Abstract
Background
Data on associations between abdominal fat depot mass and subclinical atherosclerosis are limited, especially in women with HIV.
Methods
We assessed cross-sectional associations of dual X-ray absorptiometry scan-derived estimates of visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) with 3 measures of subclinical carotid artery atherosclerosis-- carotid artery stiffness (Young’s modulus of elasticity), presence of carotid artery lesions, and carotid artery intima-media thickness (cIMT)-- in a subsample of participants in the Women’s Interagency HIV Study. Statistical models adjusted for demographic variables, HIV serostatus, behavioral variables, and cardiovascular risk factors.
Results
There were 244 women with and 99 without HIV infection (median age 42, 62% black). VAT mass (but not SAT) was associated with greater carotid artery stiffness in a fully adjusted linear regression model, including adjustment for SAT (β = 11.3 log 103 N*M−2 per kg VAT, 95% confidence interval [1.0, 21.7]). Greater SAT mass was associated with lower odds of having a carotid artery lesion in a fully adjusted model, including adjustment for VAT (adjusted odds ratio, 0.49 per kg of SAT [0.25, 0.94]). Neither VAT nor SAT was associated with cIMT. The VAT/SAT ratio was not statistically associated with any of the outcomes after covariate adjustment.
Conclusions
In our cross-sectional study of women, the majority of whom had HIV, greater VAT mass was associated with increased carotid artery stiffness whereas greater SAT mass was associated with a reduced odds of prevalent carotid artery lesions.
Keywords: subclinical atherosclerosis, visceral adipose tissue, subcutaneous adipose tissue, HIV-1, women
Introduction
Excess visceral adiposity has been associated with cardiometabolic abnormalities in the general population, often independently of the quantity of total or subcutaneous fat1–3. Increased visceral adipose tissue (VAT) is associated with greater risk of diabetes mellitus, coronary heart disease, and death4–6. In contrast, greater abdominal subcutaneous adipose tissue (SAT) may be cardioprotective, especially in those with excess VAT 7. Data from the Framingham Heart Study suggest that the relative proportions of VAT and SAT, as assessed by VAT/SAT ratio, correlate more strongly with cardiometabolic risk than does VAT alone8.
In people living with HIV, greater VAT is associated with higher Framingham risk scores9 and increased all-cause mortality10. Few studies of subclinical atherosclerosis, however, have used radiographic techniques to accurately measure abdominal fat depots11, and there is specifically a dearth of data among women with HIV, who may be at disproportionally higher risk of cardiovascular disease relative to HIV seronegative controls 12.
Quantification of VAT and SAT has traditionally been by single slice computed tomography (CT) or magnetic resonance imaging (MRI), but these modalities are limited by radiation exposure and high cost, respectively. Using standard methodologies, dual X-ray absorptiometry (DXA) scanning can quantify central versus limb fat but cannot distinguish VAT from SAT. Recent advances in DXA software, however, have enabled this methodology to estimate VAT and SAT depot size13. The lower cost and radiation exposure from DXA scanning consequently make it an attractive modality to quantify VAT and SAT. We used DXA-derived estimates of abdominal fat depots to test the hypothesis that VAT is independently associated with greater subclinical atherosclerosis assessed by carotid artery ultrasound in women with and without HIV in the Women’s Interagency HIV Study (WIHS). We also examined associations of both SAT and VAT/SAT ratio with subclinical atherosclerosis.
Methods
The WIHS is a prospective cohort study of primarily minority women with HIV and demographically similar women initially enrolled at six urban sites in the United States14,15. Participants were recruited initially in 1994–95 (n=2,623) and subsequently in 2001–02 (n=1,143). Detailed demographic, clinical, and behavioral information is collected at each semi-annual study visit. All participants were invited to participate in a Cardiovascular Substudy in which carotid artery ultrasound was performed between 2004 and 200516. Women from the Bronx, Chicago, and San Francisco sites were also invited to participate in a Metabolic Substudy if they met specific entry criteria, including weight < 119.7 kg, height < 1.85 m, and no recent use of corticosteroids, exogenous hormones, or drugs for osteoporosis17. Participants in the Metabolic Substudy had DXA scans done between 2003 and 200517. The 244 women with HIV and 99 HIV seronegative women with available data from both substudies constituted the study sample for the present analyses.
Informed consent was obtained from all participants and human experimentation guidelines of the U.S. Department of Health and Human Services and those of the authors’ institutions were followed in the conduct of this research.
Outcome of Interest: Subclinical Atherosclerosis
High resolution B-mode carotid artery ultrasound imaged six locations in the right carotid artery: the near and far walls of the common carotid artery (CCA), carotid bifurcation, and internal carotid artery as previously described18. All study sites followed a standardized protocol16 and the ultrasounds were read at a centralized reading center (University of Southern California). Carotid artery intima-media thickness (cIMT) was measured in the right distal common carotid artery by automated computerized edge detection software as previously described19. We defined a lesion (plaque) as an area with localized intima-media thickness (IMT) >1.5 mm in at least one of the six carotid artery locations20.
Brachial artery blood pressure was measured contemporaneously with the carotid ultrasound, and the average of five blood pressure measurements was used to calculate pulse pressure (PP). To assess arterial stiffness, Young’s elastic modulus (in units of 105*Newtons*meters−2) was calculated as previously described19 using the formula: PP/DD × 0.5 × DD/cIMTD, where PP = pulse pressure, DD = percent arterial dilation over the cardiac cycle, DD = arterial diameter at diastole, and cIMTD = common carotid artery intima-media thickness at diastole. A higher value of Young’s modulus corresponds to a stiffer blood vessel.
Exposures of Interest: DXA-Derived Adipose Tissue Depots
DXA scans for assessments of regional fat mass were performed using GE/Lunar Prodigy machines (Madison, WI, USA)17. The validated CoreScan™ algorithm estimated VAT and SAT size based on detection of the width of the SAT layer on the lateral abdomen and the anterior-posterior thickness of the abdomen obtained by DXA13;21. These parameters were used to estimate android SAT, which was subtracted from total android fat to yield the estimate of VAT. The DXA scans were read at a central location (Image Reading Center, Inc., New York, NY).
Covariates
Self-reported race and ethnicity were categorized as: White (including Hispanic and non-Hispanic), Black (including Hispanic and non-Hispanic) and Other (predominantly women who self-identified as Hispanic but not White or Black). Diabetes mellitus was defined based on: 1) self-reported use of diabetes medication, or 2) self-report of diabetes, fasting glucose > 126 mg/dL or hemoglobin A1c ≥ 6.5% and confirmation by subsequent report of diabetes medication or laboratory parameter22. Hypertension diagnosis was based on self-report, systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current receipt of antihypertensive medication. Menopausal status was defined as self-reported amenorrhea at two consecutive visits (12 months or more) for women aged ≥ 45 years old. Alcohol intake was categorized based on the average number of drinks reported per week during the preceding 6 months: Light use (1–3 drinks/week), moderate use (4–7 drinks/week) and heavy use (>7 drinks/week). Combination antiretroviral therapy (cART) was defined as any highly active antiretroviral therapy regimen in contemporaneous Department of Health and Human Services antiretroviral guidelines.23
Total body fat was calculated based on height, weight, resistance, and reactance, the latter two of which were measured by bioelectrical impedance analysis (RJL Systems, Inc, Detroit, MI, USA)24.
Plasma HIV-1 RNA level (viral load) was assayed by isothermal nucleic acid sequence based amplification method (NASBA/Nuclisens, bioMerieaux, San Diego CA) with a detection limit of 80 copies/ml, and CD4 T-cell counts were measured by standard flow cytometry methods. Hepatitis C virus (HCV) infection was assessed by testing for antibody to HCV by second or third-generation EIA (Ortho-Diagnostic Systems, Rochester, NY) with confirmation of reactive tests by HCV branched DNA (Quantiplex 2.0 branched chain DNA-enhanced label amplification assay; Chiron, Emeryville, CA) or by RT-PCR (COBAS Amplicor HCV Detection Kit; Roche Diagnostic Systems, Pleasanton, CA). A central laboratory measured total and HDL-cholesterol levels as well as high sensitivity C-reactive protein (hsCRP) levels, the latter by nephelometric immunoassay. Estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using serum creatinine25.
Statistical Analysis
Data were analyzed descriptively using plots, histograms, means, medians, standard deviations, skewness and kurtosis to identify erroneous values, outliers and optimal transformations. Values of hsCRP > 10 μg/ml were excluded as likely being due to acute illness rather than a marker of chronic inflammation, and the mean of two hsCRP values was taken when available. Due to right skewness, Young’s modulus of elasticity was natural logarithm transformed. Medians and first and third quartiles (Q1, Q3) summarize continuous variables. Differences between women with and without HIV infection were assessed by the Wilcoxon rank-sum test or chi-squared test as appropriate.
Spearman’s correlation evaluated associations between VAT and SAT and cardiometabolic risk factors. Linear regression evaluated associations of VAT, SAT, and the VAT/SAT ratio with both cIMT and Young’s modulus. Linear regression results are expressed as the beta-coefficient, which represents the absolute change in outcome per unit change in covariate, with 95% confidence intervals. Logistic regression evaluated associations with presence of carotid artery lesions, dichotomized as 0 or ≥ 1 lesion. Logistic regression results are expressed as odds ratios with 95% confidence intervals.
We assessed VAT and SAT in separate models, in models that included both VAT and SAT, and as the VAT/SAT ratio. We present unadjusted models (model 1) and models serially adjusted for demographic characteristics first (model 2) and then also behavioral characteristics, HIV and HCV infection status, and cardiometabolic risk factors (model 3). Our primary analysis combined women with and without HIV infection and included HIV serostatus as a covariate. We performed secondary analyses for the cIMT and Young’s modulus of elasticity outcomes stratified by HIV serostatus; however, the small number of women with carotid lesions precluded stratified analyses for this outcome. Data from stratified analyses are shown for models in which the 95% confidence intervals for the beta coefficients for the VAT and SAT exposure variables do not overlap comparing HIV seropositive with HIV seronegative women, indicative of an interaction.
Statistical analysis was performed using SAS version 9.4 software (SAS Institute Inc. Cary, NC, USA). Statistical significance was considered to be P ≤ 0.05.
Results
Baseline Characteristics
Our study sample consisted of 244 women with HIV and 99 seronegative women. The carotid artery ultrasound was performed a median of 134 days after the DXA scan in women with HIV (Interquartile range [IQR]: 269 days before, 224 days after) and 187 days after the scan for HIV-seronegative women (IQR 342 days before, 232 days after). Table 1 summarizes baseline characteristics of the study sample. Compared to the HIV seronegative group, those with HIV were older, more likely to report being post-menopausal and to report injecting drugs in the past and had a higher prevalence of HCV infection. They were also more likely to report using anti-hypertensive therapy and had both lower total and HDL cholesterol values. Among the women with HIV, 63% were taking combination antiretroviral therapy (cART); median HIV-1 RNA level was 2.76 [1.90, 3.77] log10 copies/ml and 93 (38.1%) had HIV-1 RNA levels < 80 copies/ml. Their median current and nadir CD4 counts were 386 [255, 601] and 242 [132, 350] cells/mm3, respectively. Women with HIV had lower median body mass index and SAT mass but similar VAT mass compared to the HIV seronegative women. Consequently, the VAT/SAT ratio was higher in the HIV-infected group.
Table 1.
Characteristics of Study Participants at the Time of DXA Visit
| HIV-infected (n = 244) | HIV-uninfected (n = 99) | P-value | |
|---|---|---|---|
|
| |||
| Age, years | 43.5 (38.8, 48.7) | 38.0 (31.3, 44.8) | <0.0001 |
|
| |||
| Race/ethnicity | |||
| White | 42 (17.2%) | 22 (22.2%) | 0.37 |
| Black | 152 (62.3%) | 62 (62.6%) | |
| Other | 50 (20.5%) | 15 (15.2%) | |
|
| |||
| Education < high school | 100 (41.0%) | 34 (34.3%) | 0.25 |
|
| |||
| Smoking | |||
| Current | 146 (59.8%) | 65 (65.7%) | 0.21 |
| Former | 52 (21.3%) | 13 (13.1%) | |
| Never | 46 (18.8%) | 21 (21.2%) | |
|
| |||
| Injection drug use (ever) | 96 (39.3%) | 21 (21.1%) | 0.001 |
|
| |||
| Alcohol use | |||
| None | 124 (50.8%) | 37 (37.4%) | 0.15 |
| Low | 96 (39.3%) | 48 (48.5%) | |
| Moderate | 11 (4.5%) | 6 (6.1%) | |
| High | 13 (5.3%) | 8 (8.1%) | |
|
| |||
| Post-menopausal | 69 (28.3%) | 3 (3.0%) | <0.0001 |
|
| |||
| Diabetes | 35 (14.3%) | 7 (7.1%) | 0.063 |
|
| |||
| Hypertension | 69 (28.3%) | 20 (20.2%) | 0.12 |
|
| |||
| Use of anti-hypertensive medication | 54 (22.1%) | 12 (12.1%) | 0.033 |
|
| |||
| HCV-infected | 88 (36.1%) | 17 (17.2%) | 0.0006 |
|
| |||
| On cART | 150 (61.5%) | NA | |
|
| |||
| On NRTI | 157 (64.%) | NA | |
|
| |||
| On NNRTI | 64 (26.2%) | NA | |
|
| |||
| On protease inhibitor | 86 (35.2%) | NA | |
|
| |||
| Current CD4 (cells/mm3) | 394 (258, 598) | NA | |
|
| |||
| Nadir CD4 (cells/mm3) | 248 (138, 354) | NA | |
|
| |||
| Log10 HIV RNA copies/ml | 2.78 (1.90, 3.85) | NA | |
|
| |||
| HIV RNA < 80 copies/ml | 93 (38.1%) | NA | |
|
| |||
| hsCRP (μg/ml)* | 2.03 [0.80, 4.20] | 1.95 [0.90, 4.85] | 0.77 |
|
| |||
| Body mass index (kg/m2) | 27.5 (23.4, 31.2) | 29.9 (25.2, 36.3) | 0.0005 |
|
| |||
| SAT mass (kg) | 1.32 (0.86, 1.95) | 1.90 (1.13, 3.00) | <0.0001 |
|
| |||
| VAT mass (kg) | 0.57 (0.25, 0.96) | 0.67 (0.22, 1.08) | 0.49 |
|
| |||
| VAT/SAT ratio | 0.37 (0.24, 0.62) | 0.29 (0.20, 0.47) | 0.004 |
|
| |||
| Whole body total fat (kg) | 24.6 (17.4, 32.8) | 32.5 (21.5, 43.6) | <0.0001 |
|
| |||
| Total cholesterol (mg/dL) | 166 (120, 192) [n=240] |
171 (155, 211) [n=98] |
0.011 |
|
| |||
| HDL-cholesterol (mg/dL) | 42 (34, 55) [n=240] |
52 (45, 61) [n=98] |
<0.0001 |
|
| |||
| eGFR ml/min per 1.73 m2 | 92.3 (80.2, 108.2) | 98.8 (83.7, 111.6) | 0.18 |
|
| |||
| Common carotid artery IMT (mm) | 0.73 (0.67, 0.80) [n=244] |
0.71 (0.65, 0.79) [n=99] |
0.21 |
|
| |||
| ≥ 1 carotid artery lesion | 29 (11.9%) | 11 (11.5%) | 0.91 |
|
| |||
| Young’s modulus of elasticity (105* N* m−2) | 5.35 (3.99, 7.12) [n=243] |
4.50 (3.56, 6.05) [n=98] |
0.0028 |
Data are expressed as median (Q1, Q3), or n (%). P-values are by Wilcoxon rank-sum test or Chi-squared test as appropriate.
Data on hsCRP available on n = 226 HIV-infected and 92 HIV-uninfected women.
Abbreviations: cART, combination antiretroviral therapy; DXA, dual X-ray absorptiometry; IMT, carotid intima-media thickness; eGFR, estimated glomerular filtration rate; hsCRP, high sensitivity C-reactive protein; N, Newton; NA, not applicable; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
On carotid ultrasound, cIMT was similar by HIV status, as was the prevalence of carotid artery lesions, which was 11% in both groups. Young’s modulus of elasticity was higher in women with HIV, indicative of less elasticity (5.35 [3.99, 7.12] versus 4.50 [3.56, 6.05] × 105*N*m−2, P = 0.028).
Correlations Among Body Composition Measurements, Carotid Artery Parameters, and Cardiometabolic Risk Factors
Table 2 summarizes Spearman correlations among VAT, SAT, cardiometabolic risk factors, and carotid artery parameters by HIV serostatus. Age was positively correlated with VAT but not SAT in both groups of women. Both VAT and SAT were positively correlated with systolic blood pressure in both groups. Whereas neither VAT nor SAT was statistically correlated with total cholesterol in either group, HDL-cholesterol was inversely correlated with VAT in both women with HIV (r = −0.26, p < 0.001) and HIV-seronegative women (r = −0.49, p < 0.001). HDL-cholesterol, however, was inversely statistically correlated with SAT in the HIV-seronegative group only (r = −0.32, p < 0.001 versus r = −0.09, p = 0.08 in those with HIV). VAT was correlated with SAT in both groups of women (r = 0.57, p < 0.001 in those with HIV and r = 0.77, p < 0.001 in HIV-seronegative, respectively). The correlation between VAT and total body fat, the latter assessed by bioelectrical impedance analysis, tended to be stronger in the HIV seronegative group, whereas the correlation between SAT and total body fat was similar in each group.
Table 2.
Correlations Among Visceral Adipose Tissue Mass, Subcutaneous Adipose Tissue Mass, Cardiometabolic Risk Factors and Carotid Artery Ultrasound Parameters
| Visceral Adipose Tissue (VAT) mass | Subcutaneous Adipose Tissue (SAT) mass | |||
|---|---|---|---|---|
| HIV-infected (N=244) | HIV-uninfected (N=99) | HIV-infected (N=244) | HIV-uninfected (N=99) | |
| Age (years) | 0.18** [0.05, 0.30] | 0.27** [0.08, 0.44] | −0.055 [−0.18, 0.07] | −0.024 [−0.22, 0.17] |
| Systolic blood pressure (mm Hg) | 0.12 [−0.007, 0.24] | 0.32** [0.13, 0.49] | 0.13* [0.007, 0.25] | 0.20 [−0.001, 0.38] |
| SAT (kg) | 0.57*** [0.48, 0.65] | 0.77*** [0.68, 0.84] | -- | -- |
| VAT (kg) | -- | -- | 0.57*** [0.48, 0.65] | 0.77*** [0.68, 0.84] |
| Total body fat by BIA (kg) | 0.56*** [0.49, 0.64] | 0.79*** [0.70, 0.86] | 0.93*** [0.90, .94] | 0.96*** [0.94, 0.97] |
| Total cholesterol (mg/dL) | 0.095 [−0.03, 0.22] | 0.13 [−0.07, 0.32] | 0.049 [−0.08, 0.18] | 0.098 [−0.10, 0.29] |
| HDL-cholesterol (mg/dL) | −0.26*** [−0.37, −0.12] | −0.49*** [−0.63, −0.33] | −0.094 [−0.28, 0.03] | −0.32*** [−0.48, −0.13] |
| hsCRP (mg/L)† | 0.28*** [0.16, 0.40] | 0.69*** [0.56, 0.78] | 0.22*** [0.094, 0.34] | 0.61*** [0.47, 0.73] |
| Common carotid artery IMT (μm) | 0.076 [−0.05, 0.20] | 0.19 [−0.01, 0.37] | 0.092 [−0.03, 0.22] | −0.051 [−0.25, 0.15] |
| Young’s modulus of elasticity (105* N* m−2) | 0.22*** [0.10, 0.34] | 0.16 [−0.04, 0.35] | 0.16* [0.04, 0.28] | 0.28** [0.09, 0.45] |
Values are Spearman correlation coefficients with 95% confidence intervals. P-values:
< 0.05,
< 0.01,
< 0.001
n = 226 HIV-infected; n = 92 HIV-uninfected
Abbreviations: BIA, bioelectrical impedance analysis; IMT, carotid intima-media thickness; eGFR, estimated glomerular filtration rate; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Among women with HIV, the correlations between VAT, SAT, cardiometabolic risk factors and carotid artery ultrasound parameters were similar in those with and without detectable HIV viremia (data not shown), with the exception of the correlations between VAT and SAT as follows. The correlation between VAT and SAT was stronger among the subset with undetectable viral loads (r = 0.71, p < 0.001 versus r = 0.28, p < 0.01 for those with detectable HIV viral loads) and approximated that of the HIV-seronegative group (r = 0.77).
There was no statistically significant correlation between cIMT and VAT or SAT in either group of women. There were positive correlations between SAT and Young’s modulus of elasticity in both groups (r = 0.16, p < 0.05 in women with HIV and 0.28, p < 0.01 in women without HIV, respectively), whereas VAT was statistically correlated with Young’s modulus only in the HIV-infected group (r = 0.22, p < 0.001). hsCRP was correlated more strongly with both VAT and SAT among women without HIV (r = 0.69 and 0.61, respectively) compared with women with HIV (r = 0.28 and 0.22, respectively). While correlations with hsCRP were all highly statistically significant (P < 0.001), the 95% CIs of the correlation coefficients comparing the two groups of women did not overlap.
Adjusted Associations of Abdominal Fat Measures with Carotid Artery Stiffness
Figure 1 shows associations between body composition measures and subclinical atherosclerosis as defined by carotid arterial stiffness, carotid artery lesions, and cIMT. Separate models examined SAT alone, VAT alone, both SAT and VAT, and the VAT/SAT ratio as the main exposure variables.
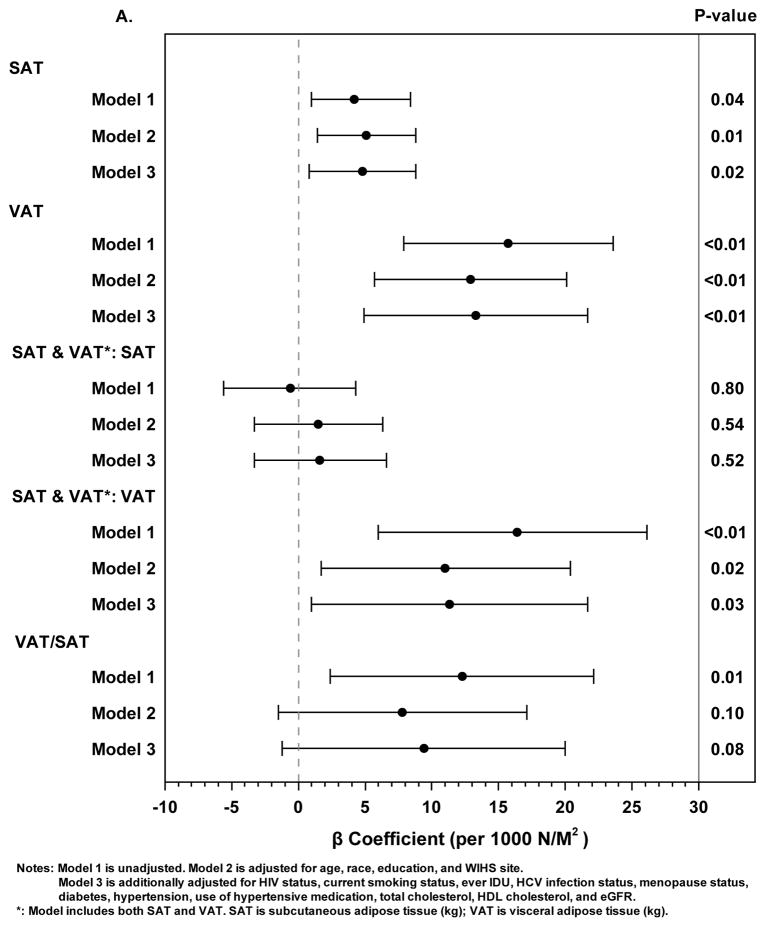
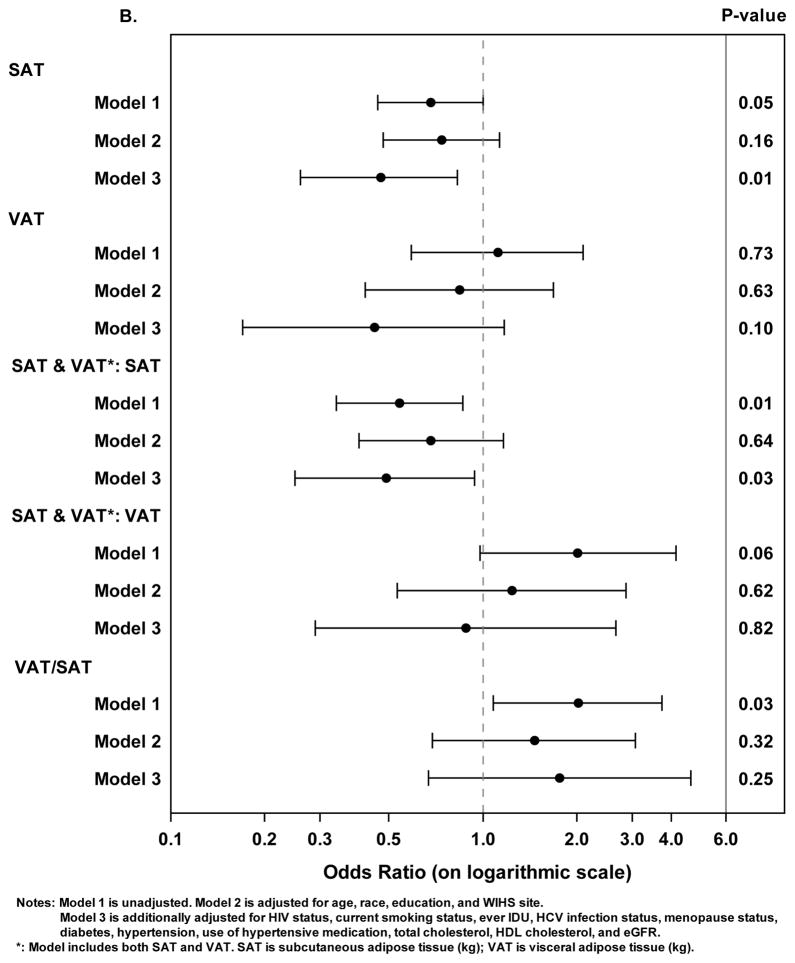
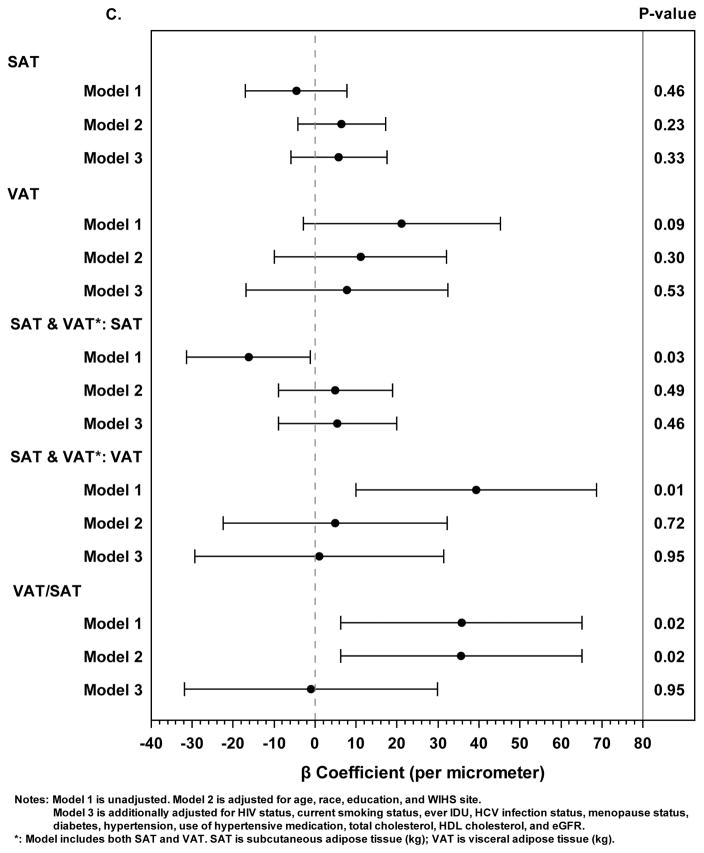
Figure 1. Associations Between Abdominal Adipose Tissue Depots and Subclinical Carotid Artery Atherosclerosis.
A. Linear regression models with the outcome of Young’s modulus of elasticity (x Log 105 Newtons*m−2). Solid circles represent beta coefficients and horizontal lines represent 95% confidence intervals.
B. Logistic regression models with the outcome of presence of carotid artery lesions. Solid circles represent odds ratios and horizontal lines represent 95% confidence intervals.
C. Linear regression models with the outcome of carotid intima-media thickness (μm). Solid circles represent beta coefficients and horizontal lines represent 95% confidence intervals.
In separate models, SAT and VAT were each positively associated with carotid arterial stiffness as assessed by Young’s modulus; these associations persisted in fully adjusted models (Figure 1A). The beta coefficient for VAT was 2–3 times higher on a per kg basis for VAT compared with SAT in these models, albeit women had approximately one-third the amount of VAT relative to SAT. When adjusted for each other in a third model, VAT, but not SAT, remained statistically associated with Young’s modulus. The VAT/SAT ratio was statistically associated with Young’s modulus but only in the unadjusted model.
In models stratified by HIV serostatus, the 95% CIs around the beta coefficients for VAT, SAT, and VAT/SAT overlapped in all models (data not shown).
Adjusted Associations of Abdominal Fat Measures with Carotid Artery Lesions
In fully adjusted analyses with the outcome defined as having at least one carotid artery lesion (versus having no lesions), greater SAT mass was statistically associated with a lower odds of having a carotid artery lesion (Figure 1B) (adjusted OR 0.47 per kg of SAT, 95% CI [0.26, 0.83]; p = 0.01). VAT alone was not associated with carotid artery lesions in any model. Furthermore SAT, when adjusted for VAT, remained statistically associated with lower odds of carotid artery lesions in the otherwise unadjusted model (OR 0.54 per kg of SAT, 95% CI [0.34, 0.86]; p = 0.01) and fully adjusted model (OR 0.49 per kg of SAT, 95% CI [0.25,0.94]; p = 0.03). In contrast, higher VAT/SAT ratio was associated with increased odds of having carotid artery lesions but in the unadjusted model only.
Adjusted Associations of Abdominal Fat Measures with cIMT
When considered as the only body composition parameter in separate regression models, neither SAT nor VAT was statistically associated with cIMT in unadjusted or adjusted models (Figure 1C). When both SAT and VAT were included in the same unadjusted model (i.e. only adjusted for each other), greater SAT remained significantly associated with lower cIMT, whereas greater VAT was no longer associated with higher cIMT. These associations were also no longer statistically significant after adjustment for demographic factors and study site (model 2). Adjustment for age alone also attenuated these associations such that they were no longer statistically significant (data not shown). Similarly, the VAT/SAT ratio was associated with cIMT but only in the unadjusted model.
In analyses stratified by HIV serostatus, in only one of the adjusted models did the 95% CIs of the effect estimates for abdominal fat exposure variables not overlap comparing HIV seropositive and seronegative women. In the model assessing cIMT that contained both SAT and VAT as exposures, the values for SAT did not overlap and were 18.4 [0.52, 36.2] and −33.2 [−62.3, −4.2] for HIV seropositive and seronegative, respectively whereas those for VAT overlapped (−13.3 [−48.9, 22.2] and 68.0 [0.55, 135.5], respectively).
Discussion
Using DXA-derived estimates of abdominal fat, we found some independent associations of VAT and SAT mass with carotid artery markers of subclinical atherosclerosis in this cross-sectional study of a demographically diverse cohort of women with and without HIV. In fully adjusted analyses, greater VAT tended to remain associated with greater arterial stiffness, whereas higher SAT tended to remain associated with less plaque. On the other hand, VAT/SAT ratio was, on the whole, uninformative despite being predictive of cardiovascular risk in other studies of the general population8;26;27 The potential protective effect of greater SAT is consistent with data from the general population, including studies of people with diabetes and survivors of acute lymphocytic leukemia 28–31. While the mechanisms by which SAT may protect against atherosclerosis are not known, accumulation of SAT may represent healthier expansion of nonpathogenic or less pathogenic adipocytes relative to adipocytes in VAT, the latter which generally secrete more proinflammatory cytokines and are less lipolytic 7.
We evaluated carotid artery elasticity, cIMT, and the presence of carotid artery atherosclerotic lesions as measures of subclinical atherosclerosis. Both cIMT and carotid arterial elasticity are well established markers of atherosclerosis in various anatomic sites and predict cardiovascular risk in the general population 32–34. The presence of carotid artery plaque is also predictive of future cardiovascular events in the general population35;36. Data are limited, however, on the value of carotid artery assessments in the prediction of cardiovascular events in HIV-infected populations. In a recent analysis of data on 209 people living with HIV from a multi-institutional registry in Boston, investigators found that the presence of plaque in the carotid artery by computed tomography was associated with a 3-fold increased risk of cardiovascular disease events and a 4-fold increased risk of stroke 37.
To our knowledge, this is the first study to evaluate DXA-derived SAT and VAT estimates in a study that included people with HIV. We found the expected positive correlations between VAT and age, as well as cardiometabolic parameters, including systolic blood pressure and HDL-C. Furthermore, we found the expected positive correlations between VAT and SAT, which were stronger among women without HIV. Of note, the correlation coefficients for the associations between VAT and SAT were similar when comparing women with HIV who had undetectable viral loads and HIV-seronegative women.
We found disparate associations of VAT and SAT with the three different parameters of carotid artery structure and function assessed by B mode ultrasound. Reduced carotid artery elasticity is thought to be an early manifestation of atherosclerosis, and the relationships of elasticity with both intima-media thickness and atheromatous plaque are complex38. This cross-sectional study was premised on the assumption that subclinical arterial disease was more likely a result, rather than a cause, of increased adiposity. Longitudinal data are needed, however, to confirm and clarify the associations found here between VAT mass and reduced elasticity and the protective association between SAT mass and the development of carotid artery lesions.
To maximize statistical power, our primary analyses pooled HIV seropositive and seronegative women. We constructed secondary analyses stratified by HIV serostatus for the cIMT and carotid artery elasticity outcomes. In the stratified models of Young’s modulus of elasticity, the 95% CIs of the effect estimates for SAT and VAT overlapped, suggesting that HIV serostatus did not modify the associations between abdominal fat and elasticity. In the pooled study population, neither SAT nor VAT was statistically associated with cIMT when both SAT and VAT were included in the same analytic model. In the analogous fully adjusted stratified models, however, SAT was positively associated with cIMT among HIV seropositive women but was negatively associated with cIMT among seronegative women. In contrast, in models that contained SAT and VAT together, while VAT was not statistically significantly associated with cIMT among seropositive women, it was positively associated with cIMT among the seronegative women. The 95% CIs for VAT in these latter models did overlap, however, indicating that the coefficients did not differ statistically. Although it is possible that associations between SAT, VAT, and cIMT truly differ by HIV serostatus due to effects of HIV itself or its therapy, our results may be spurious and either due to type 1 error from multiple comparisons or type 2 error related to smaller sample sizes in the subgroups.
Some investigators have examined associations between fat distribution and carotid ultrasound or cardiac CT-measured subclinical atherosclerosis in persons living with HIV. Most studies, however, have lacked imaging to define fat distribution and instead are limited by reliance on subjective, clinical characterization of abdominal fat accumulation and/or lipoatrophy39,40,41. In a study that used abdominal CT to quantify abdominal fat depots in 199 HIV-infected subjects, VAT but not SAT correlated positively with cIMT, but the association did not persist after adjustment for age42. Guaraldi et al performed a median of two coronary artery calcium (CAC) assessments a median of 13 months apart on 132 HIV-infected men at a cardiometabolic clinic in Italy and found that VAT volume by single slice CT imaging was positively associated with CAC progression as a dichotomous outcome43. Lastly, in a cross-sectional analysis of men from the Multicenter AIDS Cohort Study who underwent cardiac CT scanning with coronary angiography and single slice CT scanning of the abdomen, Palella et al demonstrated that among men with HIV, greater VAT was associated with the presence of non-calcified coronary artery plaque after adjustment for traditional CVD risk factors, whereas SAT was inversely associated with the extent of plaque and CAC score11. Taken together, while these results are somewhat discrepant in the setting of differences in both methodologies and study populations, there is a suggestion that greater VAT and less SAT may be associated with subclinical atherosclerosis in individuals with HIV infection. Of note, existing studies consisted mostly, if not exclusively, of men. Our data corroborate the potential presence of these signals in a population of women, most of whom have HIV.
Our study has several strengths, including the focus on a racially and ethnically diverse group of HIV positive and HIV negative women with similar behavioral characteristics and standardized carotid ultrasound and DXA assessment with central reading. Study limitations include the cross-sectional design and the modest sample size particularly of HIV-seronegative women. Furthermore, we did not validate the estimates of VAT and SAT by comparing them to those derived by a different imaging modality, such as CT or MRI. The low prevalence of viral suppression among women with HIV, likely due in part to routine deferral of cART initiation during the era of this study, may also limit generalizability to current clinical settings. While the relatively high BMIs in both the HIV seropositive and seronegative women in this study may preclude generalization to populations with different anthropometric features, HIV-infected women in the WIHS are demographically representative of women living with HIV in the United States. Therefore our study is generalizable to this sizable population. The DXA scans and carotid ultrasounds were not done at the same time; the interval between them was relatively short, however, and given the expected slow natural history of changes in body composition and carotid artery parameters, this should not have a major impact on our findings. Lastly, our primary statistical models included both women with and without HIV due to sample size constraints. Therefore, we are unable to conclude whether the influence of fat distribution on carotid artery parameters may have differed across HIV serostatus groups. We note that HIV positive individuals were more likely than HIV seronegative subjects to have an adverse fat distribution pattern, in particular lower SAT and higher ratio of VAT to SAT, although the higher ratio was driven primarily by the lower SAT and not greater VAT in HIV positive subjects.
In conclusion, DXA-derived VAT and SAT masses remained independently associated with different measures of subclinical carotid artery atherosclerosis after adjustment for demographic and clinical factors in our cross-sectional analysis of women with and without HIV. VAT was independently associated with carotid artery stiffness, considered to be an early marker of atherosclerosis, whereas SAT remained associated with reduced odds of prevalent carotid artery lesions, consistent with a putative protective effect of this fat depot. Future longitudinal studies should clarify the time course of these associations and identify pathogenetic mechanisms, such as those potentially mediated by insulin resistance and inflammation.
Acknowledgments
The authors thank Xiaotao Cai for programming assistance. Data in this manuscript were collected by three sites of the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA). A.S. was supported by K23 AR06199301. R.C.K. was supported by NIH grant R01HL126543, 5R01HL132794, 1R01HL095140, 1R01HL083760. D.B.H. was supported by K01-HL-137557. Analysis of the DXA scans was supported by a supplement from the National Institute of Aging.
References
- 1.Gast KB, den HM, Smit JW, et al. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis. 2015;241:547–554. doi: 10.1016/j.atherosclerosis.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Sironi AM, Petz R, De MD, et al. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet Med. 2012;29:622–627. doi: 10.1111/j.1464-5491.2011.03503.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee JJ, Pedley A, Hoffmann U, et al. Cross-Sectional Associations of Computed Tomography (CT)-Derived Adipose Tissue Density and Adipokines: The Framingham Heart Study. J Am Heart Assoc. 2016;5:e002545. doi: 10.1161/JAHA.115.002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 5.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 6.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55:2622–2630. doi: 10.1007/s00125-012-2639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lake JE, Wohl D, Scherzer R, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23:929–938. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherzer R, Heymsfield SB, Lee D, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25:1405–1414. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palella FJ, Jr, McKibben R, Post WS, et al. Anatomic Fat Depots and Coronary Plaque Among Human Immunodeficiency Virus-Infected and Uninfected Men in the Multicenter AIDS Cohort Study. Open Forum Infect Dis. 2016;3:ofw098. doi: 10.1093/ofid/ofw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 15.Bacon MC, von WV, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Tian F, Yin MT, Keller MJ, Cohen M, Tien PC. Association of regional body composition with bone mineral density in HIV-infected and HIV-uninfected women: women’s interagency HIV study. J Acquir Immune Defic Syndr. 2012;61:469–476. doi: 10.1097/QAI.0b013e31826cba6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y, Ergun DL, Wacker WK, Wang X, Davis CE, Kaul S. Relationship between dual-energy X-ray absorptiometry volumetric assessment and X-ray computed tomography-derived single-slice measurement of visceral fat. J Clin Densitom. 2014;17:78–83. doi: 10.1016/j.jocd.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with Incident Diabetes Mellitus: Impact of using Hemoglobin A1C as a Criterion for Diabetes. J Acquir Immune Defic Syndr. 2012;61:334–40. doi: 10.1097/QAI.0b013e31826bfc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2017. [Accessed July 17, 2017]. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 24.Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996;64:489S–497S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunimura A, Ishii H, Uetani T, et al. Impact of adipose tissue composition on cardiovascular risk assessment in patients with stable coronary artery disease. Atherosclerosis. 2016;251:206–212. doi: 10.1016/j.atherosclerosis.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Figueroa AL, Takx RA, MacNabb MH, et al. Relationship Between Measures of Adiposity, Arterial Inflammation, and Subsequent Cardiovascular Events. Circ Cardiovasc Imaging. 2016;9:e004043. doi: 10.1161/CIRCIMAGING.115.004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung CH, Kim BY, Kim KJ, et al. Contribution of subcutaneous abdominal fat on ultrasonography to carotid atherosclerosis in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2014;13:67. doi: 10.1186/1475-2840-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narumi H, Yoshida K, Hashimoto N, et al. Increased subcutaneous fat accumulation has a protective role against subclinical atherosclerosis in asymptomatic subjects undergoing general health screening. Int J Cardiol. 2009;135:150–155. doi: 10.1016/j.ijcard.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Bouchi R, Takeuchi T, Akihisa M, et al. High visceral fat with low subcutaneous fat accumulation as a determinant of atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:136. doi: 10.1186/s12933-015-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siviero-Miachon AA, Spinola-Castro AM, de Martino Lee ML, et al. Subcutaneous adipose tissue plays a beneficial effect on subclinical atherosclerosis in young survivors of acute lymphocytic leukemia. Vasc Health Risk Manag. 2015;11:479–488. doi: 10.2147/VHRM.S86883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 33.van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 34.Simons PC, Algra A, Bots ML, Grobbee DE, Van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease) Circulation. 1999;100:951–957. doi: 10.1161/01.cir.100.9.951. [DOI] [PubMed] [Google Scholar]
- 35.Cao JJ, Arnold AM, Manolio TA, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116:32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 36.Gepner AD, Young R, Delaney JA, et al. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janjua SA, Staziaki PV, Szilveszter B, et al. Presence, Characteristics, and Prognostic Associations of Carotid Plaque Among People Living With HIV. Circ Cardiovasc Imaging. 2017:10. doi: 10.1161/CIRCIMAGING.116.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol. 1997;23:157–164. doi: 10.1016/s0301-5629(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 39.Coll B, Parra S, Alonso-Villaverde C, et al. HIV-infected patients with lipodystrophy have higher rates of carotid atherosclerosis: the role of monocyte chemoattractant protein-1. Cytokine. 2006;34:51–55. doi: 10.1016/j.cyto.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 40.van Vonderen MG, Smulders YM, Stehouwer CD, et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: the role of HIV, antiretroviral therapy, and lipodystrophy. J Acquir Immune Defic Syndr. 2009;50:153–161. doi: 10.1097/QAI.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 41.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–227. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Freitas P, Carvalho D, Santos AC, et al. Carotid intima media thickness is associated with body fat abnormalities in HIV-infected patients. BMC Infect Dis. 2014;14:348. doi: 10.1186/1471-2334-14-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guaraldi G, Zona S, Orlando G, et al. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2012;28:935–941. doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]