Abstract
Introduction
Continued development and refinement of resting state functional connectivity (RSFC) fMRI techniques in both animal and clinical studies has enhanced our comprehension of the adverse effects of stress on psychiatric health. The objective of the current study was to assess both maternal behavior and resting state functional connectivity (RSFC) changes in these animals when they were dams caring for their own young. It was hypothesized that ECSS exposed dams would express depressed maternal care and exhibit similar (same networks), yet different specific changes in RSFC (different individual nuclei) than reported when they were adult females.
Methods
We have developed an ethologically relevant transgenerational model of the role of chronic social stress (CSS) in the etiology of postpartum depression and anxiety. Initial fMRI investigation of the CSS model indicates that early life exposure to CSS (ECSS) induces long term changes in functional connectivity in adult nulliparous female F1 offspring.
Results
ECSS in F1 dams resulted in depressed maternal care specifically during early lactation, consistent with previous CSS studies, and induced changes in functional connectivity in regions associated with sensory processing, maternal and emotional responsiveness, memory, and the reward pathway, with robust changes in anterior cingulate circuits.
Limitations
The sample sizes for the fMRI groups were low, limiting statistical power.
Conclusion
This behavioral and functional neuroanatomical foundation can now be used to enhance our understanding of the neural etiology of early life stress associated disorders and test preventative measures and treatments for stress related disorders.
Introduction
Continued development and refinement of resting state functional connectivity (RSFC) fMRI techniques in both animal (Zhang, Rane et al. 2010; Liang, King et al. 2011; Liang, Liu et al. 2015) and clinical studies has enhanced our comprehension of the adverse effects of stress on psychiatric health. RFSC measures intrinsic neural interactions through the measurement of spontaneous fluctuations in blood oxygen level dependent (BOLD) activity in different brain regions (Biswal, Zerrin Yetkin et al. 1995; Raichle and Snyder 2007), and facilitates the simultaneous assessment of chronic changes in multiple neural circuits involved in psychiatric illness etiology. The use of this technique in conscious rodents augments etiological relevance through the longitudinal assessment of the effects of stress on multiple neural networks across the life span, similar to clinical studies assessing multiple risk factors across time. Comparisons of clinical and animal model RSFC data will enhance our understanding of susceptibility and resilience, pathological etiology, effective preventative measures, and treatment response. These comparisons will be particularly effective when animal models possess high degrees of construct and face validity.
We have developed an ethologically relevant transgenerational model of the role of chronic social stress (CSS) in the etiology of postpartum depression and anxiety (Nephew and Bridges 2011; Coverdill, McCarthy et al. 2012; Carini, Murgatroyd et al. 2013; Carini and Nephew 2013; Murgatroyd and Nephew 2013; Babb, Carini et al. 2014; Murgatroyd, Peña et al. 2015; Murgatroyd, Taliefar et al. 2015; Murgatroyd, Babb et al. 2016). Exposure of F0 dams to the chronic social stress of a daily exposure to a novel male intruder depresses maternal care even in the absence of the male intruder and depresses offspring care and lactation in both the F0 dams (Nephew and Bridges 2011; Murgatroyd, Taliefar et al. 2015) and their female F1 offspring (Carini and Nephew 2013; Murgatroyd and Nephew 2013; Murgatroyd, Peña et al. 2015). This stressor represents an early life challenge for the F1 generation (early life CSS, ECSS) and also has transgenerational effects on the social, stress, anxiety, and depression behavior of the F2 generation (Babb, Carini et al. 2014; Murgatroyd, Babb et al. 2016; Nephew, Carini et al. 2017). These endocrine, behavioral, and genetic studies of the F1 and F2 offspring of stressed F0 dams report several changes that closely parallel clinical studies of early life stress, depression, anxiety, and autism (Murgatroyd and Nephew 2013; Babb, Carini et al. 2014; Murgatroyd, Peña et al. 2015; Murgatroyd, Babb et al. 2016; Nephew, Carini et al. 2017).
The objective of the current study was to assess functional connectivity changes in these animals when they were dams caring for their own young. Initial fMRI investigation of the CSS model indicates that early life exposure to CSS induces long term changes in functional connectivity in adult female F1 offspring (Nephew, Huang et al. 2017). CSS exposed F1 females exhibit broad changes in stress and social behavior nuclei which are components in the limbic, reward, salience, and socioaffective networks. The most substantial changes in connectivity were observed in the prefrontal cortex, nucleus accumbens, hippocampus, and somatosensory cortex, supporting the conclusion that exposure to early life CSS has persistent effects in stress, social behavior, depression, and anxiety related nuclei. Given the extensive neural plasticity related changes which occur in the female brain during gestation, parturition, and lactation (Kinsley, Bardi et al. 2008; Kinsley and Lambert 2008; Hillerer, Jacobs et al. 2014; Bridges 2016; Woodside 2016), it was hypothesized that CSS exposed dams would express depressed maternal care and exhibit similar (same networks), yet different specific changes in RSFC (different individual nuclei) than reported when they were adult nulliparous females (Nephew, Huang et al. 2017).
Methods
Animals
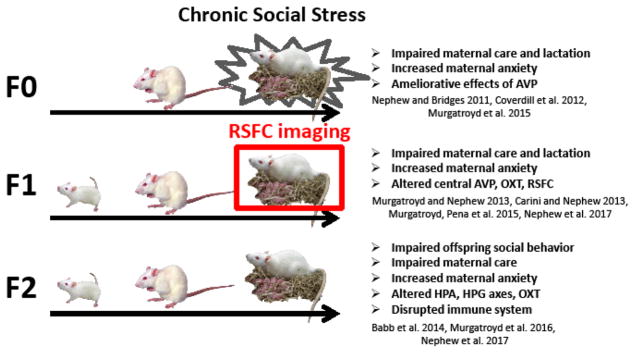
Sprague Dawley rats (Charles River, Wilmington, MA) in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts University and University of Massachusetts Institutional Animal Care and Use Committees. For an overview of the CSS paradigm, see Fig. 1. “CSS dams” refers to the adult females exposed to CSS during lactation (F0), and “ECSS females” refers to the female offspring of the CSS dams (F1); the focus of the present study. F0 and F1 dams were bred with 10 established breeder males from Charles River. The F0 CSS stage of the study was conducted at Tufts University and the F1 pups were transported 6 miles at 30–40 days of age to the University of Massachusetts Center for Comparative Neuroimaging. Early lactation refers to dams at lactation day 2–5, mid lactation refers to lactation days 8–11, and late lactation was defined as days 15–18. Behavioral testing was conducted one day prior to fMRI imaging at each stage of lactation. At the F1 dam stage, the focus of the present study, the samples sizes for the behavioral data were 10 for F1 control dam group and 12 for the ECSS dam group for all three stages of lactation. Because of movement induced loss of scans and due to little variation between early and mid-lactation groups (all p’s >0.05), fMRI scans for early and mid-lactation rats were grouped and compared to late lactation animals. Thus, final imaging group sizes were as follows: 7 control, 12 ECSS animals for early and mid-lactation and 8 control and 10 ECSS for late lactation.
Fig. 1.
Diagram of the transgenerational Chronic Social Stress (CSS) model. The different sized rats represent the juvenile, adult, and maternal stages in each generation. F0 females are mated and exposed to the social stress of the daily intrusion of a novel male intruder for 15 days during lactation (days 2–16). This represents an early life stress for the F1 pups, which were the focus of the current resting state functional connectivity (RSFC) study at the dam stage. The effects of CSS on dams and offspring at several stages have been reported in the listed studies.
ECSS model: creation of F0 dams and F1 females
F0 Dams (Charles River, Wilmington, MA) mated at Tufts University were subjected to the CSS protocol at the Cummings School (previously described) (Nephew and Bridges 2011; Carini, Murgatroyd et al. 2013), which consisted of placing a similarly sized (220–300g) novel male intruder into a lactating female’s home cage for 1 h from days 2–16 of lactation. Control dams were not exposed to the CSS protocol, and were only tested for maternal care and maternal aggression between 0800 and 1200 on days 2, 9, and 16 of lactation (both control and CSS dams were tested for maternal care and maternal aggression on these days). The F1 pups were left in the cage during the intruder presentation and the CSS F1 pups were exposed to depressed maternal care from their F0 mothers and the daily conflict between the mother and the male intruder (Early life CSS, ECSS) (Carini and Nephew 2013; Murgatroyd and Nephew 2013; Murgatroyd, Peña et al. 2015). The F1 control and ECSS females of the current study were the offspring of the F0 control and CSS dams; the differences between the treatments of the control and ECSS F1 females were limited to the exposure of the ECSS F1 females to depressed maternal care and daily conflict between their F0 mothers and the male intruders during age 2–16 days. The F1 control and ECSS animals were treated identically after the age of 16 days. All F1 (CSS and control) females were transported to the UMass CCNI at 30–40 days of age (6 miles from the Cummings School), quarantined for 21 days, and then acclimated to the imaging procedures for 8 days.
Acclimation to Imaging Procedures
A total of 27 F1 adult nulliparous females (65–90 days old) were exposed to the imaging protocol (13 CSS and 14 control). Before the imaging experiment, these rats were acclimated to the environment and imaging acoustic noise produced by the MR scanner using the procedure previously described (Zhang, Rane et al. 2010). Briefly, rats were anesthetized with isoflurane (2%) and secured in a head holder using plastic bite bar and ear bars. EMLA cream (Lidocaine2.5% and Prilocaine 2.5% cream, Hi-Tech Pharmacal Co., Inc.) was topically applied to relieve any pain associated with the head holder. Animals were then placed into a black opaque tube (mock scanner) with tape-recorded scanner noise played. Animals were acclimated for eight days, one session per day. The time of acclimation was 15 min on day 1 with an increment of 15 min per day up until day 4. A maximum of 60 min was used on days 4–8, and all animals completed the acclimation procedures successfully.
Adult and Postpartum Imaging
Animals were imaged at 65–90 days of age to assess the long term effects of early life stress on adult RSFC (Nephew, Huang et al. 2017). One week later, they were then mated with breeder males and later imaged during early, mid, and late lactation. These ranges of days were necessary to accommodate the imaging schedule at the CCNI.
All MR images were acquired on a 4.7T/40 cm horizontal magnet (Oxford, UK) interfaced with a Biospec Bruker console (Bruker, Germany) and equipped with a 20G/cm magnetic field gradient. A custom built 1H radiofrequency (RF) volume coil was used. Anatomical images were acquired using a multi-slice fast spin-echo sequence (RARE) with the parameters: repetition time (TR) = 3000 ms; RARE factor = 8; effective echo time (TE) = 50 ms; matrix size = 256 × 256 × 20; in-plane field of view (FOV) = 3.2cm × 3.2 cm; slice thickness = 1 mm; n of averages = 4. Functional images were acquired using echo-planar imaging (EPI) with the parameters: TR = 1089 ms; Flip Angle = 60°; TE = 30 ms; matrix size = 64 × 64 × 20; in-plane FOV = 3.2 cm × 3.2 cm; slice thickness = 1 mm; voxel size 0.5 mm × 0.5 mm × 1 mm. Each EPI scan had 600 repetitions, lasting about 10 min.
F1 Maternal Care Testing
F1 Maternal care was assessed at early (days 2–5), mid (days 8–11), and late lactation (days 15–18) between 0900 and 1200 h one day prior to RSFC fMRI in all dams to assess the effects of early life CSS at different time points during lactation as previously reported (Murgatroyd and Nephew 2013). Animals were transported from a central colony room to a separate behavioral testing room. After a 60-minute pup removal, maternal care testing was performed, consisting of the re-introduction of all eight pups to the home cage and video recording the dam for 30 minutes. These 30 minute behavioral observations produce consistent and substantial behavioral data that are comparable to observations of undisturbed maternal care over 30 and 60 minutes (Byrnes et al., 2000 and Johnson et al., 2011). Frequencies and durations of pup retrieval, pup grooming, nursing, nesting, self-grooming, rearing, and general locomotor activity were scored by an observer who was blind to the treatment using ODLog behavioral analysis software (Macropod Inc., USA). Nursing behavior scoring was started when the dam had been motionless over the litter for longer than 10s and stopped whenever she moved off the pups. Nursing duration is the cumulative nursing behavior during the 30 minute maternal care observation. Nesting was defined as manipulation of the nesting material with mouth or paws. Total maternal care included the combined durations of pup grooming and nursing. The composite measure of non-maternal behavior included the durations of self-grooming, nesting, and rearing. F2 pup weights were not recorded in the present study, but pup weights have been assessed in several other previous studies of the F1 and F2 generations, with no effects of CSS on initial litter sizes, litter weights, or growth (Carini and Nephew 2013; Babb, Carini et al. 2014; Murgatroyd, Babb et al. 2016; Hicks-Nelson, Beamer et al. 2017).
Image processing
Brain masks were manually created using ITKSNAP (www.itksnap.org) and these were used for a brain extraction step. The resultant cropped images were aligned with a rat brain template using the FMRIB Software Library linear registration program flirt (Jenkinson, Bannister et al. 2002). Registration matrices were saved and used to subsequently transform functional datasets into atlas space for preprocessing and analysis. Functional images were corrected for motion, slice timing delays, and time series DC spikes were removed using Analysis of Functional NeuroImages (AFNI) (Cox 1996). Linear and quadratic detrending, spatial blurring, and intensity normalization was also performed. Six head motion parameters, and cerebroventricular and white matter signals were removed from all datasets. A voxelwise temporal band-pass filter (between 0.01 Hz and 0.1 Hz) was applied to remove brain signals that contain cardiac and respiratory frequencies. Spontaneous BOLD signals were extracted from a total 150 regions of interest (ROIs) (75 ROI in the left and 75 in the right hemisphere) based on the atlas-guided seed location. Signals were averaged from voxels within ROI located in each hemisphere. Voxel-wise cross correlations between the extracted fMRI signals per each ROI were carried out to create correlation coefficient (Pearson r) maps. The first 9 images in each functional time series were not used in the cross correlation step. Pearson r maps were then subjected to a voxelwise z-transformation. Two correlation maps were averaged per subject to generate a single correlation map subsequently used for statistical comparisons. False discovery rate (FDRq=0.1) was used to control the level of false positive rates in the final composite maps.
Functional network analysis
Brain networks were analyzed in Matlab using Brain Connectivity Toolbox (Rubinov and Sporns 2010). Adjacency matrices were thresholded to generate networks with equal graph densities (15% of all possible pairs). All matrices were normalized by the highest z-score, such that all matrices had edge weights in a range from 0 to 1. Network metrics were calculated for both binary and weighted graphs. These included: node strength, clustering coefficient, modularity, average shortest path length, and small worldness (Newman 2003; Boccaletti, Latora et al. 2006; Saramäki, Kivelä et al. 2007).
To assess graph topology we followed the small world framework (Watts and Strogatz 1998). We generated ten null-hypothesis networks per rat and averaged these to statistically compare the topological features of their brain networks. The average null hypothesis clustering coefficients and path lengths were calculated and compared to the rat brain network results. The ratio of clustering coefficient of the rat brain to the null hypothesis is referred to as γ. For a small world network this parameter should be larger than 1, indicating that a small world network contains larger local connectivity (Humphries and Gurney 2008). The ratio of path lengths of the rat brain to the null hypothesis is referred to as λ. For a small world network this parameter should be close to 1. Finally, the small world parameter is the ratio of γ/λ. For this parameter, a number larger than 1 indicates a small world network while a value closer to one indicates a random network. The brain networks were visualized using BrainNet (Xia, Wang et al. 2013). The 3D networks were generated with edges that correspond to correlation scores between nodes larger than 0.3.
Data analysis
The effects of early life CSS on maternal care behaviors were tested using 2×3 repeated measures ANOVAs with stress and lactation stage (repeated time factor, early, mid, late lactation). Bonferroni post hoc tests were used to make pairwise comparisons on individual lactation days when there were significant interactions between treatment and lactation day. For imaging data, a 2-way ANOVA was used with stress and lactation stage (combined early and mid vs. late lactation) as independent factors.
Results
Maternal Care
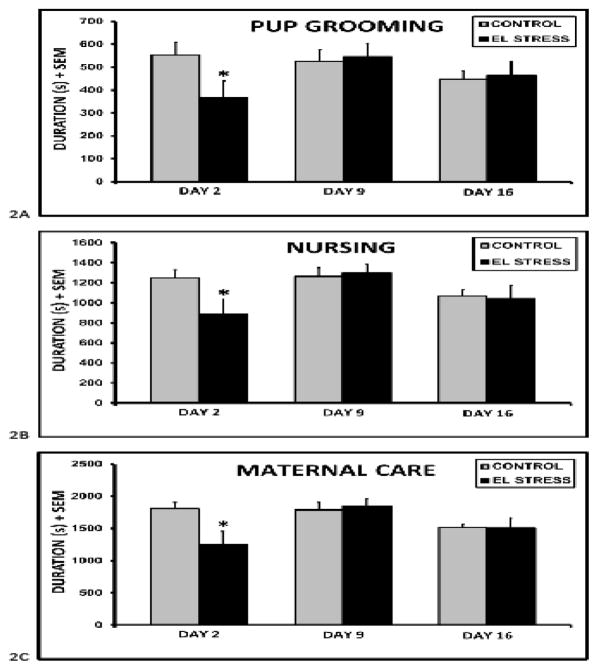
Due to the presence of large nests which extended through most of the cage, pup retrieval could not be assessed. CSS F1 dam spent less time grooming (stress x lactation stage F2,65 = 4.2, p<0.05) and nursing (stress x lactation stage F2,65 = 4.3, p<0.05) their pups during the 30 min maternal care tests during early lactation (fig. 2). The combined differences in grooming and nursing led to a 30% decrease in total maternal care (stress x lactation stage F2,65 = 5.7 p<0.01) during this period. There were effects of lactation stage on nursing (F2,65 = 5.4, p<0.01) and total maternal care (F2,65 = 6.0, p<0.01), with the longest durations of both on day 9. There were no differences in locomotor activity, and no effects of treatment or interactions between treatment and lactation day on maternal care during mid or late lactation (all p’s > 0.1). However, the combined duration of nesting, self grooming, and rearing, was increased in CSS F1 dams (162.5 ± 40.2 vs. 94.7 ± 18.8 1-tailed t, p<0.05) during late lactation.
Fig. 2.
Mean ± SEM durations of pup grooming (A), nursing (B), and total maternal care (C), pup grooming and nursing combined) during a 30 minute maternal care test in F1 control and early life chronic social stress (EL STRESS) exposed dams. *Significant difference between control and stress dams (t-test, p < 0.05).
RSFC fMRI
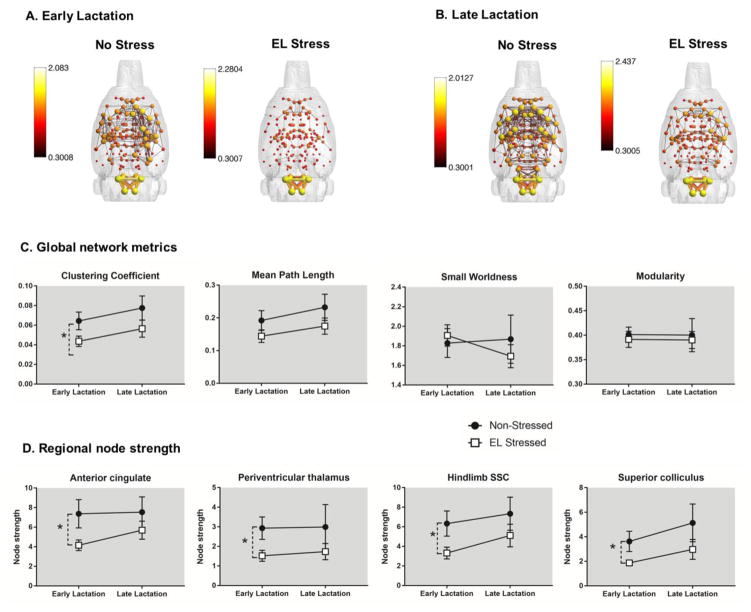
Composite maternal rat brain networks are shown in figure 3A–B. In these maps, node size and color is scaled by node strength and edges (the thickness of connecting lines) are scaled by the correlation coefficients. The figure illustrates brain functional connectivity patterns in stressed and non-stressed dams in early and late lactation. There is a notable effect of early life stress exposure, which resulted in reduced functional connectivity across a number of brain networks in both early and late lactation. The overall effect of stress appeared stronger in early than in late lactation. This is supported in our results for both network metrics and node strength (fig. 3C–D). We analyzed network metrics using a two factor (2 × 2) ANOVA (postpartum stage x stress condition interaction). This initial analysis did not reveal any significant interaction between postpartum stage x stress condition, but showed a significant main effect of stress condition. Thus, we observed a main effect of stress on clustering coefficient (F1,33 = 5.7, p = 0.02) but not mean path length, small worldness and modularity (fig. 3C). We also observed a main effect of stress on node strength in the anterior cingulate cortex (ACC) (1,33 = 5.5, p = 0.02), and the trunk (F1,33 = 7.2, p = 0.01), shoulder (F1,33 = 4.8, p = 0.04) and hindlimb (F1,33 = 5.1, p = 0.03) divisions of the somatosensory cortex (SSC), the periventricular (F1,33 = 4.6, p = 0.04) and parafasicular thalamic areas (F1,33 = 4.9, p = 0.03) and the superior colliculus (F1,33 = 4.9, p = 0.03) (fig. 3D). We conducted an additional analysis to determine the effects of stress conditions separately on early and late lactation stages. This additional analysis using an unpaired t-test with heteroscedastic variances showed that in early lactation stage dams stress reduced clustering coefficient (p = 0.009) and also reduced node strength ACC (p = 0.02), SSC trunk (p = 0.007), SSC shoulder (p = 0.04), and SSC hindlimb areas (p = 0.02), periventricular (p = 0.02) and parafascicular thalamus (p = 0.01) and superior colliculus (p = 0.02).
Fig. 3.
Composite 3D functional connectivity maps comparing control and early life social stress (EL Stress) exposed maternal rats in early (A) and late (B) lactation. Scale bar indicates lower and upper bounds statistical threshold for 3D network maps. (C) Graph network measures in non-stressed and stressed exposed maternal rats in early and late lactation. (D) Node strength in various regions of interest. Data presented as mean ± standard error. *Significantly different (two way ANOVA, p <0.05).
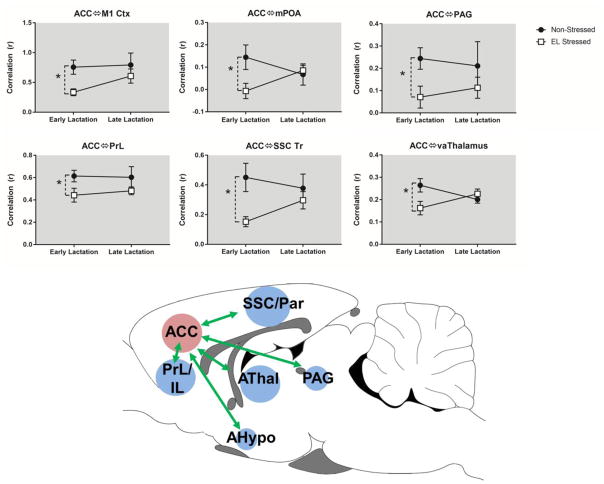
Fig. 3A–B shows maps of functional connectivity between ACC and several brain areas in early lactating non-stressed and stressed rats. Statistical comparison of stress and nonstress groups showed significant differences in functional connectivity with the ACC, including ROI’s in the SSC, primary motor (M1) cortex, striatum, septum, periventricular thalamus, superior colliculus and periaqueductal grey (PAG) (p <0.05 t-test comparing no stress vs stress in early lactation) (fig. 3C).
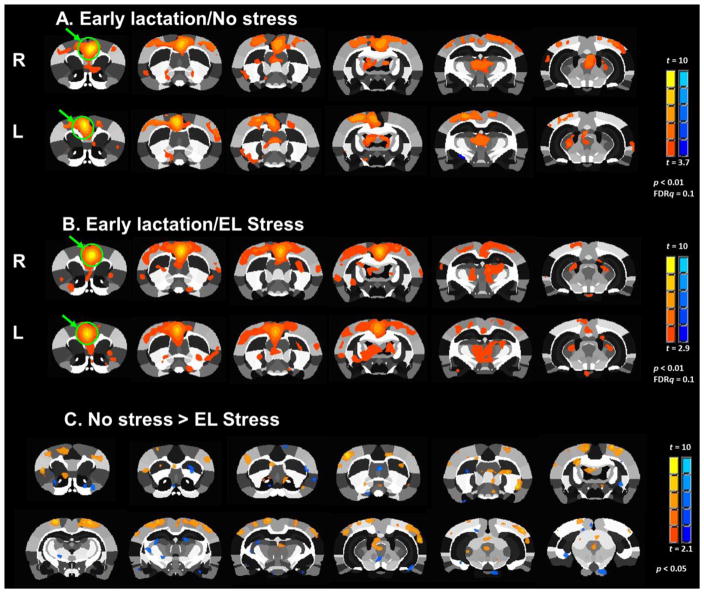
In addition to the above network metrics, we also assessed seed based functional connectivity between a ACC seed and other brain regions. A two factor ANOVA revealed reduced functional connectivity between ACC and medial preoptic area (mPOA; stress x stage interaction, F1,33 = 4.6, p = 0.04) and the ventral anterior (VA) thalamus (stress x stage interaction, F1,33 = 5.5, p = 0.02). Consistent with the above results, we observed a main effect of stress alone (no significant stress x stage interaction) on functional connectivity between ACC and the prelimbic cortex (PrL; main effect of stress, F1,33 = 5.0, p = 0.03) and the SSC (main effect of stress, F1,33 = 7.7, p = 0.009). We conducted an additional analysis to determine the effects of stress conditions separately on early and late lactation stages. This additional analysis using an unpaired t-test with heteroscedastic variances showed that in early lactation stage dams stress reduced functional connectivity between the ACC and mPOA (p = 0.02), VA thalamus (p = 0.04), PAG (p = 0.03), SSC (p = 0.003), and M1 cortex (p = 0.002). Fig. 5 shows a summary schematic representation of these differences between stressed and non-stressed animals in early lactation.
Fig. 5.
Functional connectivity with the anterior cingulate cortex is reduced by stress during early lactational period. Top, composite statistical maps of functional connectivity with the anterior cingulate cortex (green arrows show seed region). Bottom, functional connectivity between anterior cingulate cortex and several regions of interest. Data presented as mean ± standard error. *Significantly different (two way ANOVA, p <0.05).
Discussion
Early life exposure to CSS in F1 dams resulted in depressed maternal care specifically during early lactation, consistent with previous CSS studies (Carini and Nephew 2013; Murgatroyd and Nephew 2013) and induced changes in functional connectivity in regions associated with sensory processing, maternal and emotional responsiveness, memory, and the reward pathway in general. Behavioral results confirm that the ECSS model is not adversely affected by the fMRI acclimation procedure or institutional variations in behavioral testing and animal husbandry. This behavioral and functional neuroanatomical foundation can now be used to enhance our understanding of the neural etiology of early life stress associated disorders and test preventative measures and treatments for stress related disorders.
ECSS depressed both pup grooming and nursing only during early lactation, and the lack of effects during mid and late lactation suggest that ECSS exposed dams were adjusting their maternal responsiveness across lactation, possibly in response to increased vocalizations from pups (Okabe, Nagasaw et al. 2013). Similar to the effects of cocaine on maternal care (Mattson, Williams et al. 2003; Seip and Morrell 2007), the effects of ECSS may be a combination of direct depression of maternal care and effects on offspring solicitation of maternal care. This behavioral change is paralleled by the RSFC data, where the majority of differences in clustering coefficient, node strength, and connectivity were in the early and mid lactation imaging group. It is postulated that the differences in connectivity between the groups would have been more extensive (more similar to the data from imaging of the animals as nulliparous adults (Nephew, Huang et al. 2017)) with adequate sample sizes for early, mid and late lactation, and that the alterations in RSFC across lactation mediated the improvement in maternal care.
Previous fMRI research in the maternal rat used paradigms to evoke blood oxygenation level dependent (BOLD) fMRI activation in brain circuits (Febo, Numan et al. 2005; Ferris, Kulkarni et al. 2005; Febo, Stolberg et al. 2008; Febo, Shields et al. 2009; Febo and Pira 2011). These previous studies almost exclusively focused on delivering affective stimuli to dams (i.e., drugs, pups, predator odors) and they defined functional activation properties of the maternal reward system. Complementary to evoked responses in standard fMRI studies, intrinsic resting state activity reflects ongoing, continuous functional interactions between communicating brain circuits (Raichle 2011) and may thus offer a biologically meaningful measure to corroborate changes in maternal brain activity linked to chronic disease states such as perinatal depression. Nephew and colleagues were the first to apply resting state fMRI to determine the effects of chronic stress on nulliparous female brain functional circuitry of the F1 offspring of CSS dams used in the present study (Nephew, Huang et al. 2017). They reported a complex pattern of increased or reduced functional connectivity involving prefrontal striatal, basal forebrain, amygdalar, hippocampal, midbrain, somatosensory and midbrain regions. Of note were significant increases in functional connectivity between the NAc and perirhinal cortex (PRC) (spatial/olfactory/social memory) (Lévy, Keller et al. 2004; Bachevalier and Nemanic 2008; Feinberg, Allen et al. 2012), suppressed neural interactions between the NAc and hippocampus, and a shift in positive to negative correlated activity between the SSC and MC. Changes in functional interactions between these regions could arise from alterations in spatial, olfactory, and social memory and reward processing in the ECSS female rat brain.
In the present study we utilized graph theory metrics to assess functional network organization across 75 ROI’s of maternal rats (also F1 ECSS offspring of F0 CSS dams). Analyzing pairwise correlations between nodes in a symmetrical graph using these simple algorithms facilitates the general assessment of overall brain network organization (i.e., how pairwise interactions are organized across the maternal rat brain). Results show that the potential for efficient communication (average path length) and community structure (modularity) is unaffected in ECSS dams. However, clustering coefficient, which describes the tendency for nodes to group, is reduced in early lactation. This reduction in clustering coefficient could have a deleterious impact on expression of maternal behaviors reliant upon neural interactions between regions forming such clustered functional networks. To assess which regions might be affected by ECSS we analyzed node strength for each ROI. We found that the ACC, along with the PVT, SSC, and superior colliculus showed reduced node strength in early lactation and the prelimbic and temporal cortices in late lactation. Since node strength is the sum of correlation coefficients of all ROI connected with a node, these results reflect overall changes in functional connectivity in these regions. As stated in the preceding sections, the ACC showed significant changes in functional connectivity with other regions, namely, the MC, mPOA, PAG, PrL, SSC, and thalamus. Several of these regions overlap with results obtained in these animals tested under similar conditions prior to mating (Nephew, Huang et al. 2017). Thus, some prepregnancy alterations in neural interactions seem to persist through the early postnatal period, likely the result of substantial effects of chronic social stress during early life on the neurodevelopment of structural connectivity.
The maternal brain goes through substantial changes during pregnancy, parturition, and lactation, so it is generally not surprising that the RSFC results from F1 adult females (Nephew, Huang et al. 2017) and dams differ. The unifying feature of both sets of imaging data is the changes in SSC RSFC. The SSC has previously been identified as a key region in the conscious neural response of lactating dams to male intruders and vasopressin, a key maternal hormone (Nephew, Caffrey et al. 2009; Caffrey, Nephew et al. 2010). The changes in SSC connectivity are also generally consistent with neural imaging studies of other rodent models of early life stress (Holschneider, Guo et al. 2016) and depression (Ben-Shimol, Gass et al. 2015; Henckens, van der Marel et al. 2015). One study that investigated the impact of transgenerational early life stress reported decreased connectivity in the ACC and MC of female F2 mice (Razoux, Russig et al. 2016), and the substantial behavioral deficits in F2 offspring in the CSS model (Babb, Carini et al. 2014) suggest that social stress is having similar effects on connectivity, possibly through inflammatory mechanisms (Murgatroyd, Babb et al. 2016). The present results confirm that CSS has long term effects on neural connectivity which vary based on developmental stage and indicate that these changes mediate the temporally specific behavioral effects of social stress on offspring. Changes in node strength and/or functional connectivity in several regions will be discussed.
Anterior Cingulate and Prelimbic Cortices
The ACC plays a particularly important role in mediating appropriate responses to infant stimuli. In response to infant cry, mothers with lower depressive symptoms have increased activity in the dorsal ACC (Laurent and Ablow 2012; Laurent and Ablow 2012). ACC activity is associated with empathy, and it is suggested that difficulty in evaluating and responding to infant needs may be due to ACC dysfunction (Decety and Meyer 2008). In support of this hypothesis, parents show enhanced neural activation in the AC in response to the stimuli of their own infants compared to responses to unknown infants (Strathearn, Li et al. 2008; Swain, Tasgin et al. 2008). Imaging of depressed mothers vs. healthy controls reports decreases in the ACC (Deligiannidis, Sikoglu et al. 2013), and prenatal maternal depression, which can be considered an ELS for infants, is associated with RSFC changes in the ACC of the infants at six months (Qiu, Anh et al. 2015). Early life stress is specifically associated with thinning of the ACC in females (Gupta, Labus et al. 2016), and later childhood ELS is also associated with reductions in ACC volume (Cohen, Grieve et al. 2006; Baker, Williams et al. 2013). While RFSC was not assessed in these studies, it is likely that such large morphological changes altered RFSC. Therefore, the majority of studies report reduced activity in the ACC of mothers that exhibit decreased responsiveness to their infants. This hypoactivity in the ACC may be accompanied by reduced communication between this structure and other downstream regions, including cortical areas critical to responding to infant sensory cues (e.g. the somatosensory cortex). It is interesting to note that node strength was significantly reduced with early life stress, along with the clustering coefficient. These provide an overall assessment of the integrative capabilities of the functional network and suggest that the ACC displays impaired network integration.
In rodent models, gestational restraint stress induces postpartum depressive behavior and this behavioral effect is associated with altered structural plasticity in the NAc (Haim, Sherer et al. 2014). Similar actions could be occurring within the ACC of ECSS dams, resulting in decreased connectivity. The present decrease in ACC connectivity supports the clinical observations and further investigations attempting to ameliorate the negative effects of CSS, such as with intranasal oxytocin (IN OXT), can focus on the ACC as a potential predictor of treatment efficacy. IN OXT administration in human mothers increases functional connectivity between the amygdala and ACC, and it is proposed that OXT decreases negative emotions and increases the salience of infant cues through this change in connectivity (Riem, van Ijzendoorn et al. 2012). However, the effects of exogenous OXT are complex and/or not clear (Bales, Perkeybile et al. 2013; Miller, Bales et al. 2013; Weinstein, Bales et al. 2014; Perkeybile, Delaney-Busch et al. 2015; Steinman, Duque-Wilckens et al. 2015; Shamay-Tsoory and Young 2016), and exogenous peripartum oxytocin exposure is associated with an increased risk of depressive and anxiety disorders (Kroll-Desrosiers, Nephew et al. 2017). Increased coordinated study of the effects of OXT on behavior/mood and ACC function in both animal models and humans may clarify the behavioral role of this peptide. Thus, it will be interesting in future studies to determine the effects of OXT on ACC connectivity and integration, and whether chronic early life stress might modify the modulatory role of OXT on brain network connectivity.
Gestational stress in rats decreases spine density and alters spine morphology in mPFC pyramidal neurons (Leuner, Fredericks et al. 2014), and SSRI antidepressant administration is effective in reversing these stress induced changes in the NAc and mPFC (Haim, Albin-Brooks et al. 2016). Postnatal days 2–11, which covers the ECSS exposure period, is a critical period for 5-HT mediated development in the mPFC. It is suggested that increased 5-HT signaling during this period mediated anxiety and fear related changes in this area (Rebello, Yu et al. 2014). Decreased PFC activity and connectivity has been documented in a broad range of clinical studies in mothers with affective symptoms (Moses-Kolko, Perlman et al. 2010; Laurent and Ablow 2012; Schechter, Moser et al. 2012; Moser, Aue et al. 2015). Furthermore, glutamate dysregulation in the mPFC is associated with PPD (McEwen, Burgess et al. 2012). ACC – PrL connectivity was lower in ECSS dams during early lactation, suggesting that PrL connectivity, but not node strength, may be another neural alteration involved in the depressed maternal care.
Periventricular thalamus
While this region has not been a major focus of maternal related studies, artificial stimulation of the vagina and cervix in sheep increases c-Fos expression in the periventricular complex (Da Costa, Broad et al. 1997). However, it has been implicated in the control of reward mediated behavior (Hamlin, Clemens et al. 2009; Igelstrom, Herbison et al. 2010), and the changes in maternal care in the current study are clearly indicative of impaired responding to the reward of pup based cues. Orexin expression in the PVT mediates its role in reward based feeding, and while ECSS dams exhibit decreased orexin and orexin receptor expression in the supra optic nucleus (Murgatroyd, Peña et al. 2015); expression in the PVT has not been examined. It is possible that similar changes in orexin in the PVT are involved in the decreases in node strength and maternal care during early lactation.
Somatosensory Cortex
While the SSC has not been a central focus of ELS research, ELS exposed mice exhibit an increase in the turnover rate of mushroom spines in the SSC of both juveniles and adults (Takatsuru, Yoshitomo et al. 2009). The authors conclude that ELS destabilizes synaptic formation in this region, which adversely impacts somatosensory functions through a general depression in neural activity and reduced communication with other regions. This mechanism be may be responsible for the decreased RSFC between the ACC and SSC in the CSS F1 dams, mediating the depressed maternal care during early lactation. Previous studies have used maternal separation to induce changes in the SSC and the effects of CSS may have involved a similar mechanism, as F1 animals are exposed to decreased maternal care from their F0 mothers. It has been suggested that ELS induced developmental changes in the SSC are mediated by microglia (Takatsuru and Koibuchi 2015), and that is also a likely neural mechanism for the neural effects of CSS. Behaviorally, it is postulated that CSS may impair maternal responding in F1 dams through a reduction in the salience of multiple pup related olfactory, auditory, and somatosensory stimuli; similar to observations of human mothers (Decety and Meyer 2008; Laurent and Ablow 2012; Laurent and Ablow 2012; Musser, Kaiser-Laurent et al. 2012). While pup contact with the ventral surface elicits a robust reward response and associated high levels of pup directed behavior in control dams, it may induce less of a response in CSS F1 dams which results in decreased durations of pup grooming and nursing. The temporal expression of both the RSFC changes in the ACC-SSC circuit, which reflect overall change in the clustering coefficient, and the changes in maternal care are coordinated; both are only present during early lactation. It is postulated that the behavioral change across lactation (amelioration of depressed maternal care) is also neuronally mediated by associated changes in RSFC. While pup vocalization data are not available, it is suggested that increased pup calling during mid and late lactation in response to the deficient maternal care during early lactation mediates the changes in RSFC and maternal care. This hypothesis could be tested with pup stimulus based fMRI (tactile, olfactory, auditory) and cross fostering studies.
Superior Colliculus
Rodent studies have reported that dopamine actions in the SC modulate attention towards salient stimuli (Bolton, Murata et al. 2015), and investigations in primates have established that this region is involved in the establishment of visual saliency (White, Berg et al. 2017). The decrease in SC node strength in ECSS dams, possibly in combination with the several other reward related changes in neural activity and connectivity, may lower the saliency of pup cues, resulting in the depressed levels of pup care during early lactation.
Primary Motor Cortex
Connectivity between the SSC and motor regions was disrupted in these rats as virgin females (Nephew, Huang et al. 2017). The decreased ACC-M1 connectivity in these animals as dams may impair the physical coordination necessary for typical levels of pup grooming and nursing during early lactation. This type of neural alteration could also affect pup retrieval, which was unable to be assessed in the present study but has been disorganized in previous studies of ECSS dams (Carini and Nephew 2013).
Medial Preoptic Area
Oxytocin neurons in the in the mPOA regulate dopamine function in the ventral tegmental area, mediating the reward salience of pups (Shahrokh, Zhang et al. 2010; Lippard, Jarrett et al. 2015). In AVP deficient Brattleboro rats which exhibit impaired maternal care, cFOS expression in the mPOA is increased (Fodor, Klausz et al. 2012), and this may indicate disrupted functional connectivity with other regions. In addition, rats that display low levels of maternal care exhibit lower oxytocin receptor binding in the mPOA (Francis, Champagne et al. 2000). Previous study of ECSS dams has revealed maternal care and anxiety associated disruptions in both oxytocin and vasopressin related gene expression in the amygdala and PVN; expression in the mPOA has not been examined (Murgatroyd, Peña et al. 2015). The disrupted connectivity between the ACC and mPOA in ECSS dams may be mediated by changes in oxytocin receptor expression.
Periaqueductal Gray
Regions of the PAG are differentially involved in the control of rodent maternal care (Lonstein and Stern 1997; Lonstein, Simmons et al. 1998; Lonstein and Stern 1998). In human mothers, increased PAG activity is observed in response to pictures of a mother’s own child vs. an unknown child (Bartels and Zeki 2004; Noriuchi, Kikuchi et al. 2008). The decrease in ACC-PAG connectivity in ECSS dams may decrease the reward salience of pups, resulting in depressed maternal care during early lactation. The improvement in connectivity from early to late lactation (similar levels between groups during late lactation) may be the result of cumulative exposure to pup related stimuli, similar to what is observed when nulliparous rats are induced to express maternal care by exposure to foster pups (Cohen and Bridges 1981).
Conclusions
Previous investigations of the CSS model combined with the current study present both neural and behavioral targets for the testing of potential preventative measures and treatments for the adverse behavioral effects of early life stress on mothers, their offspring and future generations. The results underscore important differences between adult females and dams, adding to concerns about the need for data on sex and life history stage dependent differences in stress related disease etiology (McCullough, de Vries et al. 2014; Prendergast, Onishi et al. 2014; Klein, Schiebinger et al. 2015). Extrapolating neuroimaging data from adult to maternal females may be misleading and specific focus on developmental stage is clearly needed in future imaging work. In maternal imaging, stage of lactation also needs to be considered given the present differences between early and late lactation and established transitions in maternal care across lactation (Reisbick, Rosenblatt et al. 1975). It is likely that changes in both maternal care and associated neural connectivity that are limited to early lactation, which is the key period for natural variation in care (Meaney 2001; Champagne, Francis et al. 2003), induce long term deficits in the physical and mental health of offspring. Future neuroimaging studies should attempt to combine detailed anatomical scans, including diffusion tensor imaging with RSFC and stimulus dependent imaging in longitudinal studies of ethologically relevant animal models.
One potential therapeutic manipulation for ameliorating the adverse effects of CSS on SSC connectivity and behavior is increased physical contact through enhancing maternal care and/or the use of artificial offspring grooming. An ongoing investigation is testing the effects of intranasal oxytocin and vasopressin on maternal care in F1 dams and F2 offspring, and there are beneficial behavioral effects on F2 offspring, possibly mediated by enhanced F1 maternal care. The manipulation of tactile stimulation in rodent pups has established effects on both maternal care (Gonzalez and Fleming 2002) and other social behavior (Melo, Lovic et al. 2006), and in humans, tactile stimulation of infants mediates the adverse effects of antenatal anxiety on children (Sharp, Hill et al. 2014; Murgatroyd, Quinn et al. 2015). While the obvious benefit of detailed fMRI studies is neural targeting for potential treatments, they may also provide critical behavioral insight and validation.
Fig. 4.
Composite 2D seed based functional connectivity maps of early lactation dams. Seed region is the anterior cingulate cortex (highlighted by green circle and arrows). Maps show both left and right representations and are FDR corrected (q=0.1, p<0.01). (A) maps of control dams. (B) maps of early life social stressed dams (EL Stress). (C) Statistical comparison of EL stres vs control dams (t-test, uncorrected p <0.05).
Highlights.
Resting state functional connectivity fMRI was used to assess long term effects of social stress.
Early life social stress depressed maternal care during early lactation.
Resting state functional connectivity was decreased during early lactation.
Decreased connectivity was observed in reward, emotion, memory, and sensory circuits.
Acknowledgments
The authors would like to thank Dr. Jessica Babb for assistance with the CSS protocol at the Cummings School and Kelly Tam for experimental logistics at the CCNI. The laboratory animal medicine service staff at the Cummings School and UMass provided exceptional care of our rodents and were extremely helpful in coordinating the required logistics. This work was supported by a National Institutes of Health award (NICHD R00 HD059943) and a Brain and Behavior Research Foundation NARSAD Young Investigator Award to BCN, NIH S10 OD018132-01 to the UMass CCNI, and a McKnight Brain Foundation postdoctoral fellowship for LCP.
Footnotes
Contributors
BCN designed and initiated the study, supervised the animal and imaging work, and contributed to the manuscript. MF conducted the analysis of the fMRI data and contributed to the manuscript, WH and LP conducted the fMRI experiments, GP assisted with data analysis and revision of the manuscript, OG completed the behavioral analyses, and JK supervised the fMRI work.
Conflict of interest
The authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babb JA, Carini LM, et al. Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav. 2014;65(4):386–393. doi: 10.1016/j.yhbeh.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Baker LM, Williams LM, et al. Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging and Behavior. 2013;7(2):196–203. doi: 10.1007/s11682-012-9215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, et al. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ben-Shimol E, Gass N, et al. Reduced connectivity and inter-hemispheric symmetry of the sensory system in a rat model of vulnerability to developing depression. Neuroscience. 2015;310:742–750. doi: 10.1016/j.neuroscience.2015.09.057. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boccaletti S, Latora V, et al. Complex networks: Structure and dynamics. Physics Reports. 2006;424(4–5):175–308. [Google Scholar]
- Bolton Andrew D, Murata Y, et al. A Diencephalic Dopamine Source Provides Input to the Superior Colliculus, where D1 and D2 Receptors Segregate to Distinct Functional Zones. Cell Reports. 2015;13(5):1003–1015. doi: 10.1016/j.celrep.2015.09.046. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Hormones and Behavior. 2016;77:193–203. doi: 10.1016/j.yhbeh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey MK, Nephew BC, et al. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology. 2010;58(1):107–116. doi: 10.1016/j.neuropharm.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, et al. Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp. 2013;(76):e50324. doi: 10.3791/50324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Nephew BC. Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Hormones and Behavior. 2013;64(4):634–641. doi: 10.1016/j.yhbeh.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, et al. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology & Behavior. 2003;79(3):359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Cohen J, Bridges RS. Retention of maternal behavior in nulliparous and primiparous rats: Effects of duration of previous maternal experience. J Comp and Physiol Psychol. 1981;95:40–45. [Google Scholar]
- Cohen RA, Grieve S, et al. Early Life Stress and Morphometry of the Adult Anterior Cingulate Cortex and Caudate Nuclei. Biological Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Coverdill A, McCarthy M, et al. Effects of Chronic Central Arginine Vasopressin (AVP) on Maternal Behavior in Chronically Stressed Rat Dams. Brain Sciences. 2012;2(4):589–604. doi: 10.3390/brainsci2040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Da Costa APC, Broad KD, et al. Olfactory memory and maternal behaviour-induced changes in c-fos and zif/268 mRNA expression in the sheep brain. Molecular Brain Research. 1997;46(1):63–76. doi: 10.1016/s0169-328x(96)00272-0. [DOI] [PubMed] [Google Scholar]
- Decety J, Meyer M. From emotion resonance to empathic understanding: A social developmental neuroscience account. Development and Psychopathology. 2008;20(4):1053–1080. doi: 10.1017/S0954579408000503. [DOI] [PubMed] [Google Scholar]
- Deligiannidis KM, Sikoglu EM, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: A preliminary study. Journal of Psychiatric Research. 2013;47(6):816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Numan M, et al. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Pira AS. Increased BOLD activation to predator stressor in subiculum and midbrain of amphetamine-sensitized maternal rats. Brain Research. 2011;1382:118–127. doi: 10.1016/j.brainres.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Shields J, et al. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Research. 2009;1302:183–193. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Stolberg TL, et al. Nursing stimulation is more than tactile sensation: It is a multisensory experience. Hormones and Behavior. 2008;54(2):330–339. doi: 10.1016/j.yhbeh.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg LM, Allen TA, et al. Recognition memory for social and non-social odors: Differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiology of Learning and Memory. 2012;97(1):7–16. doi: 10.1016/j.nlm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, et al. Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A, Klausz B, et al. Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Hormones and Behavior. 2012;62(4):539–551. doi: 10.1016/j.yhbeh.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, et al. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12(12):1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Fleming AS. Artificial rearing causes changes in maternal behavior and c-fos expression in juvenile female rats. Behav Neurosci. 2002;116(6):999–1013. doi: 10.1037//0735-7044.116.6.999. [DOI] [PubMed] [Google Scholar]
- Gupta A, Labus J, et al. Interactions of early adversity with stress-related gene polymorphisms impact regional brain structure in females. Brain Structure and Function. 2016;221(3):1667–1679. doi: 10.1007/s00429-015-0996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Albin-Brooks C, et al. The effects of gestational stress and SSRI antidepressant treatment on structural plasticity in the postpartum brain - a translational model for postpartum depression. Hormones and Behavior. 2016;77:124–131. doi: 10.1016/j.yhbeh.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Sherer M, et al. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. European Journal of Neuroscience. 2014;40(12):3766–3773. doi: 10.1111/ejn.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, et al. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. European Journal of Neuroscience. 2009;29(4):802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, van der Marel K, et al. Stress-induced alterations in large-scale functional networks of the rodent brain. NeuroImage. 2015;105:312–322. doi: 10.1016/j.neuroimage.2014.10.037. [DOI] [PubMed] [Google Scholar]
- Hicks-Nelson A, Beamer G, et al. Transgenerational Social Stress Alters Immune–Behavior Associations and the Response to Vaccination. Brain Sciences. 2017;7(7):89. doi: 10.3390/brainsci7070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Jacobs VR, et al. The Maternal Brain: An Organ with Peripartal Plasticity. Neural Plasticity. 2014;2014:20. doi: 10.1155/2014/574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Guo Y, et al. Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats. Neurobiology of Stress. 2016;3:8–22. doi: 10.1016/j.ynstr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Gurney K. Network ‘small-world-ness’: a quantitative method for determining canonical network equivalence. PLoS ONE. 2008;3(4):e0002051. doi: 10.1371/journal.pone.0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom KM, Herbison AE, et al. Enhanced c-Fos expression in superior colliculus, paraventricular thalamus and septum during learning of cue-reward association. Neuroscience. 2010;168(3):706–714. doi: 10.1016/j.neuroscience.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Bardi M, et al. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Archives of sexual behavior. 2008;37(1):43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. Reproduction-Induced Neuroplasticity: Natural Behavioural and Neuronal Alterations Associated with the Production and Care of Offspring. Journal of Neuroendocrinology. 2008;20(4):515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, et al. Opinion: Sex inclusion in basic research drives discovery. Proceedings of the National Academy of Sciences. 2015;112(17):5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll-Desrosiers AR, Nephew BC, et al. Association of peripartum synthetic oxytocin administration and depressive and anxiety disorders within the first postpartum year. Depression and Anxiety. 2017;34(2):137–146. doi: 10.1002/da.22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci. 2012;7(2):125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. The missing link: mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behav Dev. 2012;35(4):761–772. doi: 10.1016/j.infbeh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, et al. Chronic Gestational Stress Leads to Depressive-Like Behavior and Compromises Medial Prefrontal Cortex Structure and Function during the Postpartum Period. PLoS ONE. 2014;9(3):e89912. doi: 10.1371/journal.pone.0089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy F, Keller M, et al. Olfactory regulation of maternal behavior in mammals. Hormones and Behavior. 2004;46(3):284–302. doi: 10.1016/j.yhbeh.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Liang Z, King J, et al. Uncovering Intrinsic Connectional Architecture of Functional Networks in Awake Rat Brain. The Journal of Neuroscience. 2011;31(10):3776–3783. doi: 10.1523/JNEUROSCI.4557-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Liu X, et al. Dynamic resting state functional connectivity in awake and anesthetized rodents. NeuroImage. 2015;104:89–99. doi: 10.1016/j.neuroimage.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippard ETC, Jarrett TM, et al. Early postpartum pup preference is altered by gestational cocaine treatment: Associations with infant cues and oxytocin expression in the MPOA. Behavioural Brain Research. 2015;278:176–185. doi: 10.1016/j.bbr.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, et al. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behavioral Neuroscience. 1998;112(6):1502–1518. doi: 10.1037//0735-7044.112.6.1502. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(9):3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal and aggressive behaviors in rats. Brain Research. 1998;804(1):21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, et al. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacolgy. 2003;167(1):1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, de Vries GJ, et al. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biology of Sex Differences. 2014;5(1):15. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen AM, Burgess DT, et al. Increased Glutamate Levels in the Medial Prefrontal Cortex in Patients with Postpartum Depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24(1):1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Melo AI, Lovic V, et al. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Dev Psychobiol. 2006;48(3):209–219. doi: 10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- Miller M, Bales KL, et al. Oxytocin and Vasopressin in Children and Adolescents With Autism Spectrum Disorders: Sex Differences and Associations With Symptoms. Autism Research. 2013;6(2):91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser DA, Aue T, et al. The relation of general socio-emotional processing to parenting specific behavior: a study of mothers with and without posttraumatic stress disorder. Frontiers in Psychology. 2015;6(1575) doi: 10.3389/fpsyg.2015.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, et al. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. The American journal of psychiatry. 2010;167(11):1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Quinn JP, et al. Effects of prenatal and postnatal depression, and maternal stroking, at the glucocorticoid receptor gene. Transl Psychiatry. 2015;5(5):140. doi: 10.1038/tp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Babb JA, et al. Transgenerational Social Stress, Immune Factors, Hormones, and Social Behavior. Frontiers in Ecology and Evolution. 2016:3. doi: 10.3389/fevo.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology. 2013;38(2):219–228. doi: 10.1016/j.psyneuen.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Peña CJ, et al. Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care. Neuropeptides. 2015;52:103–111. doi: 10.1016/j.npep.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Taliefar M, et al. Social stress during lactation, depressed maternal care, and neuropeptidergic gene expression. Behavioural Pharmacology. 2015;26:642–653. doi: 10.1097/FBP.0000000000000147. (7 - Special Issue Pharmacological Approaches To The Study Of Social Behaviour - Part 2: Social Modul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, et al. The neural correlates of maternal sensitivity: an fMRI study. Dev Cogn Neurosci. 2012;2(4):428–436. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14(6):677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Caffrey MK, et al. Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. European Journal of Neuroscience. 2009;30(5):934–945. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Carini LM, et al. Intergenerational accumulation of impairments in maternal behavior following postnatal social stress. Psychoneuroendocrinology. 2017;82:98–106. doi: 10.1016/j.psyneuen.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Huang W, et al. Altered neural connectivity in adult female rats exposed to early life social stress. Behavioural Brain Research. 2017;316:225–233. doi: 10.1016/j.bbr.2016.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. The Structure and Function of Complex Networks. SIAM Review. 2003;45(2):167–256. [Google Scholar]
- Noriuchi M, Kikuchi Y, et al. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008;63(4):415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Okabe S, Nagasaw M, et al. Pup Odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behavioral Neuroscience. 2013 doi: 10.1037/a0032395. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Delaney-Busch N, et al. Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Frontiers in Behavioral Neuroscience. 2015;9:191. doi: 10.3389/fnbeh.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, et al. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014:40. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Qiu A, Anh TT, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry. 2015;5(2):e508. doi: 10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The Restless Brain. Brain Connectivity. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. NeuroImage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Razoux F, Russig H, et al. Transgenerational disruption of functional 5-HT1AR-induced connectivity in the adult mouse brain by traumatic stress in early life. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.146. [DOI] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, et al. Postnatal Day 2 to 11 Constitutes a 5-HT-Sensitive Period Impacting Adult mPFC Function. The Journal of Neuroscience. 2014;34(37):12379. doi: 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbick S, Rosenblatt JS, et al. Decline of maternal behavior in the virgin and lactating rat. Journal of Comparative and Physiological Psychology. 1975;89(7):722–732. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- Riem MME, van Ijzendoorn MH, et al. No Laughing Matter: Intranasal Oxytocin Administration Changes Functional Brain Connectivity during Exposure to Infant Laughter. Neuropsychopharmacology. 2012;37(5):1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Saramäki J, Kivelä M, et al. Generalizations of the clustering coefficient to weighted complex networks. Physical Review E. 2007;75(2):027105. doi: 10.1103/PhysRevE.75.027105. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, et al. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc Cogn Affect Neurosci. 2012;7(8):969–979. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacolgy. 2007;194(3):309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, et al. Oxytocin-Dopamine Interactions Mediate Variations in Maternal Behavior in the Rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Young LJ. Understanding the Oxytocin System and Its Relevance to Psychiatry. Biol Psychiatry. 2016;79(3):150–152. doi: 10.1016/j.biopsych.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp H, Hill J, et al. Maternal antenatal anxiety, postnatal stroking and emotional problems in children: outcomes predicted from pre- and postnatal programming hypotheses. Psychol Med. 2014;28:1–15. doi: 10.1017/S0033291714001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, et al. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry. 2015;19(15):00824–00820. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, et al. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics. 2008;122(1):40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, et al. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(10):1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuru Y, Koibuchi N. Alteration of somatosensory response in adulthood by early life stress. Frontiers in Molecular Neuroscience. 2015;8:15. doi: 10.3389/fnmol.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuru Y, Yoshitomo M, et al. Maternal separation decreases the stability of mushroom spines in adult mice somatosensory cortex. Brain Res. 2009;19:45–51. doi: 10.1016/j.brainres.2009.07.092. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Weinstein TAR, Bales KL, et al. Early involvement in friendships predicts later plasma concentrations of oxytocin and vasopressin in juvenile rhesus macaques (Macaca mulatta) Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Berg DJ, et al. Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. 2017;8:14263. doi: 10.1038/ncomms14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside B. Mood, Food, and Fertility: Adaptations of the Maternal Brain. Compr Physiol. 2016;6(3):1493–1518. doi: 10.1002/cphy.c150036. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, et al. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Rane P, et al. Mapping resting-state brain networks in conscious animals. Journal of Neuroscience Methods. 2010;189(2):186–196. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]