Abstract
Breast cancer affects 1 out of 8 women in the US and is the second highest cause of death from cancer for women, leading to considerable research examining the causes, progression, and treatment of breast cancer. Over the last two decades, sphingosine-1-phosphate (S1P), a potent sphingolipid metabolite, has been implicated in many processes important for breast cancer including growth, progression, transformation and metastasis, and is the focus of this review. In particular, one of the kinases that produces S1P, sphingosine kinase 1 (SphK1), has come under increasing scrutiny as it is commonly upregulated in breast cancer cells and has been linked with poorer prognosis and progression, possibly leading to resistance to certain anti-cancer therapies. In this review, we will also discuss preclinical studies of both estrogen receptor (ER) positive as well as triple-negative breast cancer mouse models with inhibitors of SphK1 and other compounds that target the S1P axis and have shown good promise in reducing tumor growth and metastasis. It is hoped that in the future this will lead to development of novel combination approaches for effective treatment of both conventional hormonal therapy-resistant breast cancer and triple-negative breast cancer.
Keywords: sphingosine-1-phosphate, sphingosine kinase, estradiol, breast cancer, FTY720/fingolimod
INTRODUCTION
Breast cancer affects nearly 1 out of every 8 women over their lifetime and is the second leading cancer cause of death for women behind lung cancer in the US. Fortunately, over the last 30 years, breast cancer death rates have been dropping due to increased awareness of the disease, advances in detection, and better treatments. A large factor in these better treatments has been development of hormonal therapies to directly target specific receptors in the cancer cells such as estrogen (ER) and progesterone receptors (PR) that are present in roughly 70% of breast cancers. ER positive tumors in particular can be treated with estrogen antagonists such as tamoxifen to great effect with less side effects than traditional chemotherapy. The human epidermal growth factor receptor 2 (HER2), that is upregulated in 10 to 15% of breast cancers tumors and can also be treated with a monoclonal antibody. However, there are still 15 to 20% of tumors that are ER/PR/HER2 negative, termed triple negative breast cancer (TNBC), which are usually more aggressive and metastatic with significantly worse prognosis. Therefore, current cancer research is also focused on deeper understanding of novel signaling pathways that can contribute to breast cancer growth and metastasis. In the last 20 years, it has become apparent that the bioactive sphingolipid metabolite, sphingosine-1-phosphate (S1P), regulates processes important for breast cancer including inflammation that can drive tumorigenesis, angiogenesis, which provides cancer cells with nutrients and oxygen, cell growth and survival, as well as migration and invasion important for metastasis (Espaillat et al., 2017; Maczis et al., 2016; Nagahashi et al., 2014; Newton et al., 2015; Pyne et al., 2016; Pyne et al., 2014; Pyne and Pyne, 2010). In this review, we will summarize current research findings on S1P in breast cancer and examine the roles of the S1P/sphingosine kinase 1 (SphK1) axis in breast cancer signaling, prognosis, progression and as a possible target for future treatments, especially for TNBC and tumors that show resistance to typical first line treatments.
FORMATION OF SPHINGOSINE-1-PHOSPHATE
Sphingolipids are important membrane constituents of all eukaryotic cells that also generate bioactive metabolites, such as S1P. The formation of S1P from sphingosine, produced by degradation of sphingolipids, begins with the activation of one of two enzymes, SphK1 or SphK2, resulting in the former case in its translocation from the cytosolic compartment to the plasma membrane where its substrate sphingosine resides (Hannun and Obeid, 2008). Numerous growth factors such as EGF, hormones, such as estradiol (E2), and pro-inflammatory cytokines such as IL-1 and IL-6 activate SphK1 (Gao et al., 2015; Maceyka et al., 2012; Maceyka and Spiegel, 2014; Maczis et al., 2016). In many cases, it has been shown that this is due to stimulation of extracellular signal-regulated kinases 1/2 (ERK1/2) that in turn phosphorylates SphK1 on Ser225 allowing for its specific targeting to the plasma membrane (Pitson et al., 2003). This is in contrast to SphK2 that also resides in intracellular compartments, including the nucleus, and produces S1P there (Hait et al., 2009). As with other potent mediators, S1P is rapidly turned over either by dephosphorylation back to sphingosine by phosphatases or irreversibly cleaved by S1P lyase to ethanolamine phosphate and hexadecenal (Aguilar and Saba, 2012; Hannun and Obeid, 2008; Maceyka and Spiegel, 2014).
SPHINGOSINE-1-PHOSPHATE SIGNALING IN BREAST CANCER
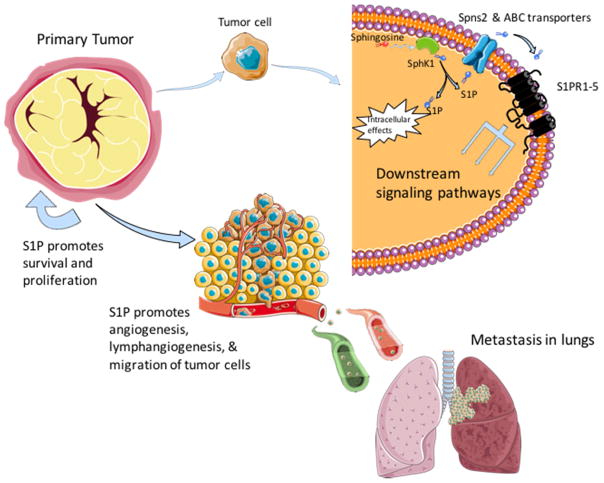
Following activation of SphK1 and restricted formation of S1P, the majority of the effects mediated by S1P occur after its export from the cell by the specific transporter called spinster 2 (Spns2) or by ATP-binding cassette transporters ABCA1, ABCC1, and ABCG2. S1P then can bind to one of five specific G protein-coupled cell surface S1P receptors (S1PR1-5) in an autocrine/paracrine manner, termed “inside-out” signaling. This leads to stimulation of downstream signaling mediated by overlapping G-proteins (Maceyka et al., 2012; Maczis et al., 2016; Takabe et al., 2008) (Fig. 1). A complete description of all of the interconnected signaling pathways that are activated by S1P is beyond the scope of this review, and this area has been extensively reviewed (Kihara et al., 2014; Pyne et al., 2016). Therefore, we will mainly focus on S1PR1 and S1PR3, two receptors that have been linked to breast cancer progression.
Figure 1.
Role of the SphK1/S1P axis in breast tumor progression and metastasis.
Intriguingly, S1PR1 has been linked to persistent activation of signal transducer and activator of transcription 3 (STAT3). STAT3 has been shown to be involved in many aspects of tumor growth and metastasis by activating a wide range of pathways promoting proliferation, survival, inflammation, invasion, and angiogenesis (Yu et al., 2014). STAT3 also enhances transcription of S1PR1 and activation of S1PR1 by S1P reciprocally activates STAT3 (Alshaker et al., 2014; Alshaker et al., 2015; Espaillat et al., 2017; Lee et al., 2010; Liang et al., 2013; Nagahashi et al., 2014). In breast cancer in particular, persistent STAT3 activation seems to be mainly due to upregulation of the pro-inflammatory cytokine IL-6 and S1PR1 (Alshaker et al., 2014; Alshaker et al., 2015; Lee et al., 2010). Moreover, IL-6 can activate SphK1 leading to a strong feed-forward mechanism promoting cancer cell progression (Lee et al., 2010). This signaling pathway is further complicated in ER negative breast cancer cells. as the adipokine leptin, a product of adipocytes, has also been shown to upregulate STAT3 and SphK1. SphK1 in turn induces production of IL-6, which then activates STAT3 (Alshaker et al., 2014; Alshaker et al., 2015). Pharmacological and molecular approaches further demonstrated that leptin-induced SphK1 activity and expression are mediated by activation of ERK1/2 and Src family kinase pathways, but not by the major pathways downstream of the leptin receptor, janus kinase 2 (JAK2) (Alshaker et al., 2015). As obesity is a risk factor for breast cancer and related to poorer prognosis, these studies could have implications for ER-negative breast cancer.
Binding of S1P to S1PR1 has also been shown to activate various receptor tyrosine kinases (RTKs) important for angiogenesis and proliferation such as VEGFR, EGFR, and PDGFR. This can result in “criss-cross” pathway activations as the growth factors that activate these RTKs can also activate SphK1. For example, EGF activation of SphK1 plays an important role in the migration of breast cancer cells towards EGF along with increased cell growth (Sarkar et al., 2005). S1P also potentiates the EGFR signaling pathway by insulin-like growth factor binding protein 3 (IGFBP-3), a growth promoter associated with poorer prognosis, suggesting that inhibition of both EGFR and SphK1 could have beneficial therapeutic effects in TNBC (Martin et al., 2014). Moreover, VEGF-mediated activation of SphK1 plays an essential role in regulating angiogenesis and lymphangiogenesis (Anelli et al., 2010; Nagahashi et al., 2012).
As for S1PR3, its activation via S1P was linked to the activation of the Notch signaling pathway along with p38MAPK in breast cancer stem cells (BCSCs) leading to proliferation and tumorigenicity (Hirata et al., 2014). BCSCs can also be activated by carcinogens, such as benzyl butyl phthalate, which has been shown to increase SphK1 expression leading to S1PR3 activation, implying that S1PR3 is a determinant of pollutant-driven breast cancer metastasis (Wang et al., 2016).
Most of S1PR3’s cancer promoting and pro-survival effects can be attributed to sustained activation of ERK1/2 and AKT/PI3K pathways, key regulators of cell cycle progression, survival, and proliferation mechanisms in breast cancer cells (Datta et al., 2014; Wang et al., 2016; Watson et al., 2010). In triple-negative MDA-MB-231 breast cancer cells, early and sustained phosphorylation of both ERK1/2 and AKT/PI3K was inhibited by a SphK1 inhibitor while only sustained activation was inhibited by pertussis toxin, a potent G protein inhibitor, suggesting that S1PRs are crucial only for sustained activation (Datta et al., 2014). Aside from activating its own downstream signaling cascade, the AKT/PI3K pathway is involved in crosstalk with several other pathways, including RAS/RAF/MEK and ER, further strengthening the interconnecting pro-survival and progression pathways (Maiti et al., 2017). Another study in TNBC cells substantiated a link between sphingosine, SphK1, and the protein kinase C (PKC) serine/threonine kinase family, important regulators of cell proliferation and survival (Kotelevets et al., 2012). This study also showed that targeting SphK1 in triple-negative MDA-MB-231 breast cancer cells decreased proliferation and survival by compromising PKC activity and cytokinesis (Kotelevets et al., 2012). While the exact mechanisms of these pathways have not been elucidated, they support the significance of SphK1 as a target for cancer therapy. A recent study with MDA-MB-231 cells looked at how S1P signaling affected adhesion and invasion via the tumor cell microenvironment. It was reported that extracellular matrix rigidity-dependent S1P secretion regulates metastatic cancer cell invasion and adhesion (Ko et al., 2016). These results suggest that alterations in the mechanical environment of the extracellular matrix surrounding the tumor cells actively regulate secretion of S1P, which in turn, may contribute to cancer progression. In summary, many of the pathways modulated by the SphK1/S1P/S1PR axis in breast cancer cells are overlapping, promoting their growth, survival, proliferation, and metastasis (Fig. 1).
In addition to the very well-known functions of S1P as a ligand for S1PRs, recent studies suggest that S1P also has important intracellular actions (Maceyka et al., 2012). Especially relevant is the observation that SphK2 is present in the nucleus of many breast cancer cell lines (Hait et al., 2009; Igarashi et al., 2003; Sankala et al., 2007) where it produces S1P that inhibits class I histone deacetylases (HDACs) (Hait et al., 2009). Thus, it was suggested that HDACs are direct intracellular targets of S1P and link nuclear sphingolipid metabolism and S1P to epigenetic regulation of expression of specific genes (Hait et al., 2009). Recently, we found that FTY720 is also phosphorylated in breast cancer cells by nuclear SphK2 and accumulates there. Moreover, like S1P, nuclear FTY720-P is also a potent inhibitor of class I HDACs. Furthermore, we observed that high fat diet increased triple-negative spontaneous breast tumors and HDAC activity in MMTV-PyMT transgenic mice that was suppressed by oral administration of FTY720. Interestingly, this treatment not only inhibited HDACs, it also reversed high fat diet-induced loss of ER and PR in advanced carcinoma (Hait et al., 2015). Furthermore, treatment with FTY720 also re-expressed ER and increased therapeutic sensitivity of TNBC syngeneic breast tumors to tamoxifen in vivo more potently than a known HDAC inhibitor. This work suggests that in combination, FTY720 could be an effective treatment of both conventional hormonal therapy-resistant breast cancer and triple-negative breast cancer (Hait et al., 2015).
SPHINGOSINE KINASE 1 AND ESTROGEN RECEPTOR SIGNALING
Nearly 80% of breast cancers are ER positive, meaning they are dependent on estrogens such as 17β-estradiol (E2) to signal growth, proliferation and metastasis. E2 normally binds to ER in the cytoplasm and after dimerization, translocates to the nucleus. In the nucleus, the ER dimers bind to estrogen response elements and act as transcription factors to activate or repress gene transcription (Klinge, 2001). E2 can also induce rapid, non-genomic cellular changes through membrane ERs that are still ill defined, including the splice variant ER36 and the G protein-coupled receptor GPR30 (Wang and Yin, 2015; Zhou et al., 2016). These membrane ERs have been shown to activate SphK1, producing S1P and activate signaling pathways downstream of S1PRs leading to increased cell growth, higher microvessel density in tumors, and enhanced resistance to anti-cancer drugs in response to hormonal therapies (Maczis et al., 2016; Sukocheva et al., 2006; Sukocheva et al., 2013; Takabe et al., 2010). GPR30 was suggested to activate SphK1 as its downregulation by anti-sense oligonucleotides inhibited E2-mediated activation of SphK1 in MCF-7 breast cancer cells (Sukocheva et al., 2006). However, the identity of the responsible receptor has not yet been conclusively established. E2-mediated formation of S1P led to rapid release of S1P from breast cancer cells via the ABCC1 and the ABCG2 transporters (Takabe et al., 2010) and “inside out” signaling by S1P (Maczis et al., 2016; Sukocheva et al., 2006; Sukocheva et al., 2013). Furthermore, inhibiting these transporters blocked E2-induced activation of ERK1/2 (Takabe et al., 2010). It was convincingly demonstrated that activation of S1PR3 by S1P transactivated EGFR through a pathway mediated by Src and matrix metalloproteases. This switch from E2/ER-mediated growth to SphK1/EGFR activation has also been thought to contribute to resistance to hormonal therapies such as tamoxifen (Maczis et al., 2016; Sukocheva and Wadham, 2014; Sukocheva et al., 2006).
SphK1 activity has also been linked to the effects of several microRNAs that are regulated by ER. miR-515-5P, a tumor suppresser, was shown to reduce SphK1 activity and loss of miR-515-5P resulted in increased oncogenic SphK1 activity. In addition, E2 treatment downregulated miR-515-5P levels, and miR-515-5p is downregulated in ER-positive compared to ER-negative breast cancers (Pinho et al., 2013).
SPHINGOSINE KINASE 1 AND BREAST CANCER PROGNOSIS
Over the last few years, new evidence from several studies has illuminated the multi-factorial role of the SphK1/S1P axis in breast cancer and its link with worse prognosis and overall outcomes (Maczis et al., 2016; Ruckhaberle et al., 2008). It also usually corresponds with upregulation of associated S1PRs and chemotherapeutic resistance (Gao et al., 2015). In one study, 62.5% of tumors analyzed (20 out of 32) had at least a 2-fold increase in SphK1 mRNA expression compared to surrounding normal breast tissue (Datta et al., 2014). Furthermore, ER negative tumors had higher SphK1 levels than ER-positive tumors and the deadlier, triple-negative tumors had the highest levels of SphK1 expression of all tumor types examined. Overall, the analysis revealed an inverse correlation between SphK1 levels and survival of breast cancer patients. One of the possible causes investigated in this study was resistance to doxorubicin and docetaxel-based chemotherapies, mainstays for treatment of ER positive breast cancer, and it was found that non-responders to treatment had significantly higher SphK1 mRNA levels. This infers that SphK1 does not just promote progression and growth of tumors but also impacts survival through its effects on drug resistance (Datta et al., 2014). Patients with high levels of cytoplasmic Sphk1 compared to low SphK1 had a nearly 8-years shorter mean time to recurrence on tamoxifen (12.61 years with low SphK1 and 4.65 years with high SphK1 expression). Further investigations examined expression of S1PR1 and S1PR3 in particular and it was noted that patients with high membrane S1PR1 had a roughly 3 years shorter mean time to recurrence on tamoxifen and just over 8 years shorter disease-specific survival. It has been speculated that these differences in recurrence and survival could be due to E2 activation of SphK1 leading to the activation of the ERK1/2 pathways downstream of S1PR3 (Watson et al., 2010). Similar observations were made in another study (Ohotski et al., 2013).
Interestingly, S1P levels in breast cancer patients with lymph node metastasis that correlate with poor prognosis were significantly higher than those with negative lymph nodes, consistent with the notion that S1P plays an important role in angiogenesis, lymphangiogenesis, and metastasis (Tsuchida et al., 2016). Another interesting finding was that SphK1 levels determined by immunohistochemistry in deadlier TNBC tumors were lower, in contrast with some earlier studies. However, the S1P levels were higher, possibly suggesting the tumor microenvironment is responsible for the increase in S1P, not the tumor itself. This agreed with their observation of higher levels of S1P in patients with increased white blood cells, and suggested that since TNBCs are more immunogenic and immune cells express SphK1 and secrete S1P, they could increase S1P levels in the microenvironment (Tsuchida et al., 2016).
In sum, high levels of SphK1 expression and resulting high levels of S1P are most likely related to poorer prognosis for most patients. This could be due the ability of SphK1/S1P axis to promote cancer cell growth, proliferation, survival, and drug resistance. Thus, decreasing SphK1 expression and activity and S1P production could represent a new approach to improve prognosis of breast cancer.
SPHINGOSINE KINASE 1 IN ANIMAL MODELS OF BREAST CANCER PROGRESSION AND METASTASIS
Most of the data on SphK1 and its relationship to breast cancer in humans have come from analysis of tumor samples combined with patient follow-up data. An increasing number of studies have used mouse models to examine the role of the SphK1/S1P axis in breast cancer progression. The first observation was that breast cancer cells stably overexpressing SphK1 formed more and larger tumors in mice than vector transfectants with higher microvessel density in their periphery (Nava et al., 2002). Similar results were obtained by orthotopically implanting 4T1-luc2 murine breast cancer cells into the mammary fat pads of immune competent female mice (Nagahashi et al., 2012). The 4T1-luc2 tumors are rapidly growing and metastasize first to the lymph nodes and then the lungs, reminiscent to human breast cancer progression. Interestingly, circulating levels of S1P in tumor bearing mice were also significantly increased. Treatment of these mice with the specific SphK1 inhibitor SKI-1 decreased plasma S1P levels concomitantly with significant reductions in tumor volume, weight, and mitotic activity as well as lymph node and lung metastsis (Nagahashi et al., 2012). Moreover, cancer stem cells overexpressing SphK1 had increased ability to develop tumors in nude mice. Tumorigenicity of these cancer stem cells was inhibited by S1PR3 knockdown or a S1PR3 antagonist indicating that S1P promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation (Hirata et al., 2014).
Growth of tumors to beyond a certain size requires the formation of new blood vessels, termed angiogenesis, to continue to feed the rapidly growing and dividing cells (Nagahashi et al., 2012). To further spread throughout the body, the tumor cells usually extravasate and travel through the lymph system while also promoting formation of new lymph vessels through lymphangiogenesis. Both tumor size and metastasis are crucial in determining the staging and prognosis of a cancer (Nagahashi et al., 2016). There are also many cellular factors that contribute to angiogenesis and lymphangiogenesis, and perhaps others still to be discovered. However, it is becoming clear that S1P plays an important role in these processes. The angiogenic and lymphangiogenic actions of S1P are likely mediated via activation of S1PR1 on endothelial cells (Anelli et al., 2010; Nagahashi et al., 2012). As discussed above, S1P is commonly elevated in cancer tissues and in the circulation and also in lymph interstitial fluid from human breast cancer tumors (Nagahashi et al., 2016).
SPHK1/S1P/S1P RECEPTOR AXIS AS A THERAPEUTIC TARGET FOR BREAST CANCER
With such strong connections between SphK1/S1P/S1PR axis and the growth and progression of breast cancer cells, SphK1 and S1PR offer new and novel targets for possible future treatment avenues aimed at treating breast cancer, especially TNBC. Several preclinical studies have used mouse breast cancer models to investigate the effects of SphK1 inhibiters or S1PR modulators on tumor growth. A combination of the non-specific SphK inhibitor SKI-II with gefitinib, an EGFR inhibitor, significantly inhibited growth of xenograft MDA-MB-468 TNBC tumors whereas neither SKI-II or gefitinib alone had any effects (Martin et al., 2014). Another SphK1 inhibitor, SKI-5C, also significantly reduced growth of tumors from another TNBC cell line, MDA-MB-231, in xenografted SCID mice (Datta et al., 2014). Using an improved syngeneic breast cancer cell implantation method that mimics human breast cancer biology better than conventional xenograft subcutaneous implantation, treatment with the specific SphK1 inhibitor SK1-I suppressed tumor growth of murine 4T1 breast cancer cells and S1P levels and reduced metastases to lymph nodes and lungs (Nagahashi et al., 2012).
Lastly, one of the most promising possible future avenues for breast cancer treatments that target the S1P axis is Fingolimod (FTY720), a sphingosine analog pro-drug currently used to treat multiple sclerosis that has long been known to have beneficial effects in many preclinical breast cancer models (Azuma et al., 2002; Deng et al., 2012; Hait et al., 2015; Rincon et al., 2015). FTY720 effects are not limited only to suppressing the development and progression of breast tumors on its own but also is an effective adjuvant therapy. Treatment with FTY720 potentiated the anti-cancer effects of doxorubicin in MDA-MB-231 xenograft tumors and particularly in MDA-MB-231 cells that acquired resistance to doxorubicin (Rincon et al., 2015). FTY720 has been shown to synergize with the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reducing tumor volume and inducing apoptosis in xenograft breast cancer models without affecting normal cells (Woo et al., 2015). FTY720 has several anti-cancer targets that contribute to its multi-potent effectiveness. When phosphorylated by SphK2, FTY720-P is a S1P mimetic that acts as a functional antagonist of S1PR1, reducing the persistent activation of STAT3 (Deng et al., 2012) and thus diminishes accumulation of regulatory T cells in tumors (Priceman et al., 2014).
We found that FTY720 is phosphorylated by nuclear SphK2 in breast cancer cells. FTY720-P accumulates in the nucleus and potently inhibits class I histone deacetylases (HDACs) leading to increased histone acetylations and expression of a restricted set of genes independently of its known effects on S1PRs. We also observed that feeding a high-fat diet accelerated formation of tumors and increased triple-negative spontaneous breast tumors in MMTV-PyMT transgenic mice and that oral treatment with FTY720 inhibited development and aggressiveness of spontaneous breast tumors in these mice, reduced HDAC activity and dramatically reversed high-fat diet-induced loss of ER and PR in advanced carcinoma. Like other HDAC inhibitors, treatment of ER-negative breast cancer cells with FTY720 reactivated expression of silenced ER and sensitized them to tamoxifen. Furthermore, treatment with FTY720 also re-expressed ER and increased therapeutic sensitivity of ER-negative syngeneic breast tumors to tamoxifen in vivo more strongly than a pan HDAC inhibitor.
Unphosphorylated FTY720 also has anti-cancer actions. It inhibits SphK1 by binding to an allosteric site that exerts auto-inhibition on the catalytic site. It also induces proteasomal degradation of SphK1 and thus inhibits actions of S1P (Lim et al., 2011). Moreover, part of the effectiveness of FTY720 in tumor suppression can be attributed to its ability to activate the tumor suppressor PP2A (Perrotti and Neviani, 2013; Saddoughi et al., 2013), which is commonly inhibited in breast cancer and is crucial for maintaining tumor cell properties (Rincon et al., 2015).
Overall these studies show that FTY720 is a multi-faceted drug with the potential to work as an effective anti-cancer drug by itself and also as an adjuvant to hormonal therapies, traditional chemotherapies, and even radiation therapies to treat not only ER-positive tumors but also the more difficult TNBCs and tumors that develop resistance to chemotherapeutic agents. As FTY720 is already an FDA approved drug for treating humans, it is hoped that it can re-purposed for use as a cancer treatment.
Acknowledgments
This work was supported by grants from Department of Defense BCRP Program Award W81XWH-14-1-0086 and NIGMS grant R01GM043880 (S. Spiegel).
Abbreviations
- BCSCs
breast cancer stem cells
- ER
estrogen receptor
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal regulated kinase
- E2
17β-estradiol
- HDAC
histone deacetylase
- HER2
human epidermal growth factor receptor 2
- MAPK
mitogen activated protein kinase
- PKC
protein kinase C
- RTK
receptor tyrosine kinase
- SphK
sphingosine kinase
- S1P
sphingosine-1-phosphate
- S1PR
S1P receptor
- TNBC
triple-negative breast cancer
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar A, Saba JD. Truth and consequences of sphingosine-1-phosphate lyase. Adv Biol Regul. 2012;52(1):17–30. doi: 10.1016/j.advenzreg.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaker H, Krell J, Frampton AE, Waxman J, Blyuss O, Zaikin A, Winkler M, Stebbing J, Yague E, Pchejetski D. Leptin induces upregulation of sphingosine kinase 1 in oestrogen receptor-negative breast cancer via Src family kinase-mediated, janus kinase 2-independent pathway. Breast Cancer Res Treat. 2014;16(5):426. doi: 10.1186/s13058-014-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaker H, Wang Q, Frampton AE, Krell J, Waxman J, Winkler M, Stebbing J, Cooper C, Yague E, Pchejetski D. Sphingosine kinase 1 contributes to leptin-induced STAT3 phosphorylation through IL-6/gp130 transactivation in oestrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2015;149(1):59–67. doi: 10.1007/s10549-014-3228-8. [DOI] [PubMed] [Google Scholar]
- Anelli V, Gault CR, Snider AJ, Obeid LM. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 2010;24(8):2727–2738. doi: 10.1096/fj.09-150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T, Nonomura N, Suzuki S, Okuyama A, Katsuoka Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62(5):1410–1419. [PubMed] [Google Scholar]
- Datta A, Loo SY, Huang B, Wong L, Tan SS, Tan TZ, Lee SC, Thiery JP, Lim YC, Yong WP, Lam Y, Kumar AP, Yap CT. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5(15):5920–5933. doi: 10.18632/oncotarget.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, Shen S, Priceman SJ, Kujawski M, Pal SK, Raubitschek A, Hoon DS, Forman S, Figlin RA, Liu J, Jove R, Yu H. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21(5):642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espaillat MP, Kew RR, Obeid LM. Sphingolipids in neutrophil function and inflammatory responses: Mechanisms and implications for intestinal immunity and inflammation in ulcerative colitis. Adv Biol Regul. 2017;63:140–155. doi: 10.1016/j.jbior.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Gao F, Chen K, Tian ML, Zhao DL. Sphingosine kinase 1 as an anticancer therapeutic target. Drug Des Devel Ther. 2015;9:3239–3245. doi: 10.2147/DDDT.S83288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, Dumur CI, Zelenko Z, Gallagher EJ, Leroith D, Milstien S, Takabe K, Spiegel S. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4:e156. doi: 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hirata N, Yamada S, Shoda T, Kurihara M, Sekino Y, Kanda Y. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. doi: 10.1038/ncomms5806. [DOI] [PubMed] [Google Scholar]
- Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura SI. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278(47):46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol. 2014;171(15):3575–3594. doi: 10.1111/bph.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29(14):2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko P, Kim D, You E, Jung J, Oh S, Kim J, Lee KH, Rhee S. Extracellular Matrix Rigidity-dependent Sphingosine-1-phosphate Secretion Regulates Metastatic Cancer Cell Invasion and Adhesion. Sci Rep. 2016;6:21564. doi: 10.1038/srep21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevets N, Fabbro D, Huwiler A, Zangemeister-Wittke U. Targeting sphingosine kinase 1 in carcinoma cells decreases proliferation and survival by compromising PKC activity and cytokinesis. PLoS One. 2012;7(6):e39209. doi: 10.1371/journal.pone.0039209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16(12):1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, Takabe K, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23(1):107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, Pyne NJ. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangment in MCF-7 breast cancer cells. J Biol Chem. 2011;286(21):18633–18640. doi: 10.1074/jbc.M111.220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczis M, Milstien S, Spiegel S. Sphingosine-1-phosphate and estrogen signaling in breast cancer. Adv Biol Regul. 2016;60:160–165. doi: 10.1016/j.jbior.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Maiti A, Takabe K, Hait NC. Metastatic triple-negative breast cancer is dependent on SphKs/S1P signaling for growth and survival. Cell Signal. 2017;32:85–92. doi: 10.1016/j.cellsig.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, de Silva HC, Lin MZ, Scott CD, Baxter RC. Inhibition of insulin-like growth factor-binding protein-3 signaling through sphingosine kinase-1 sensitizes triple-negative breast cancer cells to EGF receptor blockade. Mol Cancer Ther. 2014;13(2):3163–3128. doi: 10.1158/1535-7163.MCT-13-0367. [DOI] [PubMed] [Google Scholar]
- Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, Milstien S, Spiegel S. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv Biol Regul. 2014;54C:112–120. doi: 10.1016/j.jbior.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72(3):726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M, Yamada A, Miyazaki H, Allegood JC, Tsuchida J, Aoyagi T, Huang WC, Terracina KP, Adams BJ, Rashid OM, Milstien S, Wakai T, Spiegel S, Takabe K. Interstitial Fluid Sphingosine-1-Phosphate in Murine Mammary Gland and Cancer and Human Breast Tissue and Cancer Determined by Novel Methods. J Mammary Gland Biol Neoplasia. 2016;21(1–2):9–17. doi: 10.1007/s10911-016-9354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281(1):115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, Spiegel S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp Cell Res. 2015;333(2):195–200. doi: 10.1016/j.yexcr.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohotski J, Edwards J, Elsberger B, Watson C, Orange C, Mallon E, Pyne S, Pyne NJ. Identification of novel functional and spatial associations between sphingosine kinase 1, sphingosine 1-phosphate receptors and other signaling proteins that affect prognostic outcome in estrogen receptor-positive breast cancer. Int J Cancer. 2013;132(3):605–616. doi: 10.1002/ijc.27692. [DOI] [PubMed] [Google Scholar]
- Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013;14(6):e229–238. doi: 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho FG, Frampton AE, Nunes J, Krell J, Alshaker H, Jacob J, Pellegrino L, Roca-Alonso L, de Giorgio A, Harding V, Waxman J, Stebbing J, Pchejetski D, Castellano L. Downregulation of microRNA-515-5p by the estrogen receptor modulates sphingosine kinase 1 and breast cancer cell proliferation. Cancer Res. 2013;73(19):5936–5948. doi: 10.1158/0008-5472.CAN-13-0158. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22(20):5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priceman SJ, Shen S, Wang L, Deng J, Yue C, Kujawski M, Yu H. S1PR1 is crucial for accumulation of regulatory T cells in tumors via STAT3. Cell Rep. 2014;6(6):992–999. doi: 10.1016/j.celrep.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, Jiang HR, Ubhi S, Pyne S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv Biol Regul. 2016;60:151–159. doi: 10.1016/j.jbior.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Ohotski J, Bittman R, Pyne S. The role of sphingosine 1-phosphate in inflammation and cancer. Adv Biol Regul. 2014;54:121–129. doi: 10.1016/j.jbior.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Rincon R, Cristobal I, Zazo S, Arpi O, Menendez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, Garcia-Foncillas J, Madoz-Gurpide J, Rojo F. PP2A inhibition determines poor outcome and doxorubicin resistance in early breast cancer and its activation shows promising therapeutic effects. Oncotarget. 2015;6(6):4299–4314. doi: 10.18632/oncotarget.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112(1):41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP, Senkal CE, Garrett-Mayer E, De Palma RM, Fedarovich D, Liu A, Habib AA, Stahelin RV, Perrotti D, Ogretmen B. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5(1):105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, Dent P, Milstien S, Spiegel S. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67(21):10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579(24):5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C. Role of sphingolipids in oestrogen signalling in breast cancer cells: an update. J Endocrinol. 2014;220(3):R25–R35. doi: 10.1530/JOE-13-0388. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173(2):301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Xia P. Estrogen defines the dynamics and destination of transactivated EGF receptor in breast cancer cells: role of S1P(3) receptor and Cdc42. Exp Cell Res. 2013;319(4):455–465. doi: 10.1016/j.yexcr.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285(14):10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida J, Nagahashi M, Nakajima M, Moro K, Tatsuda K, Ramanathan R, Takabe K, Wakai T. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J Surg Res. 2016;205(1):85–94. doi: 10.1016/j.jss.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Tsai CF, Chuang HL, Chang YC, Chen HS, Lee JN, Tsai EM. Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget. 2016;7(20):29563–29576. doi: 10.18632/oncotarget.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Yin L. Estrogen receptor alpha-36 (ER-alpha36): A new player in human breast cancer. Mol Cell Endocrinol. 2015;418(Pt 3):193–206. doi: 10.1016/j.mce.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, Pyne S, Pyne NJ, Edwards J. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol. 2010;177(5):2205–2215. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SM, Seo BR, Min KJ, Kwon TK. FTY720 enhances TRAIL-mediated apoptosis by up-regulating DR5 and down-regulating Mcl-1 in cancer cells. Oncotarget. 2015;6(13):11614–11626. doi: 10.18632/oncotarget.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- Zhou K, Sun P, Zhang Y, You X, Li P, Wang T. Estrogen stimulated migration and invasion of estrogen receptor-negative breast cancer cells involves an ezrin-dependent crosstalk between G protein-coupled receptor 30 and estrogen receptor beta signaling. Steroids. 2016;111:113–120. doi: 10.1016/j.steroids.2016.01.021. [DOI] [PubMed] [Google Scholar]