data analysis
Participants' behavioral data were analyzed using Matlab (MathWorks, MA). Speech onset times (SOTs) for each task (WR, CN, and VG) were measured in milliseconds time‐locked to cue onset. Trials with missed responses (0% in WR; 3.8% in CN, and 7.8% in VG) were excluded from the analyses. Responses were excluded when participants were unsuccessful in identifying the object displayed (“I don't know” or misidentification of the object). Mean SOT in WR, CN and VG were entered into a one‐way repeated‐measures analysis of variance (ANOVAs) including TASK as within‐subjects factor. For CN and VG, the relationship between selection competition and SOT was tested in two separate random‐effects regression analyses taking participant variability into account (i.e., no averaging was done across participants, cf. Alario et al., 2004 for justification of this approach) and using as predictors of interest normed cue‐related H and AG values.
2.6. fMRI data analysis
2.6.1. Independent component analysis
Spatial ICA of the fMRI data (sICA) was performed using the GIFT package implemented in the MatLab fMRI toolbox (Calhoun, Adali, Pearlson, & Pekar, 2001). In line with previous fMRI studies on language production (Geranmayeh et al., 2012, 2014; van de Ven et al., 2009), ICA was preferred over univariate, model‐based approaches for a number of notable advantages: First, sICA allows to segregate meaningful brain signal from noise associated with overt speech movement and physiological variations in respiratory oxygenation in a way that univariate, GLM‐based analyses do not (Geranmayeh et al., 2012, 2014). Second, sICA is apt to disentangle separate but locally co‐existing activation sources corresponding to functionally independent but anatomically overlapping brain networks (components) not apparent from simple subtraction analyses of speech‐related fMRI signal. Third, being an unsupervised, data‐driven analysis method makes sICA able to minimize selection biases inherent in model‐based (GLM) analyses and subsequent selective analyses using the same datasets (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009, see also supporting information for a summary of the results obtained from traditional GLM analyses of the present data).
Images were first slice time‐ and motion‐corrected, normalized to the Talairached MNI2009c template and spatially smoothed (FWHM = 8 mm). GLM analysis was applied to regress out nuisance signals including a trend signal, 12 motion parameters and mean time series of the white matter and ventricles identified from each participant's T1 image using FreeSurfer (Fischl et al. 2002). Then, spatially independent components (ICs) were computed following the standard group sICA procedure (Calhoun et al., 2001): Data from all subjects were concatenated into a single dataset and reduced using two stages of principal component analysis: one at the subject level, the other on the aggregate dataset. A spatial map and a time course of the BOLD signal change for an optimal number of ICs were acquired through the Infomax algorithm (Bell & Sejnowski, 1995). The optimal number of ICs was determined using minimum description length criteria implemented in the software (Li, Adalı, & Calhoun, 2007). The ICs identified were then inspected for evidence of residual artifacts characterized mainly by the majority of the signal being distributed around the edge of the brain or within the CSF. Only ICs exhibiting correlated signal within the brain parenchyma were retained for analysis, excluding 32 out of 75 components (42.6%). The IC maps and time courses were then back‐reconstructed for each subject from the aggregate mixing matrix. To identify clusters of activation within each IC, the spatial IC maps were averaged across subjects, and one‐sample t tests were performed for the averaged maps using a false discovery rate (FDR) corrected for the number of ICs (p FDR < .001). To establish the involvement of each IC in WR, CN and VG, GLM analysis was then applied to regress BOLD time course within each IC against the design matrix for the tasks for each subject. The beta‐weights per task per IC were then averaged across subjects and tested against activity in the REST condition using one‐sample t tests whose reliability was based on a FDR of p FDR < .005 adjusting for the number of components (N = 43) and comparisons per component (N = 6).
2.6.2. Region of interest analyses
The sICA analyses served as a basis for a more targeted region of interest (ROI) analysis aimed at explicitly probing the relationship between task‐related competition resolution and BOLD signal changes in PFC. Based on prior meta‐analyses (Bourguignon, 2014; Indefrey & Levelt, 2004), task‐related increases in selection competition were expected to recruit a processing gradient organized along the anteroposterior axis of PFC, with the VLPFC being driven by selection competition in VG only, while anterior insular and surrounding opercular tissue should be driven by selection competition in VG and CN. Other ROIs suggested by the meta‐analyses included medial PFC regions, comprising the anterior cingulate cortex (ACC) and (pre)supplementary motor area, as well as primary motor–premotor areas supporting speech articulation.
Importantly, previous research suggests that the VLPFC may be segregated into an anterior region (henceforth: aVLPFC) associated with semantic memory retrieval and a middle region (henceforth: mVLPFC) associated with postretrieval selection (Badre & Wagner, 2007; Lau et al., 2008) implying that these regions may be differentially sensitive to measures of association strength between cues and responses (retrieval) and measures of competition between responses (selection), respectively (cf. Badre & Wagner, 2007 for detail). We probed here the extent to which aVLPFC and mVLPFC may be differentially sensitive to measures of interspeaker agreement (AG) versus entropy (H), especially given AG's conceptual closeness to measures of memory retrieval (cf. Moss & Older, 1996; Nelson et al., 1998). The VLPFC region identified in the ICA analyses was therefore subdivided further into two 10‐mm radius spherical ROIs centered on the stereotaxic space coordinates reported by Badre and Wagner (2007) for aVLPFC (xyz: −48, 30, −6) and mVLPFC (xyz: −50, 25, 14).
The relationship between percent BOLD signal change and cue‐related selection competition was investigated by extracting individual participants' time‐series in CN and VG relative to REST and entering them as dependent variables into separate random‐effects linear regression analyses using normed H and AG as independent variables. As in the behavioral results, meaningful relations between regional BOLD signal change and selection competition were expected to be expressed as positive correlations with H and as negative correlations with AG. Importantly, VG is considered a more demanding task than CN given its underlying requirements for conceptual expansion and underlying lexico‐semantic search (Kurland et al., 2014). The correlations existing between BOLD signal variance and variations in selection competition may in part reflect this difference in task difficulty. To rule out this possibility, all regression analyses factored out participants' cue‐related SOT as indices of mental effort. Given the strongly hypothesis‐driven nature of these analyses, no correction for multiple tests or ROIs was applied.
3. RESULTS
3.1. Behavioral data
Participants' mean SOTs in WR (790 ms, SE = 40 ms), CN (1 079 ms, SE = 50 ms) and VG (1 384 ms, SE = 57 ms) increased in the predicted order [F 1.553,23.289 = 168.972, p < .001 after Greenhouse‐Geisser correction for violations of sphericity], with longer SOT for VG compared with both CN and WR, and longer SOT in CN relative to WR [all ps < .001, cf. Figure 2a]. To test the predictive effect of selection competition on the behavioral correlates of CN and VG, individual participants' SOT were entered as dependent variables into separate random‐effects linear regressions using normed H and AG as predictors of interest. Both measures reliably predicted SOT variance in CN and VG (all ps < .001, cf. Figure 2b and Table 1]. For both CN and VG, SOT were positively related to H and negatively related to AG, ruling out the possibility of spurious relations in the data.
Figure 2.
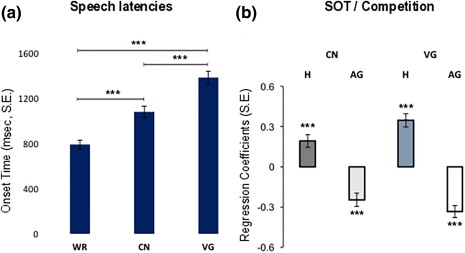
(a) Participants' mean SOTs (with standard errors) for WR, CN, and VG. (b) Standardized regression coefficients (RCs) (with standard errors, S.E.) obtained from random‐effects linear regression analyses performed between participants' SOT and entropy computed from normed entropy (H) and name and verb AG extracted from the materials of Snodgrass and Vanderwart (1980) and Kurland et al. (2014) for CN and VG, respectively. ***p < .001 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Standardized regression coefficients (RC) and associated t values obtained from random‐effects linear regression analyses performed on participants' SOT
| CN | VG | |||
|---|---|---|---|---|
| Statistics | H | AG | H | AG |
| RC | 0.2 | −0.23 | 0.35 | −0.33 |
| t | 4.23*** | −5.13*** | 7.78*** | −7.37*** |
3.2. fMRI
3.2.1. Independent component analyses
Out of the 43 ICs containing meaningful brain signal within the brain parenchyma, three of them comprised clusters of activity located in PFC regions hypothesized to support the motor and cognitive control aspects of language production (Bourguignon, 2014). The first IC (IC 1, cf. Figure 3a left and Table 2, see also Supporting information Table S4 for activation loci outside of PFC) has its two primary clusters centered on the left and right primary motor cortices (M1, BA4/6). GLM analyses performed on the time course of BOLD activity in WR, CN, and VG relative to REST showed that this component was reliably involved in WR [t 15 = 20.26, p FDR < .001], CN [t 15 = 16.9, p FDR < .001] and VG [t 15 = 15.54, p FDR = .001, cf. Figure 3b left]. Its involvement was also greater in WR compared with VG [t 15 = 4.07, p FDR = .004]. The second IC (IC 2, cf. Figure 3a middle and Table 2) had five PFC clusters centered on the ACC (BA24/32), the left and right Ant. Ins. (BA13) and the left and right superior frontal gyri (BA9). GLM analyses revealed that this component was reliably involved in CN [t 15 = 6.38, p FDR < .001] and VG [t 15 = 8.31, p FDR < .001] but not in WR [t 15 = 1.6, p FDR nonsignificant, cf. Figure 3b middle]. Furthermore, BOLD signal in this component was reliably higher in VG compared with CN [t 15 = 6.9, p FDR < .001] and reliably higher in CN compared with WR [t 15 = 4.22, p FDR = .0025]. Finally, a third component (IC 3, cf. Figure 3a right and Table 2) had its primary PFC cluster peaking on the left VLPFC (BA45/46/47). Its involvement was reliable in neither WR nor CN [t 15 ≤ 3.17, p FDR nonsignificant] but reliable in VG [t 15 = 8.03, p FDR < .001, cf. Figure 3b right]. Furthermore, its involvement in VG was reliably greater compared with both CN [t 15 = 9.12, p FDR < .001] and VG [t 15 = 9.11, p FDR < .001].
Figure 3.
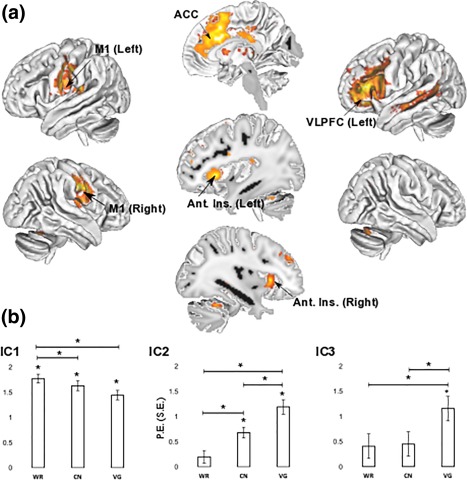
(a) ICs of interest obtained from group sICA and (b) results from the subsequent GLM analyses (parameter estimates with standard errors across participants) reflecting task‐related levels of activation of each IC relative to REST: IC 1 (Left) comprised the left and right motor‐premotor cortices (M1) active in WR, CN, and VG; IC 2 (center) comprised the left and right Anterior Insula (Ant. Ins.) and the anterior cingulate cortex (ACC); IC 3 (right) comprised a left‐lateralized cluster in the anterior parts of the left VLPFC. Results were obtained with a FDR of p FDR < .005 accounting for the number of components (43) and comparisons (6). Refer to Table 1 for details on activation clusters in PFC and Supporting information Table S4 for activation clusters outside of PFC [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Anatomical details of PFC regions revealed by IC 1 (A), IC 2 (B) and IC 3 (C). Refer to Figure 3 for visualization of each component
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Cluster A | Brodmann Area | Cluster size (voxels) | T max | x | y | z |
| •M1 (Left) | 4/6 | 1 272 | 15.8 | −50 | −10 | 30 |
| •M1 (Right) | 4/6 | 1 046 | 12.93 | 60 | −6 | 30 |
| B | ||||||
| •ACC | 24/32 | 3 047 | 16.14 | −2 | 20 | 26 |
| •Ant. Ins. (Left) | 13 | 557 | 14.67 | −42 | 16 | 0 |
| •Ant. Ins. (Right) | 13 | 542 | 11.57 | 34 | 18 | 4 |
| §SFG (Left) | 8/9 | 228 | 10.9 | −26 | 36 | 26 |
| §SFG (Right) | 8/9 | 187 | 9.32 | 30 | 44 | 32 |
| C | ||||||
| •VLPFC (Left) | 45/46/47 | 2 982 | 17.45 | −40 | 36 | −2 |
| •ACC | 32 | 375 | 13.12 | −2 | 22 | 40 |
| §SFG (Left) | 8/9 | 68 | 7.7 | −14 | 54 | 32 |
Regions marked • correspond to regions predicted in Bourguignon (2014) to form the core control gradient of speech control in PFC. Regions marked with § were subject to complementary ROI analyses described in the Supporting Information. M1, precentral gyrus; ACC, anterior cingulate cortex; Ant. Ins., anterior insula; SFG, superior frontal gyrus; VLPFC, ventrolateral prefrontal cortex. Refer also to Supporting information Table S4 for activation clusters outside PFC.
3.2.2. ROI analyses
Finally, we explicitly tested the hypothesis of a task‐related gradient of competition resolution by entering individual participants' cue‐related percent signal change in CN and VG relative to REST into random‐effects linear regression analyses using normed cue‐related entropy (H) and name or verb inter‐speaker agreement (AG) as predictors of interest. Importantly, all regressions factored out participants' SOT as indices of task‐related differences in mental effort (see Sections 1 and 2.6.2). As shown in Figure 4 and Table 3, the anterior (aVLPFC) and middle sectors of VLPFC (mVLPFC) were reliably sensitive to variations in H and AG for VG only (all ps < .03). For CN, aVLPFC exhibited marginal sensitivity to H and AG (all ps > .054), while mVLPFC did not (all ps > .6) The left Ant. Ins. exhibited sensitivity to H and AG in CN (ps < .02) and VG (ps < .01) in the left hemisphere, while its rightward homolog exhibited reliable sensitivity to H and AG only in CN (ps < .05). The ACC was reliably sensitive to H and AG in VG only (ps < .05), and neither the left or right primary motor cortices (M1) exhibited meaningful relationships with any competition measure in CN or VG (all ps nonsignificant). Note that BOLD signal covaried positively with H and negatively with AG, thus ruling out spurious relationships in the data (see also “Extended ROI analyses” presented in the Supporting Information).
Figure 4.
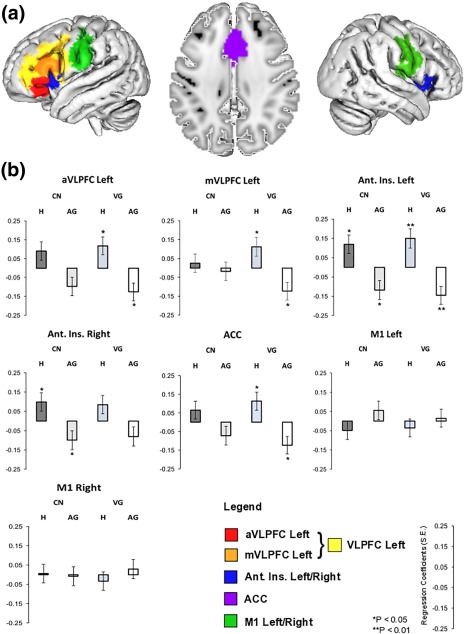
ROI‐based regression analyses of the relation between cue‐related selection competition and percent BOLD signal change in CN and VG relative to REST. (a) ROIs selected from the independent component analyses featured in Figure 3: M1, primary motor–premotor cortices (green); ACC, anterior cingulate cortex (violet); Ant. Ins., anterior insula (blue); VLPFC, ventrolateral PFC (yellow). VLPFC was subdivided further into anterior (aVLPFC, red) and middle sectors (mVLPFC, orange) through 10‐mm spheres centered on the stereotaxic coordinates given by Badre and Wagner (2007, cf. Section 2 for detail). (b) Standardized regression coefficients (RC, with standard errors, S.E.) were obtained from random‐effects linear regression analyses taking participant variability into account and factoring out SOT as indices of task difficulty. Regression analyses were performed using normed entropy (H) and name or verb agreement (AG) taken from Snodgrass and Vanderwart (1980) and Kurland et al. (2014) for CN and VG, respectively [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Regression coefficients (RC) and associated t values obtained from random‐effects linear regression analyses performed on target ROIs (cf. Figure 4) and factoring out SOTs as indices of task difficulty during CN and VG
| CN | VG | ||||
|---|---|---|---|---|---|
| ROI | Statistics | H | AG | H | AG |
| aVLPFC Left | RC | 0.091 | −0.097 | 0.118 | –0.126 |
| t | 1.86 | −1.97 | 2.36* | –2.52* | |
| mVLPFC Left | RC | 0.025 | −0.018 | 0.113 | –0.123 |
| t | 0.514 | −0.369 | 2.23* | –2.46* | |
| Ant. Ins. Left | RC | 0.119 | –0.118 | 0.148 | –0.146 |
| t | 2.43* | –2.35* | 2.946** | –2.93** | |
| Ant. Ins. Right | RC | 0.099 | –0.099 | 0.085 | −0.08 |
| t | 2.01* | –1.97* | 1.68 | −1.6 | |
| ACC | RC | 0.065 | −0.072 | 0.113 | –0.123 |
| t | 1.33 | −1.439 | 2.25* | –2.49* | |
| M1 Left | RC | −0.048 | 0.056 | −0.035 | 0.015 |
| t | −0.98 | 1.13 | −0.70 | 0.31 | |
| M1 Right | RC | 0.006 | −0.008 | −0.033 | 0.029 |
| t | 0.125 | −0.168 | −0.65 | 0.573 | |
Regression analyses were performed on normed entropy (H) and normed name or verb agreement (AG) measures obtained from Snodgrass and Vanderwart (1980) for CN and Kurland et al. (2014) for VG. aVLPFC, anterior VLPFC, mVLPFC, middle VLPFC; Ant. Ins., anterior insula; ACC, anterior cingulate cortex; M1, primary motor–premotor cortex. Significant values are listed in bold. *p < 0.05, **p < .01
4. DISCUSSION
The PFC is instrumental in many facets of human behavior, including cognitive control and language processing (Fuster, 2015; Goldberg, 2009). Amongst previous attempts to explain the neurocognitive bases of cognitive control in the particular case of language production, several authors have proposed that PFC, in particular Broca's area (BA9/44/45), plays a pivotal role in resolving competition between verbal responses during tasks of CN and VG (cf. Novick et al., 2010; Thompson‐Schill et al., 1997). This hypothesis, however, does not account for differences in PFC involvement between the two tasks and remains underspecified regarding PFC activity outside of Broca's region during VG compared with CN. This work examined whether these discrepancies may be explained by differences between these tasks' level of competition tied to their underlying lexico‐semantic operations, assuming that the requirement for conceptual expansion in VG should recruit anterior PFC sectors previously associated with controlled retrieval and selection of information from lexico‐semantic memory (Badre & Wagner, 2007; Lau et al., 2008). We explored this question using neural–computational measures of entropy previously shown to predict brain activity in competition resolution during general adaptive behavior (Koechlin et al., 2003; Yoshida & Ishii, 2006) and observed a gradient of PFC areas spanning from primary motor–premotor cortices to VLPFCs through Ant. Ins. following task‐related increases in competition resolution. We now discuss these findings in more detail and consider their implications for future research on the cognitive control of language production and its relation to general adaptive behavior.
4.1. Verb generation, ventrolateral prefrontal cortex and anterior cingulate cortex
Consistent with predictions, selection competition during VG accounted for BOLD signal variance in VLPFC. Previous research suggests a pivotal role of this region in conceptual expansion (Abraham et al., 2012), internally specified verbal responses (Tremblay & Gracco, 2006) and lexico‐semantic retrieval and selection (Badre & Wagner, 2007; Lau et al., 2008). Interestingly, previous research suggests a further functional–anatomic segregation of VLPFC into anterior regions (aVLPFC, ∼BA46/47) for semantic retrieval and middle regions (mVLPFC, ∼BA45) for postretrieval selection, respectively (Badre & Wagner, 2007; Lau et al., 2008), but recent attempts to establish this segregation in the domain of language production have been inconclusive (Snyder, Banich, & Munakata, 2011). No differences could either be noted in the present study between aVLPFC and mVLPFC's sensitivity to either H or AG, despite AG's conceptual closeness to other metrics deemed to probe memory retrieval (Moss & Older, 1996; Nelson et al., 1998). Identifying the independent effects of memory retrieval and selection has proved challenging, because of the strong correlation between measures of retrieval and selection related to the same cues (Snyder et al., 2011). Alternative interpretations suggest that retrieval and selection mechanisms are separable at the level of neural interactions within the same PFC region (Snyder et al., 2010, 2011). These proposals assume that competitive speech cues activate multiple neuronal ensembles, each representing one of several candidate responses. Competition resolution would arise when the most active of these ensembles suppresses neighboring ensembles' weighting through activation of inhibitory interneurons. Semantic retrieval, by contrast, would depend on the strength of synaptic connections between neurons coding for a given response, such that weakly associated responses require longer activation for these connections to become relevant. Pharmacological evidence exists in support of a facilitatory effect of neural inhibitors on participants' performance in VG tasks without observable effects on retrieval (Snyder et al., 2010). However, the excitatory–inhibitory neural mechanisms presumed to underlie selection and retrieval at the cognitive level still elude current models of cognitive control.
Our findings also reveal involvement of the ACC in competition resolution during VG but not in CN. The cingulate cortex has been associated with numerous subfunctions of cognitive control, including value encoding, decision‐making, reward, motivation and anticipation (Shenhav, Botvinick, & Cohen, 2013). Extensive lesions affecting the ACC often result in speech initiation deficits that can result in near‐complete mutism and reduction in the motivational aspects of speech (Paus, 2001), supporting hypotheses on the role of medial frontal structures in intrinsically energizing language production (MacNeilage, 1998). More relevant to the present findings, other research has reported selective ACC activation in VG tasks invoking many versus few competitors (Barch, Braver, Sabb, & Noll, 2000; Thompson‐Schill et al., 1997) and linked its activity to neural–computational processes of conceptual expansion (Abraham et al., 2012) and tip‐of‐the‐tongue phenomenon for retrieval and recall of declarative knowledge (Maril, Wagner, & Schachter, 2001). Together with VLPFC, ACC thus seems an integral component of the semantic system possibly participating in evaluating competition resolution success as well as speech initiation.
4.2. Verb generation, confrontation naming and anterior insula
Variations in selection competition engaged areas distributed in and around Ant. Ins. bilaterally in CN and only on the left for VG. The anterior insular cortex is implicated in both naming and generation tasks (Bourguignon, 2014; Etard et al., 2000; Indefrey & Levelt, 2004; van de Ven et al., 2009) and displays selective activation to namable as opposed to nonnameable objects, supporting its role in linking the objects perceived with corresponding lexico‐semantic representations (van Turennout, Ellmore, & Martin, 2000). Interestingly, previous correlations between uncertainty in stimulus categorization and neurophysiological activity in Ant. Ins. (Grinband, Hirsch, & Ferrera, 2006) suggests that competition resolution at the lexical level and perceptual categorization in cognitively demanding tasks share similar cortical substrates. Consistent with this view, left and right Ant. Ins. form part of a cingulo‐opercular network (IC2, cf. Figure 3) associated with maintenance of task sets (Dosenbach et al., 2007), sustained alertness under effortful perceptual discrimination (Sadaghiani & D'Esposito, 2015) and speech recognition in perceptually degraded circumstances (Vaden et al., 2013). That this network is recruited in competition resolution at the lexical level during language production is consistent with cognitive control of language production engaging domain‐general and ‐specific cortical networks (see below).
4.3. Language production and the neurocognitive architecture of adaptive behavior
This study was motivated in part from previous information‐theoretic models of the neural–computational correlates of PFC involvement in adaptive behavior at the nonverbal level (Koechlin & Summerfield, 2007), raising several questions regarding the functional and anatomical relationship between the adaptive aspects of language and complex action. In particular, these models posit the existence of a top‐down gradient of modular processes involved in guiding action selection based on the additive effects of external biasing signals, including stimulus identity (processed in motor–premotor cortices), contextual cues accompanying stimuli (processed in posterior PFC) and instructed relations between contextual cues and stimuli (processed in anterior PFC). These accounts of cognitive control as an externally driven process seem difficult to reconcile with the present findings, which rather highlight increasing levels of freedom in generating new conceptual relations as one progresses from externally specified to internally specified speech tasks (see also Tremblay & Gracco, 2006). In essence, spoken language is a generative process, and this generative capacity has already been associated with the functional anatomy of PFC (Fuster, 2015; Goldberg, 2009). However, differences in the distribution of PFC networks for action versus language, or action versus semantic memory retrieval and selection, suggest that the cognitive control of action and language may depend on partly distinct PFC networks. More specifically, evidence suggests a secondary subdivision of PFC along a dorso‐ventral axis, with limited understanding of its functional contribution to human behavior (Badre & D'Esposito, 2009). One hypothesis is that ventral PFC supports strategic retrieval and selection of representations from perceptual and long‐term storage systems, while dorsal PFC supports flexible planning and execution of actions (Badre & D'Esposito, 2009). Since language requires controlled access to representations in secondary association areas (Hickok & Poeppel, 2007)—roughly speaking: words, meanings and sounds—it is not surprising that speech production heavily relies on the ventral PFC network.
More generally, most recent research suggests a functional–anatomic dissociation of the cortex into a “multiple‐demands” system involved in attending to task‐relevant contextual information, and a “language” network supporting more specific aspects of language processing (Blank, Kanwisher, & Fedrenko, 2014; Duncan, 2013). In line with previous proposals (Lau et al., 2008), we propose that the latter network intervenes in controlling access to noncontextual representations stored in long‐term lexico‐semantic memory, contributing in part to the generative aspects of speech and language. The two systems must necessarily interact in naturalistic circumstances because language production (and language processing more generally) also requires attention to contextual information (Myachykov & Posner, 2005). This integrative account may in part explain the functional–anatomic overlap that exists between the two networks (Fedorenko, Duncan, & Kanwisher, 2012) and aligns with views of language as a collection of domain‐general and ‐specific networks reconfigurable based on task demands (Fedorenko & Thompson‐Schill, 2014).
5. CONCLUSION
Besides endorsing the importance of PFC in the regulation of human language, our results also modify prominent arguments that Broca's area is the main PFC region responsible for the resolution of competition during language production or that language processing necessarily relies on cognitive control systems supporting general adaptive behavior. Rather, speaking may depend on a specific, generative network involved in retrieving and selecting internally specified lexico‐semantic information and interacting with domain‐general control networks regulating attention to task‐relevant information specified in context.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
Bourguignon NJ, Ohashi H, Nguyen D, Gracco VL. The neural dynamics of competition resolution for language production in the prefrontal cortex. Hum Brain Mapp. 2018;39:1391–1402. 10.1002/hbm.23927
Funding information NIDCD‐R01 DC‐01250 (V.L.G.); les Fonds Québécois de Recherche, Nature et Technologie (FQRNT‐Québec, N.J.B.).
Contributor Information
Nicolas J. Bourguignon, Email: nicolas.bourguignon@UGent.be.
Vincent L. Gracco, Email: vincent.gracco@yale.edu.
REFERENCES
- Abraham, A. , Pieritz, K. , Thybusch, K. , Rutter, B. , Kröger, S. , Schweckendiek, J. , … Hermann, C. (2012). Creativity and the brain: Uncovering the neural signature of conceptual expansion. Neuropsychologia, 50, 1906–1917. [DOI] [PubMed] [Google Scholar]
- Alario, F. X. , Ferrand, L. , Laganaro, M. , New, B. , Frauenfelder, U. H. , & Segui, J. (2004). Predictors of picture naming speed. Behavior Research Methods, Instruments and Computers, 36(1), 140–155. [DOI] [PubMed] [Google Scholar]
- Badre, D. , Poldrack, R. A. , Paré‐Blagoev, E. J. , Insler, R. Z. , & Wagner, A. D. (2005). Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron, 47, 907–918. [DOI] [PubMed] [Google Scholar]
- Badre, D. , & Wagner, A. D. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. [DOI] [PubMed] [Google Scholar]
- Badre, D. , & D'esposito, M. (2009). Is the rostro‐caudal axis of the frontal lobes hierarchical? Nature Reviews Neuroscience, 10, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch, D. , Braver, T. S. , Sabb, F. W. , & Noll, D. C. (2000). Anterior cingulate and the monitoring of response conflict: evidence from an fMRI study of overt verb generation. Journal of Cognitive Neuroscience, 12(2), 298–309. [DOI] [PubMed] [Google Scholar]
- Barry, C. , Morrison, C. M. , & Ellis, A. W. (1997). Naming the snodgrass and vanderwart pictures: Effects of age of acquisition, frequency, and name agreement. The Quarterly Journal of Experimental Psychology, 50A(3), 560–585. [Google Scholar]
- Bell, A. J. , & Sejnowski, T. J. (1995). An information‐maximisation approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159. [DOI] [PubMed] [Google Scholar]
- Blank, I. , Kanwisher, N. , & Fedrenko, E. (2014). A functional dissociation between language and multiple‐demand systems revealed in patterns of BOLD signal fluctuations. Journal of Neurophysiology, 112, 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon, N. J. (2014). A rostro‐caudal axis for language in the frontal lobe: The role of executive control in speech production. Neuroscience and Biobehavioral Reviews, 47, 431–444. [DOI] [PubMed] [Google Scholar]
- Brysbaert, M. , & New, B. (2009). Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behavior Research Methods, 41(4), 977–990. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello, A. L. , & Warrington, E. K. (1989). Dynamic aphasia. The selective impairment of verbal planning. Cortex, 25, 103–114. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. F. , Fair, D. A. , Miezin, F. M. , Cohen, A. L. , Wenger, K. K. , Dosenbach, R. A. T. , … Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, J. (2013). The structure of cognition: Attentional episodes in mind and brain. Neuron, 80, 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, E. , Nagarajan, S. S. , Dalal, S. S. , Canolty, R. T. , Kirsch, H. E. , Barbaro, N. M. , & Knight, R. T. (2010). Spatiotemporal imaging of cortical activation during verb generation and picture naming. NeuroImage, 50, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmonde, T. , Giles, E. , Xuereb, J. , & Hodges, J. (1996). Progressive supranuclear palsy presenting with dynamic aphasia. Journal of Neurology, Neurosurgery, and Psychiatry, 60(4), 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard, O. , Mellet, E. , Papathanassiou, D. , Benali, K. , Houdé, O. , Mazoyer, B. , & Tzourio‐Mazoyer, N. (2000). Picture naming without Broca's and Wernicke's area. NeuroReport, 11(3), 617–622. [DOI] [PubMed] [Google Scholar]
- Fedorenko, E. , Duncan, J. , & Kanwisher, N. (2012). Language‐selective and domain‐general regions lie side by side within Broca's Area. Current Biology, 22, 2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko, E. , & Thompson‐Schill, S. (2014). Reworking the language network. Trends in Cognitive Sciences, 18(3), 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. S. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: neurotechnique automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fraisse, P. (1969). Why is naming longer than reading. Acta Psychologica, 30, 96–103. [Google Scholar]
- Fuster, J. (2015). The Prefrontal Cortex (5th ed.). London: Academic Press. [Google Scholar]
- Geranmayeh, F. , Brownsett, S. L. E. , Leech, R. , Beckmann, C. F. , Woodhead, Z. , & Wise, R. J. S. (2012). The contribution of the inferior parietal cortex to spoken language production. Brain and Language, 121, 47–57. [DOI] [PubMed] [Google Scholar]
- Geranmayeh, F. , Wise, R. J. S. , Mehta, A. , & Leech, R. (2014). Overlapping networks engaged during spoken language production and its cognitive control. The Journal of Neuroscience, 34(26), 8728–8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, E. (2009). The new executive brain: Frontal lobes in a complex world. Oxford: Oxford University Press. [Google Scholar]
- Gracco, V. L. , Tremblay, P. , & Pike, B. (2005). Imaging speech production using fMRI. NeuroImage, 26, 294–301. [DOI] [PubMed] [Google Scholar]
- Grinband, J. , Hirsch, J. , & Ferrera, V. P. (2006). A Neural representation of categorization uncertainty in the human brain. Neuron, 49, 757–763. [DOI] [PubMed] [Google Scholar]
- Herholz, K. , Reulen, H. J. , von Stockhausen, H. M. , Thiel, A. , Ilmberger, J. , Kessler, J. , … Heiss, W. D. (1997). Preoperative activation and intraoperative stimulation of language‐related areas in patients with glioma. Neurosurgery, 41(6), 1253–1262. [DOI] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Indefrey, P. , & Levelt, W. J. M. (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, J. M. , & Smith, S. M. (2002). Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Kan, I. P. , & Thompson‐Schill, S. (2004). Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognitive, Affective and Behavioral Neuroscience, 4(1), 43–57. [DOI] [PubMed] [Google Scholar]
- Koechlin, E. , Ody, C. , & Kouneiher, F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science, 302, 1181–1185. [DOI] [PubMed] [Google Scholar]
- Koechlin, E. , & Summerfield, C. (2007). An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences, 11(6), 229–235. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte, N. , Simmons, W. K. , Bellgowan, P. S. F. , & Baker, C. I. (2009). Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience, 12(5), 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman, V. , Stadthagen‐Gonzalez, H. , & Brysbaert, M. (2012). Age‐of‐acquisition ratings for 30 000 English words. Behavior Research Methods, 44, 978–990. [DOI] [PubMed] [Google Scholar]
- Kurland, J. , Reber, A. , & Stokes, P. (2014). Beyond picture naming: Norms and patient data for a verb‐generation task. American Journal of Speech‐Language Pathology, 23, S259–S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman, R. (1973). Uncertainty effects on time to access the internal lexicon. Journal of Experimental Psychology, 99(2), 199–208. [Google Scholar]
- Lau, E. F. , Phillips, C. , & Poeppel, D. (2008). A cortical network for semantics: (de)constructing the N400. Nature Reviews Neuroscience, 9, 920–933. [DOI] [PubMed] [Google Scholar]
- Li, Y. O. , Adalı, T. , & Calhoun, V. D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria, A. R. , & Tsvetkova, L. S. (1968). The mechanism of ‘dynamic aphasia’. Foundations of Language, 4(3), 296–307. [Google Scholar]
- MacNeilage, P. F. (1998). The frame/content theory of evolution of speech production. Behavioral and Brain Sciences , 21, 499–546. [DOI] [PubMed] [Google Scholar]
- Moss, H. , & Older, L. (1996). Birkbeck Word Association Norms. Hove, UK: Psychology Press. [Google Scholar]
- Maril, A. , Wagner, A. D. , & Schachter, D. L. (2001). On the tip of the tongue: An event‐related fMRI study of semantic retrieval failure and cognitive conflict. Neuron, 31, 653–660. [DOI] [PubMed] [Google Scholar]
- Myachykov, A. , & Posner, M. I. (2005). Attention in language In Itti L., Rees G. and Tsotsos J. (Eds.), Neurobiology of Attention (pp. 324–329). Waltham, MA: Academic Press, Elsevier. [Google Scholar]
- Nelson, D. L. , Schreiber, T. A. , & Xu, J. (1998). The University of South Florida word association, rhyme, and word fragment norms. http://www.usf.edu/FreeAssociation. [DOI] [PubMed]
- Novick, J. M. , Trueswell, J. C. , & Thompson‐Schill, S. L. (2010). Broca's area and language processing: Evidence for the cognitive control connection. Language and Linguistics Compass, 4(10), 906–924. [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Paus, T. (2001). Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Reviews Neuroscience, 2, 417–424. [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. , Fiez, J. A. , Videen, T. O. , MacLeod, A. M. K. , Pardo, J. V. , Fox, P. T. , & Petersen, S. E. (1994). Practice‐related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex, 4, 8–26. [DOI] [PubMed] [Google Scholar]
- Raven, J. , Raven, J. C. , & Court, J. H. (2003). Manual for raven's progressive matrices and vocabulary scales. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Robinson, G. , Blair, J. , & Cipolotti, L. (1998). Dynamic aphasia: an inability to select between competing verbal responses? Brain, 121, 77–89. [DOI] [PubMed] [Google Scholar]
- Sadaghiani, S. , & D'esposito, M. (2015). Functional characterization of the cingulo‐opercular network in the maintenance of tonic altertness. Cerebral Cortex, 25, 2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severens, E. , Van Lommel, S. , Ratinckx, E. , & Hartsuiker, R. J. (2005). Timed picture naming norms for 590 pictures in Dutch. Acta Psychologica, 119, 159–187. [DOI] [PubMed] [Google Scholar]
- Shenhav, A. , Botvinick, M. M. , & Cohen, J. D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79, 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass, J. G. , & Vanderwart, M. (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6(2), 174–215. [DOI] [PubMed] [Google Scholar]
- Snyder, H. R. , Hutchison, N. , Nyhus, E. , Curran, T. , Banich, M. T. , O'reilly, R. C. , & Munakata, Y. (2010). Neural inhibition enables selection during language processing. Proceedings of the National Academy of Sciences of the United States of America, 107(38), 16483–16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, H. R. , Banich, M. T. , & Munakata, Y. (2011). Choosing our words: Retrieval and selection processes recruit shared neural substrates in left ventrolateral prefrontal cortex. Journal of Cognitive Neuroscience, 23(11), 3470–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill, S. L. , D'Esposito, M. , Aguirre, G. K. , & Farah, M. J. (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America, 94, 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, P. , & Gracco, V. L. (2006). Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. NeuroImage, 33, 947–957. [DOI] [PubMed] [Google Scholar]
- Vaden, K. I. , Kuchinsky, S. E. , Cute, S. L. , Ahlstrom, J. B. , Dubnno, J. R. , & Eckert, M. A. (2013). The cingulo‐opercular network provides word‐recognition benefit. The Journal of Neuroscience, 33(48), 18979–18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven, V. , Esposito, F. , & Christoffels, I. K. (2009). Neural network of speech monitoring overlaps with overt speech production and comprehension networks: A sequential spatial and temporal ICA study. NeuroImage, 47, 1982–1991. [DOI] [PubMed] [Google Scholar]
- van Turennout, M. , Ellmore, T. , & Martin, A. (2000). Long lasting cortical plasticity in the object naming system. Nature Neuroscience, 3(12), 1329–1334. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1999). Wechsler abbreviated scale of intelligence (WASI). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Yoshida, W. , & Ishii, S. (2006). Resolution of uncertainty in prefrontal cortex. Neuron, 50, 781–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


