Abstract
Purpose
To explore mechanisms underlying the association of TSG-6 with osteoarthritis (OA) progression.
Methods
TSG-6-mediated heavy chain (HC) transfer (TSG-6 activity) and its association with inflammatory mediators were quantified in knee OA (n=25) synovial fluids. Paired intact and damaged cartilages from the same individuals (20 tibial and 12 meniscal) were analyzed by qRT-PCR and immunohistochemistry (IHC) for gene and protein expression of TSG-6 and components of IαI (necessary for TSG-6 activity), and for TSG-6 activity +/− spiked in IαI. Primary chondrocyte culture (n=5) +/− IL1ß or TNFα were also evaluated for gene expression. The effects of TSG-6 activity on cartilage extracellular matrix assembly were explored using quantitative hyaluronan (HA)-aggregan binding assays.
Results
TSG-6 activity was significantly associated (R>0.683, p<0.0002) with inflammatory mediators including TIMP-1, A2M, MMP3, VEGF, VCAM-1, ICAM-1 and IL-6. Although TSG-6 protein and mRNA were highly expressed in damaged articular and meniscal cartilage and cytokine-treated chondrocytes, there was little or no cartilage expression of components of the IαI complex (containing HC1). By IHC, TSG-6 was present throughout lesioned cartilage but HC1 only at lesioned surfaces. TSG-6 impaired HA-aggrecan assembly, but TSG-6 mediated HA-HC formation reduced this negative effect.
Conclusion
TSG-6 activity is a global inflammatory biomarker in knee OA synovial fluid. IαI, supplied from outside cartilage, only penetrates to the surface, restricting TSG-6 activity (HC transfer) to this region. Therefore, unopposed TSG-6 in intermediate and deep regions of OA cartilage could possibly block matrix assembly, leading to futile synthesis and account for increased risk of OA progression.
Keywords: TSG-6, hyaluronan, cartilage matrix, osteoarthritis, progression, inflammation, biomarker
Introduction
Tumor necrosis factor (TNF)-stimulated gene-6 protein (TSG-6) is a hyaluronan (HA) binding protein stimulated by pro-inflammatory cytokines, growth factors and hormones[1–6]. We previously identified TSG-6 as one of the most significantly up-regulated genes in articular cartilage in a human model system of osteoarthritis (OA) progression[7]. This secreted protein is upregulated in many cell types in inflammatory disease states in addition to OA[8–10] and significantly elevated in synovial fluids (SF) after acute knee injury in both a human cohort and a murine model[11,12].
TSG-6 contains an HA-binding LINK domain and a CUB domain[13]. The LINK domain directly binds the glycosamingoglycans HA, heparin, heparan sulfate, chondroitin sulfate and the CXC and CC family of chemokines[10,14,15]. Through its binding this family of cytokines, TSG-6 can block chemotactic signals that induce neutrophil infiltration[16–18]. The CUB domain (for complement C1r/C1s, Uegf, Bmp1) contains calcium binding sites that mediate the formation of complexes of TSG-6 with Inter-alpha-Inhibitor (IαI), a protein complex consisting of heavy chain 1 (HC1), heavy chain 2 (HC2) and one light chain (bikunin). With these two functional domains, TSG-6 can catalyze the transfer of HCs to the glycosaminoglycan HA[13,19]. TSG-6 mediated HC transfer (TSG-6 activity) plays an essential role in fertility by stabilizing the HA-rich extracellular matrix (ECM) of cumulus cell-oocyte complexes[20–22]. HA-HC formed by TSG-6 and IαI in amniotic membranes is anti-inflammatory: it induces apoptosis of inflammatory neutrophils; enhances macrophage phagocytosis[23]; and inhibits angiogenesis through inhibition of VEGF and vascular structure formation from umbilical vein endothelial cells[24]. IαI and TSG-6 also synergistically inhibit plasmin activity[6,25], a protease that degrades the ECM directly or indirectly through activation of matrix metalloproteases (MMPs), such as MMP3, MMP9, MMP12 and MMP13[26–28] and inhibits inflammatory arthritis in murine models[29,30]. Despite these beneficial effects, paradoxically, we observed that TSG-6 activity in OA SFs predicted knee OA progression[31].
We hypothesized that analyses of the role of TSG-6 in remodeling the HA-rich ECM of articular cartilage could elucidate mechanisms underlying the association of TSG-6 with OA progression that could lead to development of a novel strategy to reduce disease development or worsening. Although TSG-6 is known to be expressed by articular cartilage, this is the first study to evaluate its expression in meniscal as well as articular cartilages and to examine the potential functional consequences of HA-HC in cartilage.
Material and Methods
Human specimens
Biospecimens were acquired as anonymized surgical waste under Institutional Review Board (IRB) approval of Duke University from 20 patients (12 female, 8 male) undergoing total knee replacement for medial compartment dominant knee OA. From these 20 joint specimens, we harvested the human outer lateral (intact) and inner medial (damaged) tibial articular cartilages, and the lateral (intact) and medial (damaged) meniscal cartilages when available (12 of 20 meniscal pairs available, the remaining 8 pairs could not be analyzed due to lack of sufficient medial meniscal cartilage). All specimens were stored in liquid nitrogen within 4 hours after procurement. Additional knee OA SF (n=25 subjects, 19 female, 6 male) was provided from the EC20 cohort, a previously described completed study characterizing the in vivo extent of activated macrophages in the context of Kellgren-Lawrence grade 1–4 knee OA[32]. Table 1 summarizes the demographic data corresponding to the studied sample sets.
Table 1.
Demographic information of samples
| Samples | n= | Female % | Mean age ± SD | Stage of OA | Cohort | TSG-6 activity (U), Median (Range) |
|---|---|---|---|---|---|---|
| OA knee cartilage | 20 | 60.0 | 66.6 ± 9.9 | Final | TKA | 0.01 (0–0.24) in I, 0.04 (0–2.80) in D |
| OA meniscus | 12 | 58.3 | 65.5 ± 9.0 | Final | TKA | 0.05 (0.01–1.37) in I, 0.15 (0.03–1.59) in D |
| OA knee SF | 20 | 60.0 | 66.6 ± 9.9 | Final | TKA | 5.99 (0–30.14) |
| OA knee SF | 25 | 70.6 | 63.6 ± 15.7 | K-L grade 1–4 | EC20 | 45.55 (7.6–290) |
TKA: Samples were obtained during total joint replacement surgery
I: Intact cartilage; D: Damaged cartilage
Western blots
A representative pool of OA SF was prepared from 20 total knee joint replacement specimens. SF (1:100 diluted in PBS) samples were incubated for 1, 2, 4, 8 or 16 hours with or without 12mM EDTA at 37°C in HA coated plates. Supernatants were collected and plates washed three times with TBST (50 mM Tris-HC, 150 mM NaCl, 0.1% BSA, 0.05% Tween-20, pH 7.4). To collect the transferred HCs from immobilized HA, plates were treated with 20 μl HAse from Streptomyces hyalurolyticus (250 U/ml, H1136, Sigma-Aldrich, Mo) for 1 hour at 37°C. Diluted biological fluid samples (5μl) or transferred HC samples (20 μl) were electrophoresed on 4–15% precast polyacrylamide gels (Bio-rad, CA) under denaturing and reducing conditions. Proteins were transferred to the Immun-Blot PVDF Membrane (Bio-rad, CA). The membrane was blocked with 5% fat-free milk in TBST and incubated with specific primary antibodies against IαI (DAKO A0301; 1:10,000 dilution) or TSG-6 (SC-377277, Santa Cruz Biotechnology, Texas; 1:200 dilution) followed by their respective secondary antibodies. Bound antibodies were detected with chemiluminescence reagents (Pierce, Thermo scientific, IL).
TSG-6 protein quantification by ELISA
A commercially available human TSG-6 sandwich ELISA kit (CUSABIO, CSB-EL023959HU, China) was used to measure protein concentrations of TSG-6 in SF from the EC20 cohort. The assay was performed according to the manufacturer’s instructions. In brief, SF samples (diluted 1:10) and a serial dilution of recombinant TSG-6 protein as standards were added onto the TSG-6 specific antibody pre-coated microplate. Biotin-conjugated TSG-6 antibody, avidin-conjugated HRP and TMB were used to detect any TSG-6 protein bound by the immobilized antibody. The limit of detection range was 0.156–10 ng/ml. The inter- and intra-assay CVs were 5.37% and 2.03%, respectively.
Inflammatory biomarker measurements
SF samples of the EC20 cohort were analyzed by Myriad Rules-Based Medicine (RBM) (Austin, TX) for a panel of 47 cytokines/chemokines (the Human InflammationMAP, a bead-based multiplexed immunoassay). All assays had CVs <20% and 97% had CVs <15% (https://myriadrbm.com/company/data-quality/).
Immunohistochemical analyses
Full depth cartilage (~2cm length × 5mm width) from the tibial plateau and meniscus were fixed in 4% paraformaldehyde in PBS (Sigma-Aldrich, Mo) overnight and decalcified in 10% EDTA (Sigma-Aldrich, Mo) with a buffer change every week for four weeks. After decalcification, the tissue was embedded in paraffin and 7 μm sections were prepared, deparaffinized and hydrated, followed by quenching of endogenous peroxidase activity in 0.3% H2O2 for 30 minutes. Antigen retrieval was conducted at 37°C for 30 minutes in 100 mu/ml Chondroitinase ABC (C3667, Sigma-Aldrich, Mo) and 250 U/ml Hyaluronidase (HAse) from rom Streptomyces hyalurolyticus (H1136, Sigma-Aldrich, Mo) in PBS with 0.02% BSA. Sections were blocked with 2.5% normal horse serum at 25°C for 1 hour, followed by incubation at 4°C overnight with primary antibodies as follows: anti-heavy chain-1 (Atlas antibodies, HPA042049, Sweden; dilution 1:1000); anti-TSG-6 (SC-377277, Santa Cruz Biotechnology, Texas; dilution 1:25); or mixed normal mouse and rabbit IgG (1:1 mixed, Santa Cruz Biotechnology, Texas; dilution 1:200) as the non-immune control for anti-HC1 and anti-TSG6. After washing, sections were reacted with ImmPRESS rabbit or mouse secondary antibodies (Vector Laboratory, CA) for 30 minutes. Signal amplification and staining were performed using DAB kits (Vector Laboratory, CA) according to the manufacturer’s protocol and counterstained with hematoxylin solution Gill no. 2 (Sigma-Aldrich, Mo) for 2 mins.
Cartilage processing for gene expression and TSG-6 activity analyses
The processes of both articular and meniscal cartilage harvest, sectioning, grinding, and extraction were performed as previously described[33]. Regions of interest were sectioned and powdered under liquid nitrogen; ~100 mg of cartilage powder was used for RNA isolation with 5 ml of Trizol (Thermo Scientific, IL) and ~10 mg of cartilage powder was used for protein extraction with 200 ml of M-PER mammalian protein extraction reagent (Thermo Scientific, IL) mixed with a protease inhibitor cocktail (Thermo Scientific, IL). An adjacent section was preserved for histological evaluation of the degree of cartilage degeneration. Total RNA from primary chondrocytes was isolated as previously described[33].
qRT-PCR
A total of 1000 ng RNA from each sample was converted into cDNA using Superscript III reverse transcriptase (Invitrogen, CA). To determine expression of TSG-6 and the components comprising IαI, we performed qRT-PCR for TNF alpha induced protein 6 (TNFAIP6 known as TSG6), Inter-alpha-trypsin inhibitor heavy chain 1 (ITIH1), Inter-alpha-trypsin inhibitor heavy chain 2 (ITIH2) and alpha-1-microglobulin/bikunin precursor (AMBP) genes, plus Glyceraldehyde Phosphate Dehydrogenase (GAPDH) housekeeping gene on 40 and 24 cDNA samples from 20 and 12 OA paired damaged and non-damaged knee articular and meniscal cartilages, respectively. The qRT-PCR was performed as previously described[33]. The levels of cDNA among samples were normalized to the expression of GAPDH and analyzed with the Ct relative quantification method to identify significant variation in gene expression comparing damaged and non-damaged regions with paired t-test. If gene expression was undetectable, the Ct value of 40 was assigned, which corresponds to the Ct of water (the negative control). cDNA from HepG2 cells were used as a positive control for every qRT-PCR run. Forward (F) and Reverse (R) primer sequences used to detect expression were: ITIH1, F: CCACCCCATCGGTTTTGAAGTGTCT, R: TGCCACGGGTCCTTGCTGTAGTCT; ITIH2, F: CAACCAGGTCTCCACTCCAT, R: AATCCTGCAAGTCGTCCATC; AMBP, F: AGTGGTACAACCTGGCCATC, R: AAGCTCCAGACGTCTCCTCA); TNFAIP6, F: ACCACAGAGAAGCACGGTCT, R: CAACTCTGCCCTTAGCCATC); and GAPDH, F: GAGTCAACGGATTTGGTCGT, R: TTGATTTTGGAGGGATCTCG).
Primary chondrocyte cultures
Primary chondrocytes were obtained from the normal appearing lateral tibial articular cartilage from OA knee joints. Cartilage tissue was diced into pieces of approximately 2×2 mm and digested in 0.1% (weight/volume) collagenase type II for 16–24 hrs at 37°C. The harvested chondrocytes were resuspended in DMEM/F12 culture media (Thermo scientific, IL) containing 4.5 mg/L glucose (Sigma-Aldrich, Mo), 10% (v/v) fetal bovine serum (Thermo scientific, IL), 50 mg/L ascorbic acid (Sigma-Aldrich, Mo), 10 mM HEPES (Thermo scientific, IL), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, Mo) and cultured in a humidified, 5% CO2 incubator for 5–7 days until 80% confluent. These passage-one chondrocytes were subcultured onto 6 well-plates and incubated until 80% confluent when they were treated with or without human IL-1ß (0.05, 0.5, 1, and 10 ng/ml; R&D, MN) or 20 ng/ml TNFα (R&D, MN) for 24 hours.
Matrix Assembly Assays
To quantify binding of HA Binding Protein (HABP) and aggrecan to HA, high molecular weight HA, biotinylated through a long chain spacer (1500 kDa, HA-609, CreativePEGworks, NC; 100ng HA for HABP assay and 10ng HA for aggrecan assay), was coated for 2 hours at room temperature onto wells of NeutrAvidin high capacity plates (Thermo Scientific, IL). HA coated plates were washed three times (and between all incubation steps) with 200 μl TBST. HA coated wells were incubated for 2 hours at 37°C with and without the following reaction mixtures in 20mM HEPES, 137mM NaCl buffer, pH 7.4: recombinant human TSG-6 protein (for HABP assay 1, 10, 20 and 40nM; for aggrecan assay 30nM); and 20nM purified human IαI (Athens Research& Technology, GA). To quantify HABP binding, wells were incubated for 1 hour at room temperature with HRP-conjugated HABP (bovine cartilage origin from Corgenix, Colorado), followed by 100 μl TMB substrate (Pierce, Thermo Scientific, IL) for 20 minutes at room temperature, then 50 μl stop solution (Thermo Scientific, IL) with absorbance at 450 nm measured to reflect the amount of HABP bound to HA. To quantify aggrecan binding, wells were incubated for 1 hour at room temperature with 1 μg purified aggrecan (A1D1 fraction of bovine origin prepared as described previously[34–36]), followed by incubation for 1 hour at room temperature with anti-keratan sulfate monoclonal antibody (5D4, in house) diluted 1:10,000 in TBST as described previously[35,36]) followed by incubation with anti-mouse IgG secondary antibody with HRP conjugate (Thermo scientific; IL, 1:500 in TBST) for 1 hour at room temperature then 100 μl TMB substrate for 30 minutes at room temperature, then 50 μl stop solution, with absorbance at 450 nm measured to reflect the amount of aggrecan bound to HA.
Statistical Analyses
With the exception of MMP-3, TSG-6 protein and RNA (−ΔCt), values of all inflammatory biomarkers and TSG-6 activity approximated a normal distribution upon log10 transformation. Pearson’s correlation coefficient was used to test the associations of TSG-6 activity with the other biomarkers with the exception of MMP-3, TSG-6 protein and RNA for which Spearman’s correlation coefficients were used. Significant correlations were identified with control of the family-wise Type I error rate with Holm–Bonferroni step-down adjusted P-values[37]. This procedure controlled the family-wise Type I error rate accounting for 47 tests. Paired t-tests were used to assess differences in mean levels of expression for each gene and TSG-6 activity between damaged and undamaged cartilage and primary chondrocytes with varying levels of cytokines. P values <0.05 were considered significant.
Results
Performance of optimized TSG-6 activity assay
Our optimized TSG-6 assay demonstrated both dose (Supplementary Figure 1B, using 0–6.6 nM of spiked in TSG-6) and time (Supplementary Figure 1C, using 6.6 nM spiked in TSG-6 followed for 16 hours) dependent increases in TSG-6 mediated heavy chain transfer activity. As shown by Western blot, as IαI was depleted from the media (top panel), HCs (mainly HC1) were increasingly transferred to immobilized HA on the plate (middle panel). Transferred HC2, and HC3 signals were too weak to be detected although the antibodies used (Atlas HPA059150 and PA5-22232, respectively) could detect these proteins by Western Blot as part of IαI (HC2 containing) or PαI (HC3 containing) demonstrating the reliability of the antibodies (data not shown). In contrast to our earlier assay format[31], this optimized TSG-6 activity assay protocol reduced the time for plate preparation from 1 day to 2 hours, and eliminated one incubation step without loss of sensitivity by use of the TMB substrate. Nevertheless, results of the prior and current assay format were highly correlated (R=0.83, p=0.0019; Supplementary Figure 2). Synovial fluids from OA knees contained HA-HC, IαI, and TSG-6. HCs could be transferred to immobilized HA by endogenous IαI and TSG-6 in SF (Supplementary Figure 1D). TSG-6 activity of OA SF was significantly positively correlated with the concentration of endogenous TSG-6 protein as measured by ELISA (Spearman correlation R=0.6382, P=0.0006) (Figure 1H).
Figure 1. Correlations of TSG-6 activity and inflammatory mediators in human OA SF in EC20 cohort.

(A–B) Correlation of TSG-6 activity with protease inhibitors: TIMP-1 (R=0.890, P<0.0001) A2M (R=0.831, P<0.0001). (C–E) Correlation of TSG-6 activity with inflammatory mediators: VEGF (R=0.734, and P<0.0001), VCAM-1 (R=0.730, and P<0.0001) and ICAM-1 (R=0.688, and P=0.0001). (F) Correlation of TSG-6 activity with MMP3 (protease). Spearman’s correlation R=0.763, and P<0.0001. (G) Correlation of TSG-6 activity with cytokine IL-6. Pearson’s correlation R=0.683, and P=0.0002). (H) TSG-6 activity in SFs significantly correlated with the concentration of TSG-6 protein as measured by ELISA (assessed using Spearman correlation, n=25). The markers that were fully measurable but that were not significantly associated with TSG-6 activity were listed in the supplementary table-1.
TSG-6 activity is a global inflammatory marker
There was a strong association of TSG-6 activity with inflammatory biomarkers in OA SFs. In the OA EC20 cohort, levels of SF TSG-6 activity were significantly associated with the levels of 7 inflammatory biomarkers (P values passed the Holm–Bonferroni correction), including Tissue inhibitor of metalloprotease-1 (TIMP-1, R=0.84, P<0.0001), Alpha-2 macroglobulin (A2M, R=0.82, P<0.0001), Stromelysin-1 (MMP3, R=0.76, P<0.0001), vascular endothelial growth factor (VEGF, R=0.73, P<0.0001), Vascular cell adhesion protein-1 (VCAM-1, R=0.73, P<0.0001), Intercellular adhesion molecule-1 (ICAM-1, R=0.69, P=0.0001) and Interleukin-6 (IL-6, R=0.68, P=0.0002) (Figure 1). Although TSG-6 is known to potentiate the anti-plasmin activity of IαI[6], the presence of HA or HA fragments blocked the anti-plasmin activity of IαI (with or without TSG-6, Supplementary Figure 3) suggesting that this known anti-inflammatory effect of TSG-6 is unlikely to be relevant in the context of an OA joint where HA fragments are abundant[38] and the binding sites for bikunin and HA on TSG-6 may overlap[25] that may also reduce its anti-plasmin effect.
Presence and origin of TSG-6 and HC1 in cartilage and chondrocytes
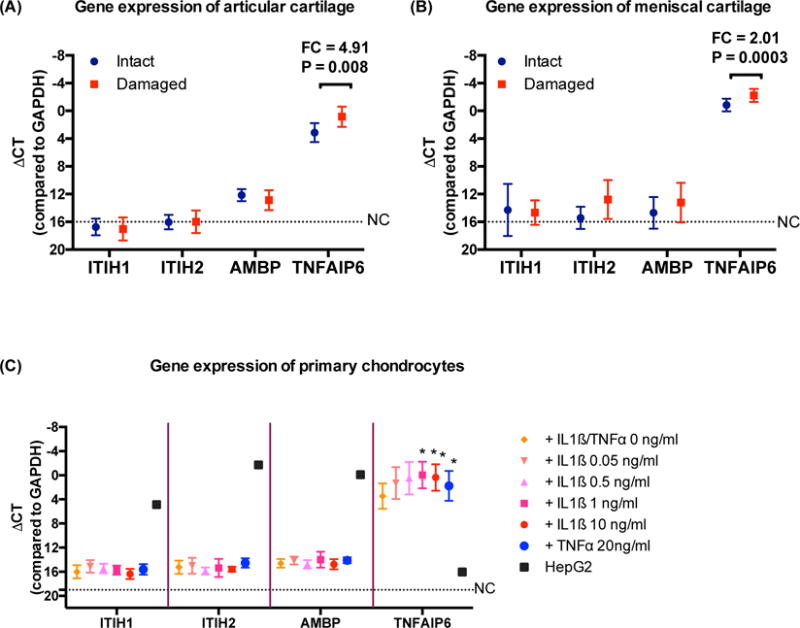
QRT-PCR was used to determine whether chondrocytes were capable of producing, in addition to TNFAIP6 (TSG-6), the three components of IαI[39]: ITIHI (HC1), ITIH2 (HC2), and AMBP (gene precursor for bikunin) (Figure 2). TSG-6 gene expression was significantly upregulated in chondrocytes from damaged compared with intact cartilage: fold-change (FC)=4.01, p=0.008 in articular cartilage; FC=2.01, p=0.0003 in meniscal cartilage. Gene expression of ITIH1 and ITIH2 was indistinguishable from the negative control in both damaged and intact articular and meniscal cartilages. AMPB expression was low in knee cartilage (damaged and intact) but undetectable in meniscal cartilage (Figure 2A and 2B). Cytokines (both IL-1ß and TNFα) greatly induced TSG-6 expression from primary chondrocytes but not ITIH1, ITIH2 or AMBP. Transcripts were successfully detected in the positive control HepG2 cells with each of the primer sets used in this study (Figure 2C).
Figure 2. Expression of TSG-6 and IαI complex related genes in OA cartilage from joint replacement and primary chondrocytes with or without cytokines treatment.
mRNAs were quantified by qRT-PCR in matched intact and damaged cartilages (20 tibial and 12 paired menisci) and primary chondrocytes (n=5) with or without IL1ß or TNFα treatment. TNFAIP6 (TSG-6) was highly and significantly differentially expressed in chondrocytes from damaged (red) compared to intact (blue) (A) tibial plateau cartilage and (B) meniscal cartilage and (C) primary chondrocytes treated with cytokines for 24 hrs. ITIH1, ITIH2, and AMBP (bikunin) were either not expressed or expressed at a low level in cartilage and chondrocytes, i.e. ~212 lower than GAPDH that was used as an endogenous control to normalize sample loading. NC represents the negative control (water in lieu of primers) with undetectable expression. FC=fold-change. P values were determined by paired t-test. Error bar = standard deviation.
Immunohistochemical staining of articular and meniscal cartilages was performed to determine the fate of transferred HC in vivo (Figure 3). Since TSG-6 is a secreted protein, as expected, high expression of TSG-6 could be found in both chondrocytes and the surrounding ECM; TSG-6 protein was detected by immunostaining at all depths of lesioned cartilage, was particularly abundant in the damaged surface regions of both articular and meniscal cartilages, but undetectable or minimally expressed in deep regions of healthy cartilage. Despite the gene expression not being detected in cartilage, HC1 was also abundant and co-localized with TSG-6 in surface regions of damaged articular and meniscal cartilages, was sparse in non-damaged cartilage, and absent in intact cartilage. Taken together with the gene expression data, these results suggest that HC1 is derived only from IαI in SF, and therefore can only transfer to HA in the surface zone of cartilage. The fact that HC1 co-localized with TSG-6 in cartilage suggests that the transfer activity is facilitated by cartilage TSG-6 that is upregulated in lesioned regions.
Figure 3. Immunolocalization of HC1 and TSG-6 in HA-rich matrices of intact and damaged regions of OA cartilages including tibial plateau and meniscus.
Antibodies against HC1 and TSG-6 were used to detect their protein expression (dark brown staining) by immunohistochemistry in the HA-rich matrices of cartilage from (A) tibial plateau and (B) meniscus. High expression levels of HC1 (yellow triangles) and TGS-6 (red arrows) were detected and co-localized on the surface of damaged cartilage, with little or none or much detected in intact cartilage. Non-immune IgGs were used as negative controls. All sections were counterstained light blue with hematoxylin. The scale bars are 0.3 mm.
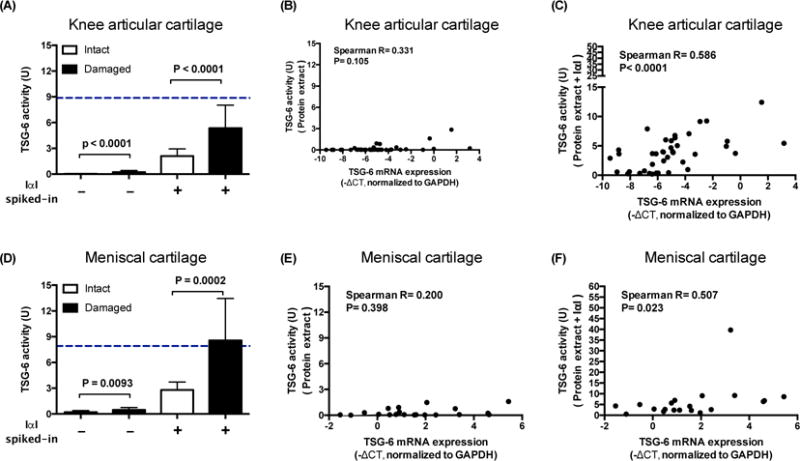
In order to test this hypothesis, proteins were extracted from articular and meniscal cartilage taken at the time of knee joint replacement for OA. TSG-6 activities were determined by incubating protein extracts with and without spiked-in exogenous IαI. Without exogenous IαI spiked-in, TSG-6 activities were low in all cartilage extracts but nevertheless, compared with intact cartilage, were significantly higher in damaged articular (p<−0.0001) and meniscal (p=0.0093) cartilage. With exogenous IαI spiked-in, TSG-6 activities increased significantly in all cartilage extracts and compared with intact cartilage, were consistently higher in damaged articular (p<0.0001) and meniscal (p=0.0002) cartilages (Figures 4A and 4D). There was no correlation of TSG-6 activity and TSG-6 gene expression until IαI was spiked-in (Figure 4B–C and 4E–F).
Figure 4. Addition of exogenous IαI increases TSG-6 activity of cartilage.

TSG-6 activities in protein extracts from cartilage with or without exogenous IαI and the correlations between TSG-6 activity and gene expression. Compared to intact cartilage, protein extracts from damaged cartilage had higher TSG-6 activities although the activities from both regions were low in (A) articular and (D) meniscal cartilage (intact articular 0.02±0.05 U, damaged articular 0.23±0.40 U; intact meniscal 0.20±0.35 U, damaged meniscal 0.46±0.57 U). With exogenous IαI spiked-in, TSG-6 activities of protein extracts increased significantly (p<0.0001 in each group between with and without IαI spiked-in) and were consistently higher in damaged compared with intact cartilage (intact articular 2.11±1.82 U, damaged articular 5.36±5.88 U; intact meniscal 2.79±1.80 U, damaged meniscal 8.57±9.51 U). Without IαI spiked-in, TSG-6 activity was not correlated with TSG-6 gene expression in the articular (B) or meniscal cartilage (E). With exogenous IαI spiked-in, TSG-6 activity increased and correlated with TSG-6 gene expression in both articular (C) and meniscal (F) cartilages. In addition, with exogenous IαI spiked-in, the TSG-6 activities of damaged cartilages were similar to the mean TSG-6 activity levels in OA synovial fluids (A and D, blue dotted lines). These data demonstrate that endogenous IαI is rate limiting for TSG-6 activity in cartilage. Error bar = standard deviation.
TSG-6 in the absence of IαI blocks matrix assembly
To assess potential effects of TSG-6 on ECM assembly, HABP or purified aggrecan were added to immobilized HA, pre-incubated with recombinant human TSG-6 alone, or TSG-6 in combination with IαI to induce heavy chain transfer to HA. The presence of TSG-6 protein alone resulted in a statistically significant dose-dependent inhibition of HABP and aggrecan binding to immobilized HA (Figure 5). Pre-incubation of TSG-6 and IαI to create HA-HC resulted in significant retention of the HA binding capacity for HABP and aggrecan.
Figure 5.

Formation of HA-HC results in retained ability of HA to bind HA binding proteins. The consequence of generating HA-HC (TSG-6 activity in the presence of IαI) for matrix assembly was examined using (A) HA binding protein (HABP) and (B) aggrecan (A1D1) binding assays. Pre-incubation of HA with increasing (A) or high (B) concentrations of TSG-6 significantly reduced binding of HABP and aggrecan to immobilized HA, respectively. HA-HC formation resulted in retained ability of immobilized HA to bind HABP and partially but significantly restored the ability of aggrecan to bind HA. Dotted and dashed lines in A represent the OD values of negative controls consisting of HA pre-incubated with buffer and 20nM IαI, respectively. These results suggest a negative effect of TSG-6 in the absence of IαI on matrix assembly capabilities. Paired t-test. **P<0.001; ***P<0.0001.
Discussion
TSG-6 reduced binding of HABP and aggrecan to HA suggesting it may have deleterious effects on cartilage matrix assembly in the context of OA due to TSG-6 being one of the most highly up-regulated genes in OA cartilage[7]. This negative effect was prevented by the presence of HC on HA suggesting that the presence of IαI is essential for preserving the matrix assembly capacity of HA. The transfer of HC to HA may serve to cross-link and maintain HA a more open and permissive structure for matrix assembly than that formed by HA in the absence of HC. Our data demonstrate that HC originates from outside cartilage and is transferred only to the surface of damaged articular cartilage. This suggests the hypothesis that high TSG-6 in the intermediate and deep zones of OA cartilage may, lacking HC, block matrix assembly and be responsible for ‘frustrated’ or ‘futile synthesis’, the process of new ECM protein synthesis but lack of protein retention in the matrix. This may in part help explain our prior observation of an association of SF TSG-6 activity with OA progression.
Our studies demonstrated that TSG-6, but not TSG-6 HC transfer activity, is increased in OA cartilage and may have a net negative effect on matrix assembly (Figure 6). Based on the demonstration 20 years ago that TSG-6 could bind to both HA and aggrecan in a pH dependent manner (strongest at low pH), the possible involvement of TSG-6 in matrix dissociation was proposed[15]. Taken together with our data, stronger inhibition of matrix assembly would be expected in inflammatory conditions due to higher expression of TSG-6 and lower pH under inflammatory conditions[15,40,41]. The preferential binding of TSG-6 to HA in low pH microenvironments (pH6) has also been postulated to potentially facilitate the transfer of newly synthesized aggrecan to higher pH microenvironments where aggrecan-HA complexes would be expected to predominate to provide a chondroprotective stimulus[Heng 2008]. Since chondrocytes reside in an acidic ECM, with further acidosis occuring in joint disease due to hypoxia and production of inflammatory cytokines[42], the physiological relevance of potential aggrecan shuttling in the matrix is unclear[41].
Figure-6. The potential role of TSG-6 in joint disease.

TSG-6 can come from cartilage or synovial macrophages in response to inflammatory mediators, including IL-1ß and TNFα. The anti-plasmin effect of IαI-TSG-6 was minor due to the presence of HA in knee joints. High expression of TSG-6 in full thickness cartilage damaged by OA causes ECM disruption due to increasing HA condensation and reduced ECM assembly. In conjunction with IαI, TSG-6-mediated heavy chain transfer activity (TSG-6 activity) could mitigate the negative effects of TSG-6 on cartilage matrix assembly and turnover. TSG-6 may promote pathology in cartilage in OA knees. However, the surface disruption that occurs from OA damage exposes the cartilage to IαI from the synovial fluid and enables TSG-6 activity to mitigate the damage.
SF TSG-6 activity also appears to be a global marker of inflammation in OA knee. Inflammation has been associated with incidence and progression of knee OA[43,44]. However, the primary effects of TSG-6 in chronic OA appear to be different than in the context of acute inflammation of acute joint injury or autoimmune arthritis. For instance, elevation of SF TSG-6 within the first 8 weeks after severe knee joint injury in humans was associated with better clinical outcomes at 3 months in the Oxford KICK study[11]. Murine models of autoimmune arthritis are also associated with anti-inflammatory and chondroprotective effects of TSG-6. TSG-6–knockout mice, for example, have enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis[45]. Treatment of collagen induced arthritis with recombinant TSG-6, or TSG-6 produced locally by T cells in the arthritic joints of transgenic mice, reduced disease incidence and potently inhibited inflammation and joint destruction; the level of improvement was comparable to that seen with anti-TNF antibody treatment[29,46,47]. To investigate its chondroprotective effect, Glant et al. generated transgenic mice expressing murine TSG-6 specifically in cartilage[48]. The induction of adjuvant induced arthritis (AIA) in these mice resulted in severe joint inflammation, but their cartilage remained intact for at least 1 week (control mice suffered major damage from day 5) and both loss of aggrecan and accumulation of MMP-generated fragments were reduced. Furthermore, after 4–5 weeks, TSG-6 transgenic mice were free of local inflammation and their cartilage was almost fully repaired (which was not the case in controls). These anti-inflammatory and chondroprotective effects have only been demonstrated in the context of acute inflammation in animal models and have not yet been tested in a clinical trial setting. Possibly, expression of TSG-6 might only protect cartilage by blocking chemotactic signals rather than repairing the integrity of cartilage already affected by arthritis[16,17,29,45,46].
The presence of soluble HA decreased the anti-plasmin activity of the combination of TSG-6 and IαI (Supplementary Figure 2). Since the N-terminal half of the TSG-6 protein (the LINK module) can bind both HA and chondroitin sulfate (present in numerous cartilage matrix components released to SF as well as in IαI)[15,49], the presence of HA and chondroitin sulfate in the fluid could sequester the TSG-6 protein and decrease the chance of TSG-6 interactions with IαI and thereby reduce the potentiation of anti-plasmin activity. This mechanism is consistent with the known overlap of bikunin and HA binding sites on TSG-6[15,25]. Taken together, the overall anti-inflammatory effects of the TSG-6 in combination with IαI in the OA joint may be relatively minor, particularly in the context of cartilage and SF that are both rich in HA and chondroitin sulfate. These results may explain why high TSG-6 activity in the context of established OA is not associated with a protective effect[31].
Transcripts of HC1, HC2, and HC3 but not AMBP were previously reported in human cartilage[50]; chain-specific HC1, HC2, HC3 and bikunin proteins were also identified in the surface of normal and OA cartilage by immunostaining[50]. Based on these results, an articular cartilage origin of HA-HCs was concluded. Our data, based on quantitative PCR, also demonstrated no expression of bikunin and little or no expression of HCs in cartilage, suggesting that HCs originate from outside cartilage with protein ‘capture’ in the surface of damaged articular cartilage as HA-HC due to the HC transfer activity of TSG-6 that is abundant and upregulated in the surface of damaged cartilage. Our study used total RNA from full depth articular cartilage for detection of IαI and TSG-6 gene expression; we therefore cannot definitively exclude the possibility that surface zone chondrocytes produce HA-HC–further investigation is needed.
In summary, SF TSG-6 activity appears to be a global marker of inflammation in the OA joint. This may in part explain our prior association of SF TSG-6 activity with OA progression. In addition, the ability of TSG-6 to block formation of hyaluronan-aggrecan complexes provides another possible mechanism underlying the association of SF TSG-6 with OA progression. Given the high expression of TSG-6 in OA cartilage, absence of IαI gene expression and only rate-limiting IαI protein concentrations provided from SF, it would be expected that TSG-6 could interfere with matrix assembly, particularly in the intermediate and deep layers of OA cartilage. Although TSG-6 is known to be anti-chemotactic for neutrophils and has been demonstrated to have other beneficial effects related to OA pathogenesis, the association of TSG-6 with inflammatory markers, and its potential to disrupt matrix production in OA cartilage suggest that the role TSG-6 plays in the context of OA pathogenesis is complex.
Supplementary Material
Acknowledgments
DAKO A0301 antibody was kindly provided by Dr. Mark Lauer from Cleveland clinic.
Role of the funding source
This study was supported by NIH/NIA P30-AG-028716.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
CH Chou carried out all experiments and data analysis, statistical analysis, histological evaluations and drafted the manuscript. DE Attarian participated in sample collection and editing of the manuscript. HG Wisniewski, P Band and VB Kraus conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final version to be published. CH Chou and VB Kraus had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interest statement
The authors declare that they have no competing interests.
References
- 1.Kehlen A, Pachnio A, Thiele K, Langner J. Gene expression induced by interleukin-17 in fibroblast-like synoviocytes of patients with rheumatoid arthritis: upregulation of hyaluronan-binding protein TSG-6. Arthritis Res Ther. 2003;5:R186–192. doi: 10.1186/ar762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee TH, Lee GW, Ziff EB, Vilcek J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol Cell Biol. 1990;10:1982–1988. doi: 10.1128/mcb.10.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier R, Wisniewski HG, Vilcek J, Lotz M. TSG-6 expression in human articular chondrocytes. Possible implications in joint inflammation and cartilage degradation. Arthritis Rheum. 1996;39:552–559. doi: 10.1002/art.1780390403. [DOI] [PubMed] [Google Scholar]
- 4.Robinson LJ, Tourkova I, Wang Y, Sharrow AC, Landau MS, Yaroslavskiy BB, et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochemical and biophysical research communications. 2010;394:12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshioka S, Ochsner S, Russell DL, Ujioka T, Fujii S, Richards JS, et al. Expression of tumor necrosis factor-stimulated gene-6 in the rat ovary in response to an ovulatory dose of gonadotropin. Endocrinology. 2000;141:4114–4119. doi: 10.1210/endo.141.11.7784. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol. 1996;156:1609–1615. [PubMed] [Google Scholar]
- 7.Chou CH, Lee MT, Song IW, Lu LS, Shen HC, Lee CH, et al. Insights into osteoarthritis progression revealed by analyses of both knee tibiofemoral compartments. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997;8:143–156. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 9.Bayliss MT, Howat SL, Dudhia J, Murphy JM, Barry FP, Edwards JC, et al. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage. 2001;9:42–48. doi: 10.1053/joca.2000.0348. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney DJ, Swales C, Athanasou NA, Bombardieri M, Pitzalis C, Kliskey K, et al. TSG-6 inhibits osteoclast activity via an autocrine mechanism and is functionally synergistic with osteoprotegerin. Arthritis Rheum. 2011;63:1034–1043. doi: 10.1002/art.30201. [DOI] [PubMed] [Google Scholar]
- 11.Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklatvala J, et al. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical Outcomes. Arthritis Rheumatol. 2016;68:2129–2140. doi: 10.1002/art.39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burleigh A, Chanalaris A, Gardiner MD, Driscoll C, Boruc O, Saklatvala J, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64:2278–2288. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 13.Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- 14.Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Jowitt TA, Day AJ, Prestegard JH. Nuclear Magnetic Resonance Insight into the Multiple Glycosaminoglycan Binding Modes of the Link Module from Human TSG-6. Biochemistry. 2016;55:262–276. doi: 10.1021/acs.biochem.5b01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getting SJ, Mahoney DJ, Cao T, Rugg MS, Fries E, Milner CM, et al. The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-alpha -inhibitor-independent manner. J Biol Chem. 2002;277:51068–51076. doi: 10.1074/jbc.M205121200. [DOI] [PubMed] [Google Scholar]
- 17.Dyer DP, Salanga CL, Johns SC, Valdambrini E, Fuster MM, Milner CM, et al. The Anti-inflammatory Protein TSG-6 Regulates Chemokine Function by Inhibiting Chemokine/Glycosaminoglycan Interactions. J Biol Chem. 2016;291:12627–12640. doi: 10.1074/jbc.M116.720953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer DP, Thomson JM, Hermant A, Jowitt TA, Handel TM, Proudfoot AE, et al. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J Immunol. 2014;192:2177–2185. doi: 10.4049/jimmunol.1300194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briggs DC, Birchenough HL, Ali T, Rugg MS, Waltho JP, Ievoli E, et al. Metal Ion-dependent Heavy Chain Transfer Activity of TSG-6 Mediates Assembly of the Cumulus-Oocyte Matrix. J Biol Chem. 2015;290:28708–28723. doi: 10.1074/jbc.M115.669838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- 21.Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, et al. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001;276:7693–7696. doi: 10.1074/jbc.C000899200. [DOI] [PubMed] [Google Scholar]
- 22.Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, et al. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281:1154–1160. doi: 10.1006/bbrc.2001.4475. [DOI] [PubMed] [Google Scholar]
- 23.He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013;288:25792–25803. doi: 10.1074/jbc.M113.479584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shay E, He H, Sakurai S, Tseng SC. Inhibition of angiogenesis by HC.HA, a complex of hyaluronan and the heavy chain of inter-alpha-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52:2669–2678. doi: 10.1167/iovs.10-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney DJ, Mulloy B, Forster MJ, Blundell CD, Fries E, Milner CM, et al. Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparin: implications for the inhibition of plasmin in extracellular matrix microenvironments. J Biol Chem. 2005;280:27044–27055. doi: 10.1074/jbc.M502068200. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Garcia S, Carrion M, Jimeno R, Ortiz AM, Gonzalez-Alvaro I, Fernandez J, et al. Urokinase plasminogen activator system in synovial fibroblasts from osteoarthritis patients: modulation by inflammatory mediators and neuropeptides. J Mol Neurosci. 2014;52:18–27. doi: 10.1007/s12031-013-0189-z. [DOI] [PubMed] [Google Scholar]
- 27.Jin T, Tarkowski A, Carmeliet P, Bokarewa M. Urokinase, a constitutive component of the inflamed synovial fluid, induces arthritis. Arthritis Res Ther. 2003;5:R9–R17. doi: 10.1186/ar606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 29.Mindrescu C, Dias AA, Olszewski RJ, Klein MJ, Reis LF, Wisniewski HG. Reduced susceptibility to collagen-induced arthritis in DBA/1J mice expressing the TSG-6 transgene. Arthritis Rheum. 2002;46:2453–2464. doi: 10.1002/art.10503. [DOI] [PubMed] [Google Scholar]
- 30.Nagyeri G, Radacs M, Ghassemi-Nejad S, Tryniszewska B, Olasz K, Hutas G, et al. TSG-6 protein, a negative regulator of inflammatory arthritis, forms a ternary complex with murine mast cell tryptases and heparin. J Biol Chem. 2011;286:23559–23569. doi: 10.1074/jbc.M111.222026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wisniewski HG, Colon E, Liublinska V, Karia RJ, Stabler TV, Attur M, et al. TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:235–241. doi: 10.1016/j.joca.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou CH, Lee CH, Lu LS, Song IW, Chuang HP, Kuo SY, et al. Direct assessment of articular cartilage and underlying subchondral bone reveals a progressive gene expression change in human osteoarthritic knees. Osteoarthritis Cartilage. 2013;21:450–461. doi: 10.1016/j.joca.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bello AE, Garrett WE, Jr, Wang H, Lohnes J, DeLong E, Caterson B, et al. Comparison of synovial fluid cartilage marker concentrations and chondral damage assessed arthroscopically in acute knee injury. Osteoarthritis Cartilage. 1997;5:419–426. doi: 10.1016/s1063-4584(97)80046-5. [DOI] [PubMed] [Google Scholar]
- 35.Seifer DR, Furman BD, Guilak F, Olson SA, Brooks SC, 3rd, Kraus VB. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage. 2008;16:1532–1538. doi: 10.1016/j.joca.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 37.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 38.Band PA, Heeter J, Wisniewski HG, Liublinska V, Pattanayak CW, Karia RJ, et al. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthritis Cartilage. 2015;23:70–76. doi: 10.1016/j.joca.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bost F, Diarra-Mehrpour M, Martin JP. Inter-alpha-trypsin inhibitor proteoglycan family–a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem. 1998;252:339–346. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- 40.Parkar AA, Kahmann JD, Howat SL, Bayliss MT, Day AJ. TSG-6 interacts with hyaluronan and aggrecan in a pH-dependent manner via a common functional element: implications for its regulation in inflamed cartilage. FEBS Lett. 1998;428:171–176. doi: 10.1016/s0014-5793(98)00523-7. [DOI] [PubMed] [Google Scholar]
- 41.Heng BC, Gribbon PM, Day AJ, Hardingham TE. Hyaluronan binding to link module of TSG-6 and to G1 domain of aggrecan is differently regulated by pH. J Biol Chem. 2008;283:32294–32301. doi: 10.1074/jbc.M804155200. [DOI] [PubMed] [Google Scholar]
- 42.Peter I, Milner RJW, John S Gibson. Cellular Physiology of Articular Cartilage in Health and Disease. In: Rothschild BM, editor. Principles of Osteoarthritis- Its Definition, Character, Derivation and Modality-Related Recognition. Vol. 2012. pp. 567–590. [Google Scholar]
- 43.Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75:390–395. doi: 10.1136/annrheumdis-2014-205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daghestani HN, Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1890–1896. doi: 10.1016/j.joca.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szanto S, Bardos T, Gal I, Glant TT, Mikecz K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004;50:3012–3022. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
- 46.Mindrescu C, Thorbecke GJ, Klein MJ, Vilcek J, Wisniewski HG. Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein. Arthritis Rheum. 2000;43:2668–2677. doi: 10.1002/1529-0131(200012)43:12<2668::AID-ANR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Bardos T, Kamath RV, Mikecz K, Glant TT. Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-alpha-stimulated gene-6) in murine models of experimental arthritis. Am J Pathol. 2001;159:1711–1721. doi: 10.1016/s0002-9440(10)63018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glant TT, Kamath RV, Bardos T, Gal I, Szanto S, Murad YM, et al. Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor alpha-stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 2002;46:2207–2218. doi: 10.1002/art.10555. [DOI] [PubMed] [Google Scholar]
- 49.Wisniewski HG, Snitkin ES, Mindrescu C, Sweet MH, Vilcek J. TSG-6 protein binding to glycosaminoglycans: formation of stable complexes with hyaluronan and binding to chondroitin sulfates. J Biol Chem. 2005;280:14476–14484. doi: 10.1074/jbc.M411734200. [DOI] [PubMed] [Google Scholar]
- 50.Yoshihara Y, Plaas A, Osborn B, Margulis A, Nelson F, Stewart M, et al. Superficial zone chondrocytes in normal and osteoarthritic human articular cartilages synthesize novel truncated forms of inter-alpha-trypsin inhibitor heavy chains which are attached to a chondroitin sulfate proteoglycan other than bikunin. Osteoarthritis Cartilage. 2008;16:1343–1355. doi: 10.1016/j.joca.2008.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


