Abstract
Dengue is the most common arboviral infection of humans, responsible for a substantial disease burden across the tropics. Traditional insecticide-based vector control programmes have limited effectiveness and the one licensed vaccine has a complex and imperfect efficacy profile. Strains of the bacterium Wolbachia deliberately introduced into Aedes aegypti have been shown to be able to spread to high frequencies in mosquito populations in release trials, and mosquitoes infected with these strains show markedly reduced vector competence. Thus, Wolbachia represents an exciting potential new form of biocontrol for arboviral diseases, including dengue. Here we review how mathematical models give insight into the dynamics of the spread of Wolbachia, its potential impact on dengue transmission and discuss remaining evaluation and development challenges.
The need for better dengue control
Dengue is an acute systemic disease caused by one of four genetically related but antigenically distinct serotypes (DENV1-4), and is the most prevalent human arboviral infection globally, with almost half of the world’s population at risk of infection [1,2]. Since 1943, when the first dengue virus serotype was isolated in Japan, dengue transmission has followed the rapid geographical expansion of its most competent vector, the Aedes aegypti mosquito, causing a substantial and ever-increasing public health and economic burden throughout the tropics [3–5]. In the absence of specific antiviral treatment and, until recently, a vaccine against dengue, disease control has long relied on the suppression of mosquito populations through environmental management, biological control and chemical interventions [6]. If rigorously applied, these traditional forms of control can be effective in reducing the size of mosquito populations and decreasing the burden of dengue at small spatial scales in the short term. Nonetheless, there is increasing evidence that such measures are both ineffective and unsustainable in the long term, with mosquito populations returning to pre-treatment levels after control interventions cease [7]. In addition, the implementation of large scale insecticide-based programmes gives rise to ecological and environmental concerns, including promoting the evolution and spread of insecticide resistance, which appears to be emerging in several countries, thus causing further detrimental effects on dengue control [8]. Several dengue vaccines are currently in development [9,10] and the first dengue vaccine (Dengvaxia), developed by Sanofi-Pasteur, was licensed in 2015. However, Dengvaxia has a complex efficacy profile, with questions around its long-term safety and efficacy in dengue-seronegative recipients [11–13]. Vaccination is therefore currently recommended exclusively in moderate to high-transmission settings [14], where 80% vaccination coverage in 9-year olds has been predicted to reduce symptomatic disease and hospitalisations by 20–30% at most [15,16]. Hence, there remains an urgent need for new interventions which have the potential to substantially reduce dengue transmission and disease burden across a wide range of settings.
Two new approaches have been recently tested in field trials. The first is based on the release of genetically modified male mosquitoes that carry a dominant lethal gene. This strategy has been shown to be able to supress mosquito populations, but requires the continuous release of large numbers of transgenic mosquitoes for several months [17]. However, in the absence of a gene drive system, questions remain about the sustainability of this intervention, as the migration of unmodified mosquitoes from neighbouring areas following local suppression of a mosquito population requires releases to be continued indefinitely, albeit at lower levels than during initial suppression [18].
The second approach involves the release of mosquitoes transinfected with the vertically transmitted intracellular bacterium Wolbachia. Wolbachia has been shown to be able to establish itself in mosquito populations [19] and to suppress arbovirus replication in mosquitoes, and so is a potentially promising means to controlling dengue transmission in endemic settings [20]. Here we review the milestones in the development of Wolbachia transinfection in Aedes aegypti as a tool for dengue control, with a focus on the mathematical models developed to date to study the invasion dynamics of Wolbachia and its impact on dengue transmission. We discuss the uncertainties and challenges that lie ahead for the use of Wolbachia as a potential new form of biocontrol for dengue.
Wolbachia transinfection
Wolbachia is an endosymbiotic intracellular bacterium that is naturally present in about 60% of all insect species [21,22], including some mosquitoes. Mosquito species known to be naturally infected with Wolbachia include Culex pipiens [23] and Aedes albopictus [24], one of the vectors for dengue virus, but not Aedes aegypti, the primary vector for this virus.
Wolbachia is transmitted maternally and alters the reproductive phenotype of infected insects to give the bacteria a reproductive advantage relative to uninfected insects. The reproductive phenotype expressed depends on both the insect species and Wolbachia strain, and can result in feminisation, male killing, parthenogenesis or, most commonly, cytoplasmic incompatibility [21].
Cytoplasmic incompatibility (CI), the phenotype expressed by the Wolbachia strains developed for Aedes aegypti transfection, renders eggs laid as the result of an uninfected female and infected male cross unviable, whereas Wolbachia-infected females lay viable Wolbachia-positive eggs regardless of the infection status of the male (Figure 1) [21]. However, depending on the Wolbachia strain, infection can impose additional fitness costs on infected insects, such as increased adult mortality [25], reduced fecundity [25], prolonged egg and larval development time [26], and reduced survival of desiccated eggs [27]. This leads to a trade-off between the reproductive advantage bestowed on Wolbachia-infected mosquitoes (relative to wild type) by CI and the fitness costs of infection, resulting in a frequency dependent invasion threshold which must be exceeded to ensure fixation of Wolbachia in a wild population [28]. This threshold will also depend on the degree of vertical transmission among Wolbachia-infected mosquitoes [29,30].
Figure 1. Invasion dynamics of Wolbachia.
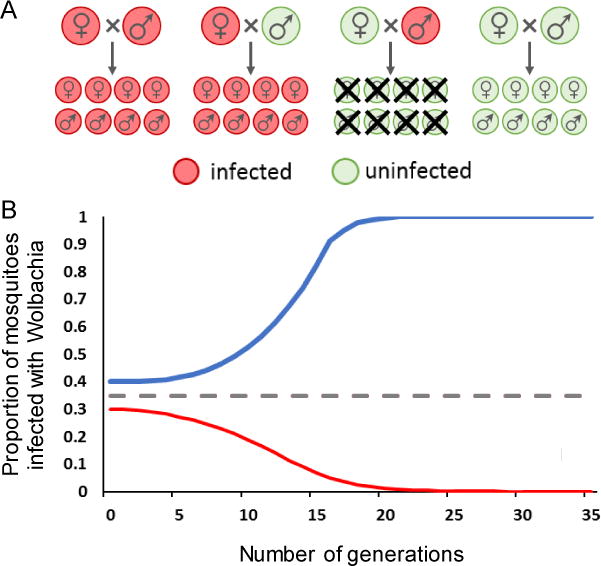
A. Illustration of how cytoplasmic incompatibility (CI) gives Wolbachia-infected females a reproductive advantage by making the progeny of infected males and wild-type females non-viable. B. CI thus allows Wolbachia-infected mosquitoes to displace wild-type (blue curve) if introduced into a population above a threshold frequency (dashed line) which is determined by the fitness costs of Wolbachia infection. If introduced below this threshold frequency (red curve), Wolbachia-infected insects are out-competed by wild-type, despite CI.
Although not naturally present in Aedes aegypti, Wolbachia was introduced into the species artificially via transinfection. This was demonstrated for the first time in 2005 by Xi et al [31], when they isolated Wolbachia-containing cytoplasm from Aedes albopictus eggs, which are naturally infected with both wAlbA and wAlbB strains, and used microinjection to transfer the bacteria into Aedes aegypti eggs, resulting in two stable wAlbB-infected Aedes aegypti lines. Laboratory experiments showed almost perfect maternal transmission and CI in these lines. The initial rationale for introducing Wolbachia into Aedes aegypti mosquitoes was to demonstrate that CI could be used as a drive mechanism to introduce transgenes into an Aedes aegypti population, rather than make use of the properties of Wolbachia itself as a dengue control measure. However, based on the observation that Drosophila melanogaster fruit flies infected with the virulent wMelPop strain have increased adult mortality [32], it was hypothesised that the translation of these fitness costs to Aedes aegypti might reduce population density and the mean lifespan of an infected mosquito (possibly to below the extrinsic incubation period of dengue virus), thus greatly reducing their potential to transmit the virus onwards after an infectious blood meal [33].
In 2009, transinfection of the wMelPop Wolbachia strain from Drosophila melanogaster to Aedes aegypti eggs was demonstrated by McMeniman et al [33]. Experiments showed a 19% reduction in fecundity and an over 50% increase in adult mortality due to wMelPop infection in Aedes aegypti. However, while initial cage invasion experiments [34] showed that with a high initial release fraction, wMelPop-infected mosquitoes can invade wild-type populations, the high fitness costs raised concerns about the viability of establishing wMelPop in the field. Thus in parallel to the work on wMelPop, a second less virulent Wolbachia strain, wMel [35], was also transinfected from Drosophila to Aedes aegypti [34], with experiments showing almost perfect CI and maternal transmission, but much lower (non-significant) fecundity and mortality costs of only 10% (vs 50% with wMelPop).
More unexpectedly, both wMel and wMelPop infection was demonstrated to dramatically reduce vector competence; wMelPop-infected mosquitoes fed with blood spiked with DENV2 virus showed no evidence of dengue virus in their salivary glands after 14 days, compared with 81% of wild-type mosquitoes [34]. Virus replication was also dramatically reduced in wMel-infected Aedes aegypti, albeit not as completely as for wMelPop, with 4.2% of mosquitoes showing DENV2 virus in their saliva 14 days after blood-feeding [34].
However, further examination revealed that each pooled sample of saliva showing DENV2 virus after 14 days contained saliva from wMel-uninfected mosquitoes, suggesting that imperfect maternal transmission may have contributed to these results [34].
Joubert et al. [36] have recently demonstrated that Wolbachia superinfection is possible, by generating a line of Aedes aegypti infected with both wMel and wAlbB and showing that the superinfection generates unidirectional CI when crossed to each single infected parental line, No significant differences in fitness were observed between the superinfected line and either single infected parental line, suggesting that the superinfection has the potential to replace single constituent Wolbachia strains already present in a mosquito population. Furthermore, upon challenge with blood meals from viremic dengue patients, the superinfected line was observed to also reduce dengue virus replication notably more efficiently than the parental wMel line [36].
From the laboratory to field releases
Cage experiments demonstrated that both wMelPop and wMel infected mosquitoes could invade wild-type populations and achieve high frequencies [33,34]. However, for Wolbachia to be a viable dengue control measure, it was necessary to demonstrate invasion was possible in field conditions. Field releases were also needed to understand the real-world dynamics of invasion and to optimise release strategies. Initial studies in two small communities near Cairns, Australia in January 2011 [19] involved the release of almost 300,000 wMel-infected mosquitoes in total over a period of 10 weeks. Counts of wMel-infected and wild type mosquitoes from traps collected 5 weeks after the end of releases indicated that the frequency of Wolbachia in the local Aedes aegypti population had reached 90%. Further analysis of the data [37] has also allowed researchers to explore the spatial patterns of Wolbachia abundance during the releases, including the effect of dwelling type and land cover on the proportion of Wolbachia-positive Aedes aegypti mosquitoes, which could guide the strategy of future releases. Follow-up studies indicated that Wolbachia infection is stable and persistent, with no measurable drift in maternal transmission or CI, and with long-term average infection frequencies over 94.0% [38]. Furthermore, they have indicated that Wolbachia infection continues to reduce vector competence following establishment in wild-type populations [39].
Field releases of wMelPop-infected Aedes aegypti were conducted near Cairns, Australia and Tri Nguyen, Vietnam [40]. In Australia, 15 releases took place in the wet season over a 4 month period starting in January 2012, with 6 additional releases carried out during the dry season in one location. In Vietnam, pupae (instead of adults) were released over a period of 23 weeks starting in April 2013, following an active Aedes aegypti suppression campaign. In all locations, the frequency of Wolbachia as measured from trapping data exceeded 90% by the end of the release period; however, by the end of the monitoring period (approximately 40–60 weeks post-initial release), the frequency had dropped to less than 20%, supporting the hypothesis that the fitness costs of wMelPop are too severe to be self-sustaining in wild populations. Following on from these releases, wMel-infected Aedes aegypti have been released into and successfully invaded all three locations within a shorter release window [40].
So far, all field releases have focused on entomological endpoints to establish the sustainability of wMel in wild Aedes aegypti populations. However, in order to demonstrate its viability as a dengue control measure, large scale releases are now occurring in Indonesia (https://clinicaltrials.gov/ct2/show/NCT03055585), Australia, Colombia and Brazil [41], Vietnam (http://www.eliminatedengue.com/vn/progress).
While in this article we focus on the use of Wolbachia as a population replacement strategy (i.e. permanently establishing Wolbachia infection in the Aedes aegypti population), the CI phenotype Wolbachia infection bestows can also be exploited as an alternative to genetic modification or radiation based sterilisation for population suppression strategies relying on the release of sterile males. The Singaporean National Environment Agency conducted small-scale field releases of Wolbachia-infected male mosquitoes, beginning in Singapore in 2016 (http://www.nea.gov.sg/public-health/environmental-public-health-research/wolbachia-technology/project-wolbachia-singapore/wolbachia-aedes-small-scale-field-study), to test the dispersal, longevity and competitiveness of Wolbachia-carrying mosquitoes compared to male Aedes aegypti in an urban setting. In addition, two US companies, MosquitoMate (http://mosquitomate.com) and Verily (https://verily.com) partnered to conduct a large scale population suppression trial using this approach in Florida in 2017. Similar approaches are also being adopted for Aedes albopictus suppression in Guangzhou, China (https://www.reuters.com/article/us-china-health-mosquitoes/chinas-mosquito-factory-aims-to-wipe-out-zika-other-diseases-idUSKCN10D0MV). However, a challenge with using Wolbachia as a sterile male technology is the need for extremely accurate sex-sorting of insects prior to release, since the accidental release of even a small proportion of Wolbachia-infected females poses the risk of turning a population suppression strategy into a population replacement one; i.e. the establishment of breeding Wolbachia-infected females in the area where the wild-type population has been supressed by the male releases.
Invasion dynamics of Wolbachia
The successful establishment and spread of Wolbachia in any given host population is dependent on the trade-off between the fitness benefits and costs incurred by infection with the bacteria. As first illustrated by Caspari and Watson [42] using a discrete generation population genetic model, this trade-off results in bistable dynamics, where two stable equilibria exist; one where infection frequency is zero and one where there is a high proportion of infected individuals (Figure 1B). To reach the non-zero equilibrium, infection frequency must exceed a critical threshold value, determined by the trade-off between the relative reduction in fecundity of infected females and the intensity of CI [29,30,42–44]. Extensions of this model predicted that if Wolbachia infection reduces the lifespan of infected hosts, a higher initial frequency of infected individuals may be needed for successful invasion [45]. In addition, if maternal transmission is not perfect, the speed of invasion may be reduced [46] and co-existence is more likely, as uninfected individuals are continually introduced into the host population [29,30]. Furthermore, when overlapping-generations are incorporated into models, the critical threshold frequency is seen to depend on the growth rate and age-structure of the population, in addition to the fitness effects of Wolbachia on the population [44]. Stochastic effects may also play an important role in the invasion dynamics, most notably when population sizes are small [47,48], as initial infection frequencies below the (deterministic) threshold frequency can lead to successful Wolbachia establishment [47] and conversely, frequencies above the threshold do not necessarily lead to establishment.
Although Wolbachia infection may establish locally, this does not necessarily guarantee spatial spread, as spread beyond the local environment depends on the initial infection frequency, the critical threshold frequency, the dispersal behaviour of the host population and features of the underlying environment [43,49–53]. Initial analysis by Turelli and Hoffmann showed that a critical frequency threshold of less than 0.5 is necessary for spatial spread to occur following local establishment [43]. Schraiber et al showed a similar result accounting for lifespan-shortening as well as fecundity reducing effects [54]. Other analysis showed that as the critical threshold approaches 0.5, wave speed slows dramatically, suggesting that, in practical terms, a critical threshold value of 0.35 or less is necessary for spatial spread [52,55]. The critical threshold value, along with the initial infection frequency and dispersal behaviour of the host population, determines the size of the release area necessary to initiate spatial spread, with larger release areas needed for higher threshold values (Figure 2A–B) [55]. Long-range dispersal, as compared with local (Gaussian) diffusion, is predicted to reduce both the size of the release area needed and the wave speed [52]. Environmental heterogeneities, such as sharp changes in population density or habitat quality, may also slow or halt invasion unless migration of infected individuals from neighbouring areas is sufficient to allow the critical threshold frequency to be exceeded at the local level [52,53,55,56].
Figure 2. Schematic illustration of factors influencing the spatial spread of Wolbachia in a mosquito population.
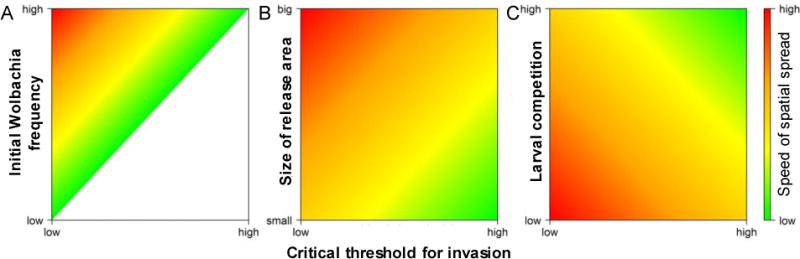
A. In the initial release area, the frequency of Wolbachia-infected mosquitoes needs to exceed the critical threshold for invasion (see Figure 1B) for invasion to occur. B. Wolbachia is more likely to establish and spread spatially if the initial release area is larger. C. More intense larval stage density-dependent competition slows initial establishment and spatial spread.
Models which consider the life cycle and demographics of mosquito populations have offered further insight into the dynamics of Wolbachia spread. Rather than primarily focusing on frequency thresholds for infection spread, population dynamics models instead consider how factors such as density-dependent competition, seasonal changes in abundance and population size affect the spread of Wolbachia through mosquito populations. The degree of density-dependent competition during the larval stage of population growth is predicted to have a considerable impact on the dynamics observed, with larger numbers of infected mosquitoes needing to be released to initiate spatial spread in populations subject to high levels of competition (Figure 2C) [57–59]. Last, seasonal variation in mosquito abundance is expected to affect establishment, with releases performed early in the wet season to coincide with early stages of population growth more likely to succeed [60]. Successful establishment in field settings will therefore depend on both the number of releases [52,58,60] and the type of release [60].
Predicted effect of Wolbachia on dengue transmission
The release of Wolbachia-infected Aedes aegypti mosquitoes has the potential to decrease, and in some settings, eliminate dengue transmission via two mechanisms: (a) by reducing mosquito population density and/or lifespan; (b) by reducing mosquito competence to transmit dengue.
Estimating the likely impact of these two effects on dengue transmission in the field from experimental data poses challenges. Early assessments of impacts on vector competence [34] used blood artificially spiked with virus and estimated almost complete suppression of viral replication in Aedes aegypti [61–63]. Such levels of suppression would be expected to achieve elimination of transmission of dengue in contexts where wMel is able to be established in the local Aedes population at close to 100% frequency, given the relatively low basic reproduction number (the average number of human infections generated by a typical human infection in an immunologically naïve population), R0, of dengue [64]. However recent, more realistic experiments used fresh blood from dengue patients [20] and found somewhat lower levels of viral suppression compared with the earlier work [20]. The wMelPop strain was still seen to confer profound resistance to dengue infection (leading to > 90% blocking of transmission), but wMel-infected (and wild-type) mosquitoes exhibited infection rates which depended on the level of viremia in the blood meal [20]. Artificial infection experiments have also shown the potential for wMel to partially supress replication of a range of other arboviruses, including Chikungunya, Yellow Fever and Zika [61–63].
When suppression is partial and dependent on viral titre in blood meals, modelling is needed to predict the overall impact of wMel infection on transmission [20]. To assess the likely impact of wMel infection on dengue, Ferguson et al. coupled data on viral dynamics within the human host with those in the vector, with the aim of translating the laboratory results into estimates of the potential impact of wMel on the R0 of the different dengue serotypes [20]. This analysis predicted that wMel infection would reduce R0 by 66% for DENV1 and 75% for DENV2/3/4, and therefore argued that widespread release of Aedes aegypti infected with wMel could eliminate dengue in low-to-moderate transmission settings (i.e. where R0 < 3–4) even if infection with Wolbachia had no fitness costs on mosquitoes.
Limited mathematical modelling has been undertaken to date on the likely impact of Wolbachia on arbovirus transmission dynamics. In theoretically focussed studies, Hancock et al. [60] examined the impact of Wolbachia on transmission of a malaria-like disease, while Hughes and Britton [65] examined the potential impact of a Wolbachia strain with perfect maternal transmission and CI on transmission of a single strain arbovirus. Both studies examined the invasion dynamics of Wolbachia and how the phenotypic parameters of Wolbachia, which affect transmission and baseline pathogen transmissibility, determined the overall impact of Wolbachia on disease transmission. Other research has used simplified compartmental models of dengue transmission to examine similar issues [66–69], including the potential impact of Wolbachia on dengue outbreaks in non-endemic settings [67].
None of the modelling studies yet undertaken to explore the impact of Wolbachia on dengue transmission have used realistic 4-strain disease models [15] or incorporated the latest estimated impacts of Wolbachia on the R0 of dengue [20]. Here we therefore adapt a published model of dengue transmission used to assess the likely impact of the Sanofi dengue vaccine on dengue disease burden [15] to incorporate wMel-infected as well as wild-type mosquitoes. We assumed that wMel infection increased adult mosquito mortality by 5% and decreased fecundity by 15% relative to wild-type, and that CI was perfect [34]. We varied two other phenotypic parameters: (a) the ability of a mosquito to transmit dengue (i.e. infectiousness) induced by wMel infection (between 20% and 100%); (b) the efficiency of maternal transmission of wMel (90%–100%). All other parameters were as previous published [15].
The predicted impact of blanket releases and successful establishment of wMel in a large human population (i.e. where exposure to dengue outside the wMel treated area makes up a negligible fraction of all dengue transmission prior to release) on dengue transmission dynamics is shown in Figure 3 (model available freely on request). We choose a deliberately conservative scenario, with wMel only reducing mosquito infectiousness (for dengue) by 40%, and maternal transmission of wMel only having 90% efficiency. The latter assumption is pessimistic, but motivated by one recent report of loss of wMel infection in some Aedes aegypti samples collected in the field[70]. Under these assumptions, following introduction, wMel only reaches a frequency of 87% due to the imperfect maternal transmission, but the overall mosquito population density is reduced by over 40% due to the fitness costs of wMel infection coupled with the impact of CI on wild-type fitness. Overall, the impact of wMel introduction in this scenario is insufficient to reduce the R0 of dengue to below 1, the threshold for permanent elimination. Nevertheless, Wolbachia introduction results in near immediate and transient elimination of dengue transmission for over 10 years post-introduction, with re-establishment of sustained (although substantially reduced) transmission only occurring more than 25 years after the initial release. These dynamics result from wMel introduction disrupting the balance between dengue transmission and host population (i.e. ‘herd’ immunity); wMel introduction reduces the effective reproduction number (the reproduction number accounting for human population immunity), R, of dengue to well below 1, causing transmission to cease. Only as herd immunity falls in the human population (due to new immunologically naïve people being born) can transmission resume.
Figure 3. Simulated time-series of monthly symptomatic dengue incidence following (or without) Wolbachia release.
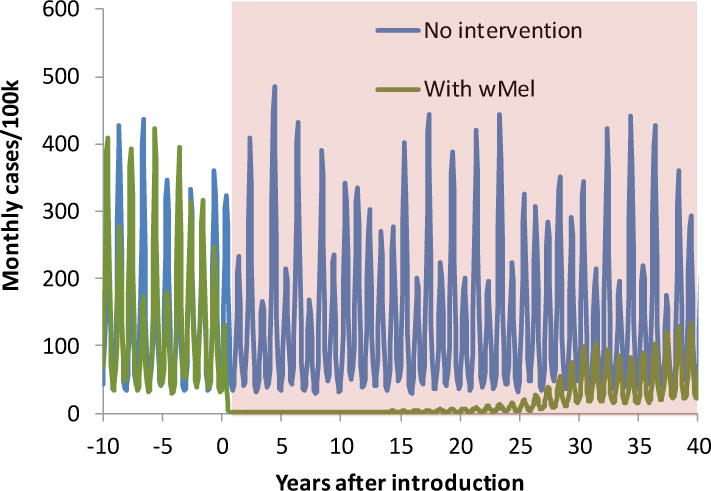
Results use the deterministic dengue transmission model published in [15]. Mean dengue R0 set at 4.5 and wMel is assumed to reduce dengue infectiousness of mosquitoes by 40% and to have 90% efficient maternal transmission. Shaded area shows dynamics after the initial release of Wolbachia at time 0.
Figure 4 shows how the long-term impact of large-scale wMel establishment on dengue disease varies with the efficiency of maternal transmission of wMel, the local transmission intensity (R0) of dengue and one further entomological factor which turns out to be important: the intensity of density-dependent regulation of Aedes aegypti larval populations. Our default scenario for larval density dependence (used by nearly all mathematical models of mosquito population dynamics) is that the per-capita rate of larval mortality is proportional to larval density. More intense larval density dependent competition (here represented by a mortality rate proportional to the square of larval density) leads to somewhat lower reductions in dengue transmission, consistent with the predicted effects of larval competition on Wolbachia establishment discussed earlier [57–59]. As expected, imperfect maternal transmission of Wolbachia also reduces impact.
Figure 4. Predicted long-term impact of large-scale Wolbachia release on symptomatic dengue incidence derived using the deterministic dengue transmission model published in [15].
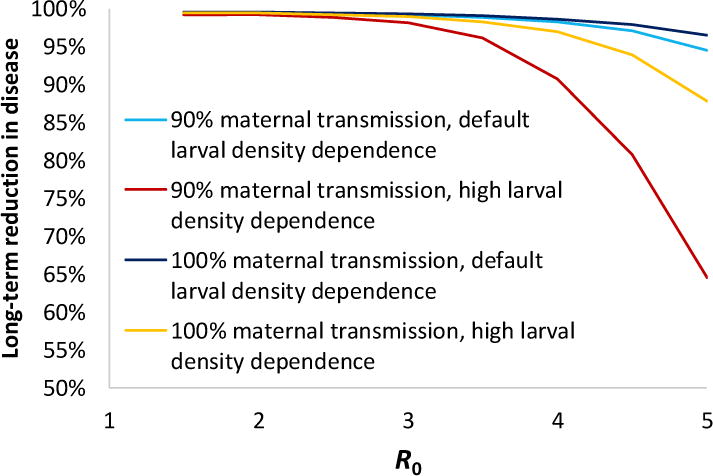
Four different scenarios using different assumptions regarding Wolbachia maternal transmission and larval competition intensity are shown. Note that the transient impact of Wolbachia release on dengue transmission (i.e. in the first 10–30 years post-release) is much larger (see Figure 3), typically giving a >95% reduction in dengue cases in that timeframe.
Future challenges
Enormous progress has been made in the last decade to bring us to the current point, with Wolbachia positioned as one of the most promising novel interventions to control dengue and other arboviruses. However, several major challenges remain (see Outstanding Questions box). Despite the large impact modelling predicts large-scale Wolbachia releases will have on dengue transmission, experimentally evaluating effectiveness in the field is not straightforward [71,72]. Dengue incidence is highly variable over time and over relatively short spatial scales, necessitating either a long duration trial in a small number of locations or a shorter trial in a large number of sites [69,70]. Being an area-based intervention, randomisation in an efficacy trial needs to occur at the site rather than the participant level, necessitating a cluster-randomized design. Furthermore, even in high transmission settings, annual incidence of clinical dengue rarely exceeds 5% in any age group, requiring large cohorts of trial participants to be recruited to achieve sufficient trial power [71]. Somewhat greater power (or smaller numbers of participants) can be achieved using seroconversion as the trial endpoint, but this necessitates bleeding participants (usually young children) at multiple timepoints over the trial. ‘Contamination’ also presents a challenge; if the Wolbachia release areas in a trial are too small, participants resident in those areas may be exposed to dengue when they travel outside those areas, or by wild-type mosquitoes migrating in. Similarly, if control areas are placed close to intervention areas, participants resident in the former may partially benefit from the protection provided by the latter, particularly if Wolbachia spreads from the intervention to the control areas over the course of a trial.
These challenges have led to consideration of other more novel study designs to evaluate impact. One promising approach is to adapt the test-negative design used for evaluating influenza vaccine effectiveness [73] to a cluster-randomised trial context. A sample of febrile patients with dengue-like symptoms seeking healthcare are recruited across both intervention and control areas, their residential location determined, and are virologically tested for dengue. If Wolbachia reduces dengue transmission, the ratio of test-positive to test-negative patients is expected to be lower in intervention areas than control areas. A cluster-randomized trial of wMel releases using a test-negative end-point design is now underway across the city of Yogyakarta in Indonesia, with results expected in late 2019 (https://clinicaltrials.gov/ct2/show/NCT03055585).
An alternative approach to evaluating effectiveness, albeit giving a lower standard of evidence, is simply to release Wolbachia across a large urban area and observe the impact upon dengue incidence trends observed through routine surveillance. Given the imperfect specificity of clinical diagnosis of dengue, enhanced virological confirmation of suspected dengue cases is desirable in such studies. While such observational approaches cannot establish causality, should the effect size be as large as the modelling discussed above would suggest, a large reduction in dengue case numbers would be expected. So long as this reduction was sustained, as more observation time accumulated it would be increasingly difficult to attribute the observed reduction to chance effects, particularly if dengue incidence outside the release area continued at comparable levels to previously. Such an approach is being adopted for the two largest scale releases of wMel currently underway – in Medellin, Colombia and Rio de Janeiro, Brazil. By late 2019, sufficient data should have accumulated in both cities to make initial assessments of effectiveness.
Optimistically assuming that the studies currently underway demonstrate that wMel release leads to large falls in dengue incidence, there are additional challenges involved in transforming Wolbachia into a fully operationalised, standardised, inexpensive vector control ‘product’, capable of large-scale highly cost-effective deployment. Facilities for the mass-production of mosquitoes have become increasingly sophisticated and resource-efficient over the last few years, due to the development and testing of both Wolbachia and genetically modified mosquitoes. However, all Wolbachia field releases to date have been very heavily monitored via fine spatial scale grids of mosquito traps, with release sizes and durations being adaptively tuned based on the observed spread of Wolbachia into the wild-type population. Such high levels of instrumentation are less feasible for routine large-scale operational deployment and would add substantially to costs, but more research is needed to optimise standardised release protocols to maximise the chance of successful establishment while minimising the duration of releases and numbers of mosquitoes needing to be released.
As a biological control, we should also expect the phenotypic properties of any one Wolbachia strain to potentially evolve over time[74], perhaps leading to reductions in the extent of transmission blocking. In addition, current Wolbachia strains introduced into Aedes aegypti may not be optimal in their fitness costs, level of blocking or temperature sensitivity [75]. It will therefore be important to maintain development of a pipeline of new strains capable of being successfully released into Aedes aegypti populations where wMel has already been established. The encouraging results from experiments examining the degree of viral suppression achieved in Aedes aegypti superinfected with both wMel and wAlbB [36] suggest that the release of superinfected mosquitoes might be an effective strategy to manage potential reductions in the capacity of single Wolbachia strains to block transmission.
Nevertheless, despite these challenges, Wolbachia represents a highly innovative and exciting new approach to vector control for arboviral diseases, and the first which offers the real prospect of dramatically reducing the global disease burden caused by those pathogens. Mathematical modelling will continue to have an important role to play in helping to overcome the challenges involved in evaluating the effectiveness of Wolbachia and transforming it into a fully operationalised, cost-effective intervention capable of being globally deployed.
Wolbachia is a vertically transmitted endosymbiotic intracellular bacterium that is naturally present in about 60% of all insect species.
With the goal of dengue transmission reduction, a number of strains of Wolbachia have been deliberately transinfected into Aedes aegypti.
In small-scale field releases, these strains have been shown to spread to high frequencies in Aedes aegypti populations.
Wolbachia offers the potential to reduce dengue transmission via two mechanisms: mosquito population density reduction (Wolbachia imposes a fitness cost) and reduced vector competence.
Mathematical modelling has guided our understanding of the spread of Wolbachia in mosquito populations, its potential impact on dengue transmission, and field study design.
City-scale studies are now underway to evaluate the effect of releasing Wolbachia-infected Aedes aegypti on dengue transmission.
What is the impact of large-scale releases of Wolbachia-infected Aedes aegypti on dengue transmission? High spatiotemporal variability in dengue incidence requires studies to be large (geographically and in terms of the enrolled population). Considerable efforts, including mathematical modelling, have been put into study design in recent years. A mixture of cluster randomized trials and observational studies are now underway across multiple cities in Latin America and Southeast Asia.
What is the best protocol for releasing Wolbachia-infected mosquitoes to ensure establishment, minimise cost and reduce the need for high levels of monitoring? Releases to date have adaptively adjusted the numbers of mosquitoes released and the duration of releases to ensure rapid establishment, making use of extensive real-time mosquito trapping data. Transforming Wolbachia into an operationalized vector control tool capable of inexpensive and wide deployment will require robust protocols for release which are not reliant on such intensive monitoring.
What will be the long-term phenotypic stability of the Wolbachia strains transinfected into Aedes aegypti in the field? As a recently transinfected biological control, it is quite possible that the Wolbachia – Aedes aegypti system will co-evolve in coming years. Should this result in reduced fitness costs or suppression of arboviral replication, new Wolbachia strains may be needed which can superinfect existing populations. As a corollary, learning how Wolbachia reduces arbovirus replication in the mosquito may allow higher efficacy strains to be developed.
If Wolbachia releases are insufficient to eliminate dengue in high transmission settings, how can Wolbachia best be combined with other control measures, notably vaccines? This is far from obvious, given the one licensed vaccine has relatively low efficacy in seronegative recipients, and Wolbachia, by driving down transmission, will decrease dengue seroprevalence in treated areas.
Acknowledgments
The authors thank the Imperial College Junior Research Fellowship scheme, the National Institute of General Medical Sciences MIDAS initiative and the Bill and Melinda Gates Foundation for research funding. The authors also thank the UK Medical Research Council for Centre and PhD studentship funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady OJ, et al. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina JP, et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014;22:138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepard DS, et al. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- 5.Stanaway JD, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achee NL, et al. A Critical Assessment of Vector Control for Dengue Prevention. PLoS Negl Trop Dis. 2015;9:e0003655. doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esu E, et al. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; Systematic review. Trop Med Int Heal. 2010;15:619–631. doi: 10.1111/j.1365-3156.2010.02489.x. [DOI] [PubMed] [Google Scholar]
- 8.Ranson H, et al. Insecticide resistance in dengue vectors. TropIKA.net. 2010;1:1–12. [Google Scholar]
- 9.Thisyakorn U, Thisyakorn C. Latest developments and future directions in dengue vaccines. Ther Adv Vaccines. 2014;2:3–9. doi: 10.1177/2051013613507862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannice KS, et al. Status of vaccine research and development of vaccines for dengue. Vaccine. 2016;34:2934–2938. doi: 10.1016/j.vaccine.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 11.Capeding MR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 12.Villar L, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 13.Hadinegoro SR, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Dengue vaccine: WHO Position Paper - July 2016. Weekly Epidemiological Record. 2016;91:349–364. [Google Scholar]
- 15.Ferguson NM, et al. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science. 2016;353:1033–1036. doi: 10.1126/science.aaf9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flasche S, et al. The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study. PLoS Med. 2016;13:e1002181. doi: 10.1371/journal.pmed.1002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho DO, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. 2015;9:e0003864. doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YJ, et al. Biological Control Strategies for Mosquito Vectors of Arboviruses. Insects. 2017;8:21. doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson NM, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:279ra37–279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werren JH, et al. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 22.Zug R, Hammerstein P. Still a host of hosts for Wolbachia : Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertig M. The rickettsia, Wolbachia pipientis (gen. et sp n.) and associated inclusions of the mosquito, Culex pipiens. Parasitology. 1936;28:453–486. [Google Scholar]
- 24.Kittayapong P, et al. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am J Trop Med Hyg. 2002;66:108–111. doi: 10.4269/ajtmh.2002.66.108. [DOI] [PubMed] [Google Scholar]
- 25.Fry AJ, et al. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity. 2004;93:379–389. doi: 10.1038/sj.hdy.6800514. [DOI] [PubMed] [Google Scholar]
- 26.Ross PA, et al. Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am J Trop Med Hyg. 2014;91:198–205. doi: 10.4269/ajtmh.13-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie SA, et al. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003930. doi: 10.1371/journal.pntd.0003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haygood R, Turelli M. Evolution Of Incompatibility-Inducing Microbes In Subdivided Host Populations. Evolution. 2009;63:432–447. doi: 10.1111/j.1558-5646.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- 29.Fine PEM. On the dynamics of symbiote-dependent cytoplasmic incompatibility in culicine mosquitoes. J Invertebr Pathol. 1978;31:10–18. doi: 10.1016/0022-2011(78)90102-7. [DOI] [PubMed] [Google Scholar]
- 30.Turelli M, Hoffmann AA. Cytoplasmic incompatibility in Drosophila simulans - dynamics and parameter estimates from natural-populations. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xi Z, et al. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 32.Min KT, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acadamy Sci USA. 1997;94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMeniman CJ, et al. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 34.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 35.Riegler M, et al. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol. 2005;15:1428–1433. doi: 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 36.Joubert DA, et al. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann AA, et al. Invasion of Wolbachia at the residential block level is associated with local abundance of Stegomyia aegypti, yellow fever mosquito, populations and property attributes. Med Vet Entomol. 2014;28:90–97. doi: 10.1111/mve.12077. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann AA, et al. Stability of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations. PLoS Negl Trop Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frentiu FD, et al. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen TH, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites & Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callaway E. Rio fights Zika with biggest release yet of bacteria-infected mosquitoes. Nature. 2016;539:17–18. doi: 10.1038/nature.2016.20878. [DOI] [PubMed] [Google Scholar]
- 42.Caspari E, Watson GS. On the Evolutionary Importance of Cytoplasmic Sterility in Mosquitoes. Evolution. 1959;13:568. [Google Scholar]
- 43.Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- 44.Turelli M. Cytoplasmic incompatibility in populations with overlapping generations. Evolution. 2010;64:232–241. doi: 10.1111/j.1558-5646.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 45.Brownstein JS, et al. The potential of virulent Wolbachia to modulate disease transmission by insects. J Invertebr Pathol. 2003;84:24–29. doi: 10.1016/s0022-2011(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 46.Schofield P. Spatially Explicit Models of Turelli–Hoffmann Wolbachia Invasive Wave Fronts. J Theor Biol. 2002;215:121–131. doi: 10.1006/jtbi.2001.2493. [DOI] [PubMed] [Google Scholar]
- 47.Jansen VA, et al. Stochastic spread of Wolbachia. Proc R Soc B Biol Sci. 2008;275:2769–2776. doi: 10.1098/rspb.2008.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu L, et al. Wolbachia spread dynamics in stochastic environments. Theor Popul Biol. 2015;106:32–44. doi: 10.1016/j.tpb.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Keeling MJ, et al. The invasion and coexistence of competing Wolbachia strains. Heredity. 2003;91:382–388. doi: 10.1038/sj.hdy.6800343. [DOI] [PubMed] [Google Scholar]
- 50.Guevara-Souza M, Vallejo EE. A computer simulation model of Wolbachia invasion for disease vector population modification. BMC Bioinformatics. 2015;16:317. doi: 10.1186/s12859-015-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schofield P, et al. Evolution of searching and life history characteristics in individual-based models of host-parasitoid-microbe associations. J Theor Biol. 2005;237:1–16. doi: 10.1016/j.jtbi.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 52.Turelli M, Barton NH. Deploying dengue-suppressing Wolbachia: Robust models predict slow but effective spatial spread in Aedes aegypti. Theor Popul Biol. 2017;115:45–60. doi: 10.1016/j.tpb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt TL, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15:e2001894. doi: 10.1371/journal.pbio.2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schraiber JG, et al. Constraints on the use of lifespan-shortening Wolbachia to control dengue fever. J Theor Biol. 2012;297:26–32. doi: 10.1016/j.jtbi.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Barton NH, Turelli M. Spatial Waves of Advance with Bistable Dynamics: Cytoplasmic and Genetic Analogues of Allee Effects. American Naturalist. 2011;178:E48–E75. doi: 10.1086/661246. [DOI] [PubMed] [Google Scholar]
- 56.Hancock PA, Godfray HCJ. Modelling the spread of Wolbachia in spatially heterogeneous environments. J R Soc Interface. 2012;9:3045–3054. doi: 10.1098/rsif.2012.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crain PR, et al. Wolbachia infections that reduce immature insect survival: Predicted impacts on population replacement. BMC Evol Biol. 2011;11:290. doi: 10.1186/1471-2148-11-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hancock PA, et al. Population Dynamic Models of the Spread of Wolbachia. American Naturalist. 2011;177:323–333. doi: 10.1086/658121. [DOI] [PubMed] [Google Scholar]
- 59.Hancock PA, et al. Predicting Wolbachia invasion dynamics in Aedes aegypti populations using models of density-dependent demographic traits. BMC Biol. 2016;14:96. doi: 10.1186/s12915-016-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hancock PA, et al. Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Negl Trop Dis. 2011;5:e1024. doi: 10.1371/journal.pntd.0001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moreira LA, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 62.Van den Hurk AF, et al. Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dutra HLC, et al. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson MA, et al. Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine. 2011;29:5860–5868. doi: 10.1016/j.vaccine.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes H, Britton NF. Modelling the Use of Wolbachia to Control Dengue Fever Transmission. Bull Math Biol. 2013;75:796–818. doi: 10.1007/s11538-013-9835-4. [DOI] [PubMed] [Google Scholar]
- 66.Supriatna AK, Anggriani N. System dynamics model of Wolbachia infection in dengue transmission. Procedia Eng. 2012;50:12–18. [Google Scholar]
- 67.Ndii MZ, et al. Modelling the transmission dynamics of dengue in the presence of Wolbachia. Math Biosci. 2015;262:157–166. doi: 10.1016/j.mbs.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Ndii MZ, et al. The effect of Wolbachia on dengue outbreaks when dengue is repeatedly introduced. Theor Popul Biol. 2016;111:9–15. doi: 10.1016/j.tpb.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Ndii MZ, et al. The effect of Wolbachia on dengue dynamics in the presence of two serotypes of dengue: symmetric and asymmetric epidemiological characteristics. Epidemiol Infect. 2016;144:2874–2882. doi: 10.1017/S0950268816000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt TL, et al. Fine-scale landscape genomics helps explain the slow spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. biorxiv doi.org. 2017 doi: 10.1101/103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolbers M, et al. Considerations in the Design of Clinical Trials to Test Novel Entomological Approaches to Dengue Control. PLoS Negl Trop Dis. 2012;6:e1937. doi: 10.1371/journal.pntd.0001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lambrechts L, et al. Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect Dis. 2015;15:862–866. doi: 10.1016/S1473-3099(15)00091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 74.Weeks AR, et al. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007;5:0997–1005. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross PA, et al. Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog. 2017;13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]


