Abstract
In cortical and hippocampal neurons of the mammalian brain, the synaptic vesicle cycle is a series of steps that tightly regulate exo- and endocytosis of vesicles. Many proteins contribute to this regulation, but lipids have recently emerged as critical regulators as well. Of all the many lipid signaling molecules, phosphatidic acid is important to the physical processes of membrane fusion. Therefore, the lipid-metabolizing enzymes that produce phosphatidic acid are vital to the regulation of the cycle. Our lab is particularly interested in the potential regulatory mechanisms and neuronal roles of two phosphatidic acid-producing enzymes: diacylglycerol kinase theta (DGKθ) and phospholipase D (PLD). We recently discovered a regulatory role of DGKθ on evoked endocytosis (Goldschmidt et al. 2016). In addition to this enzyme, studies implicate PLD1 in neurotransmission, although its precise role is of some debate. Altogether, the production of phosphatidic acid by these enzymes offer an interesting and novel pathway for the regulation of the synaptic vesicle cycle.
Keywords: Phospholipase D, Diacylglycerol Kinase, Neurons, Boutons, Mitochondria
1. Introduction
In the mammalian central nervous system, proper brain function is dependent upon efficient communication between neurons. This communication occurs at the synapse, where a signal is propagated from one neuron to another. The signal is transmitted in the form of an action potential, which stimulates the exocytic release of neurotransmitter-filled synaptic vesicles from the presynaptic neuron. Neurotransmitters travel across the synapse and bind to postsynaptic receptors, where an array of postsynaptic signaling propagates the signal further. The regulated control of synaptic vesicle exocytosis and subsequent endocytosis is crucial to proper neuronal communication while maintaining neuronal architecture. This is achieved in part by the synaptic vesicle cycle, where exocytic release of neurotransmitter-filled vesicles is balanced by the endocytosis of portions of the presynaptic membrane, which are re-acidified and filled with neurotransmitters, and prepared for further rounds of release. Each step of this cycle is vital to the efficiency of signal propagation, and so an abundance of proteins function at each step to regulate the cycle (Sudhof 1995). There has been abundant research leading to the identification of these proteins and their regulatory roles. For example, Munc13-1 is required for synaptic vesicle release, as it functions to prime and dock vesicles just before their exocytic release (Augustin et al. 1999). Synaptotagmin-2 is a crucial calcium sensor that blocks the complete binding of soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins on docked synaptic vesicles until the influx of calcium following an action potential (Garcia et al. 2000). Additionally, dynamin-1 is a crucial regulatory protein that functions to pinch vesicles off the membrane following endocytosis into the presynaptic bouton (Cocucci et al. 2014). Although these proteins and many others are crucial regulators of the synaptic vesicle cycle, lipid molecules also serve as important regulators. Lipid molecules comprise the membranes of the boutons and synaptic vesicles and therefore are the most important players in the membrane fusion events of exo- and endocytosis. Further, lipids are important signaling molecules as they interact with and regulate many proteins themselves. Two lipids that have received considerable attention in relation to the synaptic vesicle cycle are diacylglycerol (DAG) and phosphatidic acid (PtdOH) (Chasserot-Golaz et al. 2010, Puchkov et al. 2013, Davletov et al 2010, Rohrbough and Broadie 2005).
DAG is an important lipid that has well-established roles in neurotransmission. Upon binding, DAG activates protein kinase C (PKC), which targets SNARE proteins such as SNAP-25 and Munc-18 and regulates their activity via phosphorylation. DAG also binds Munc13-1, which is required for the complete priming of synaptic vesicles prior to exocytosis. Finally, DAG is the metabolic precursor to PtdOH, another signaling lipid implicated in neurotransmission. Diacylglycerol kinase theta (DGKθ) phosphorylates DAG to produce PtdOH (Ammar et al. 2013, Puchkov et al. 2013, Davletov et al 2010). Recent studies have suggested that DGKθ regulates the synaptic vesicle cycle, theoretically because of its production of PtdOH (Goldschmidt et al. 2016). It is widely understood that PtdOH is involved in exocytosis in a variety of cell types. As a cone-shaped lipid, PtdOH induces negative membrane curvature, the first step in promoting membrane fusion (Chasserot-Golaz et al. 2010, Ammar et al. 2013, Cazzolli et al. 2006, Raben 2017). In addition to this architectural role, the SNARE protein syntaxin-1a binds to and concentrates PtdOH in regions where exocytosis occurs in the membrane. This binding interaction is essential to membrane fusion; disruption of syntaxin-1a binding to PtdOH in PC12 cells depresses vesicle exocytosis (Lam et al. 2008). Additionally, PtdOH is the metabolic precursor to phosphatidylinositol 4,5-biphosphate (PIP2) and activates a PI kinase (Moritz et al. 1992) required for production of PIP2 in neurons (Volpicelli et al. 2010, Rohacs 2016). PtdOH may also be involved in mediating the function of dynamin, a protein implicated in synaptic vesicle endocytosis (Burger et al. 2000). Together, these insights suggest that PtdOH has a role in regulating the synaptic vesicle cycle. The only endogenous sources of PtdOH in neurons are DGKθ (phosphorylation of DAG), PLD (hydrolysis of phosphatidylcholine), lysophosphatidic acidacyltransferase (LPAAT, acylation of lysophosphatidic acid), and de novo synthesis (Cazzolli et al. 2006, Raben 2017). The production of PtdOH via these three enzymes offer an interesting aspect of potential synaptic vesicle cycle regulation.
2. Materials and Methods
2.1 Electron Microscopy
The following electron microscopy sample preparation was performed as described previously (Goldschmidt et al. 2016). For the physical analysis of DGKθ KO and wild-type (WT) synapses, age- and sex-matched DGKθ KO and WT mice (~6 months old) were sacrificed and perfused with phosphate buffer followed by fixative solution. Brains were removed and fixed overnight. Brain sections containing the hippocampus and cortex were then cut down into smaller pieces for subsequent preparation. Similarly, DGKθ KO and WT neurons were grown on 18mm glass coverslips and transferred to 35mm tissue cultures dishes where they were rinsed and fixed. After a second rinse, brain sections and cultured cells were post-fixed, rinsed, and stained en-bloc. Then, sections and cells were dehydrated and embedded in Eponate 12. Sections of 80nm thickness were produced by a Diatome diamond knife and were picked up onto copper grid slots and further stained with uranyl acetate and lead citrate. These grids were imaged on a Hitachi 7600 TEM operating at 80kV and a XR50, 5 megapixel CCD camera captured digital images. This sample preparation was performed by Michael Delannoy in the Johns Hopkins University School of Medicine Microscope Facility. Image acquisition and data analysis was performed by Casey Barber.
3. DGKθ KO Synapses
Recently, our lab investigated the potential function of DGKθ in the synaptic vesicle cycle. Because of its role in production of PtdOH, we hypothesized that ablation of DGKθ, and therefore a reduction in the amount of PtdOH, would affect the exocytic rate of vesicles. There are ten isoforms of DGK, all found in the mammalian central nervous system, but at the time the function of DGKθ in neurons was unknown (Goldschmidt et al. 2016). However, there was evidence that an ortholog of DGKθ in C. elegans, DGK-1, suppressed acetylcholine release at the neuromuscular junction (Nurrish et al. 1999). With this being known, our lab characterized the role of DGKθ in mammalian cortical and hippocampal neurons. DGKθ is found throughout the brain, specifically in neurons, and is located primarily pre-synaptically on excitatory neurons. Using a pH-sensitive GFP as an optical reporter of synaptic vesicle endo- and exocytosis, we found that complete ablation of DGKθ in these neurons resulted in reduced rates of evoked endocytosis. These rates further increased following more intense stimulation. Interestingly, in contrast to our hypothesis, loss of DGKθ did not affect rates of exocytosis following depolarization (Goldschmidt et al. 2016).
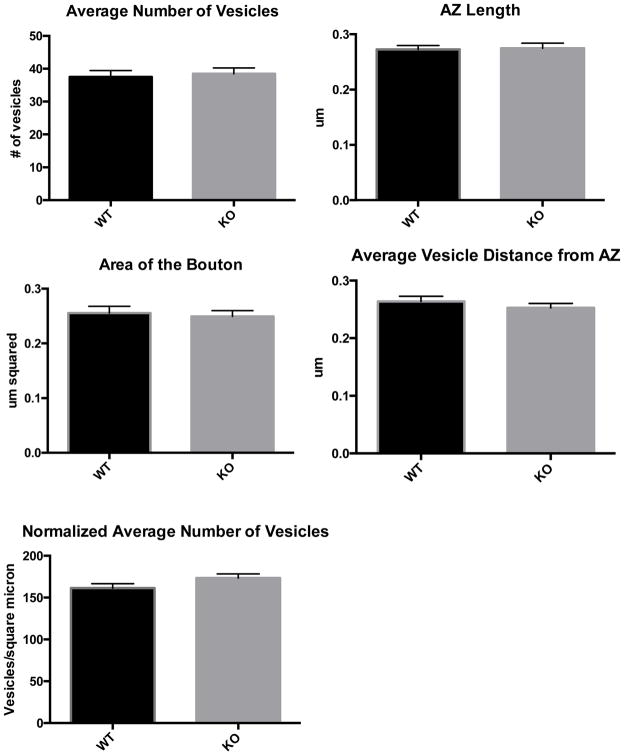
Having shown that loss of DGKθ results in reduced rates of synaptic vesicle endocytosis, we predicted that this might result in fewer synaptic vesicles within the presynaptic bouton of KO synapses and perhaps a difference in the size of the bouton. To address this, we conducted an analysis of over 200 electron micrographs of DGKθ KO and WT synapses in cortical and hippocampal brain slices and neuron cultures. Using ImageJ to characterize each synapse, we measured the number of vesicles in each synapse, the area of the bouton, the length of the active zone, and the average distance of each vesicle from the active zone (the portion of the presynaptic membrane where vesicles are exocytosed). The average of each value for each genotype reflected that there was no comparative difference between DGKθ KO and WT synapses (Figure 1). Upon further examination, we realized that these data supported our original finding that loss of DGKθ produces slower rates of evoked endocytosis. The images used for the analysis in Figure 1 were taken of unstimulated neurons and therefore reflect the basal characteristics of synapses at rest. Our data on the recycling rates of DGKθ KO synapses only suggested a difference in evoked endocytic rates, therefore a difference in the number of vesicles per synapse would perhaps only exist in depolarized neurons.
Figure 1. Cortical and hippocampal neurons from DGKθ KO and WT mouse brain slices.
Cortical and hippocampal brain slices from DGKθ KO and WT mice were subjected to TEM and the 1) number of vesicles, 2) number of vesicles normalized to the size of the bouton, 3) area of the bouton, 4) length of the active zone, and 5) average vesicle distance to the active zone were quantified. No significant differences were detected in the average or normalized number of vesicles per synapse, area of the bouton, active zone length and average vesicle distance from the active zone between DGKθ KO and WT synapses in brain slices. N= 70 for each genotype. Age ~ 6 months. Error bars = SEM. AZ = active zone.
4. Mitochondria in DGKθ KO Neurons
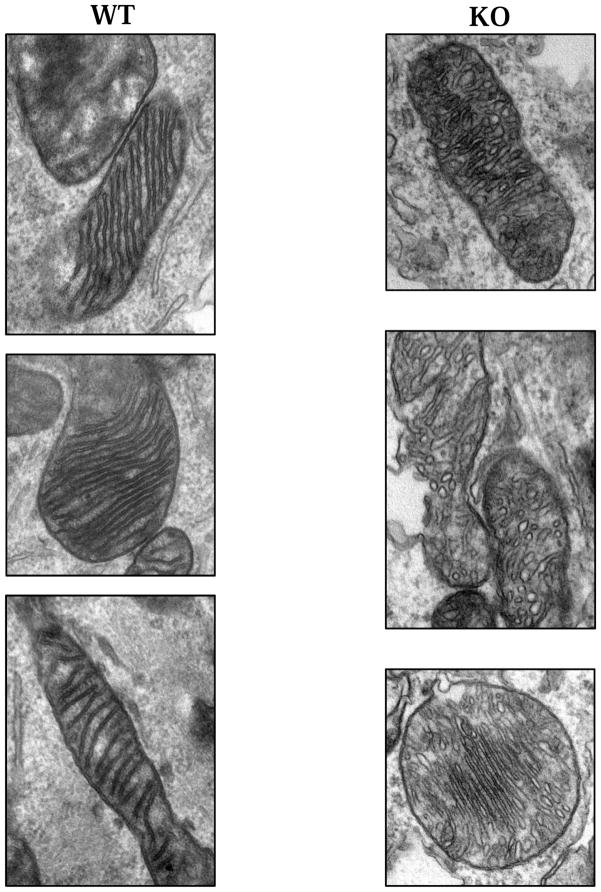
Mitochondria located near or within presynaptic boutons are critical to the synaptic vesicle cycle as they produce ATP needed to drive membrane fusion during exo- and endocytosis (Pathak et al. 2015). Upon further examination of the DGKθ and WT synapses in the electron micrographs used for analysis in Figure 1, we noticed a peculiarity in the morphology of the mitochondria found in the DGKθ KO neurons. Mitochondria in WT synapses have long, continuous, parallel cristae that are narrow and consistently darkly-stained in micrographs. Comparatively, some of the mitochondria found in the DGKθ KO synapses had strikingly different shaped cristae. They often appear disfigured, a heterogeneous mix of globular shapes. Figure 2 shows some examples of the mitochondria found in each genotype. It should be noted that this was observed in cultured neurons and that this is unlikely an artifact of sample processing as this phenomenon was observed consistently in DGKθ KO neurons but not WT neurons. It is important to emphasize that this is purely an observation without quantitative or qualitative analyses. It is unknown as to whether this morphological attribute results in any functional phenotype, but surely the disruption in cristae morphology may affect the efficiency of ATP production through the electron transport chain. Further studies are required to resolve this issue.
Figure 2. Mitochondria in DGKθ KO and WT synapses.
Cultured cortical and hippocampal neurons from DGKθ and WT mice were subjected to TEM and mitochondrial morphology was examined. In cultured mouse cortical and hippocampal neurons, the mitochondria found in DGKθ KO synapses look strikingly different from those found in WT synapses. Compared to WT, DGKθ KO mitochondria have short, globular-looking cristae. This observation is representative of three experiments. DIV14 cultures.
5. DGKθ KO Mouse Model
While further biochemical analyses are underway to examine the potential mechanism of DGKθ’s regulatory role in the synaptic vesicle cycle, we are also working to characterize the DGKθ KO mouse model. Although DGKθ KO mice appear normal upon simple observation, we hypothesize that the regulatory function of DGKθ during evoked endocytosis might have a functional phenotype in another pathway. To address this, we conducted simple assays to test for differences in hearing and sight between DGKθ KO and WT mice. Using the auditory brainstem response (ABR) assay as a crude readout of hearing ability, we found that there was no difference in hearing between the DGKθ KO and WT mice (Hunter and Willot 1987, Laumen et al. 2016, Blatchley et al. 1987). However, using electroretinographs (ERGs) to crudely study visual acuity in these mice, we discovered that there was a significant difference in the B-wave of the ERGs of the DGKθ KO mice (data not shown). The B-wave portion of ERGs is produced by bipolar cells, those cells downstream of the photoreceptors (Porciatti 2007, Pinto et al. 2007). These tests need to be replicated and further analysis should be conducted, but the recycling defect seen in DGKθ KO neurons could have a functional phenotype in these cells as well.
6. Phospholipases D
In addition to DGKθ, our lab is also interested in whether the production of PtdOH by PLD is also a point of regulation of the synaptic vesicle cycle. PLD catalyzes the hydrolysis of phosphatidylcholine (PC) to produce PtdOH. PLD was first cloned from castor beans and there are currently six known isoforms of this enzyme. The most widely studied isoforms are the mammalian PLD1 and PLD2 isoforms. Although they only have about 50% sequence similarity, their structures are quite similar. Both PLD1 and PLD2 contain a Phox domain, a pleckstrin homology domain, and a PIP2 binding domain. They also contain four highly conserved regions (CRI-IV) which are thought to come together when the protein folds to form the catalytic core. Conserved regions II and IV contain the invariant charged motif sequence HxxxxKxD (HKD domain) containing histidine (H), any amino acid (x), lysine (K), and aspartic acid (D). These two domains are responsible for catalytic activity; any mutation within these domains produces a catalytically dead enzyme. PLD3 and PLD4 are transmembrane proteins located in the endoplasmic reticulum (ER). Currently no enzymatic activity has been reported for either of these isoforms and it is thought that they instead have non-enzymatic functions in the ER. There are currently no publications on PLD5, but it is known that this isoform does not contain the typical conserved catalytic motifs of the other PLDs, so it is unlikely that this isoform has catalytic activity as well. PLD6 (MitoPLD) is specifically located on the outer membrane of mitochondria and functions to hydrolyze cardiolipin, a lipid specific to mitochondria (Bruntz et al. 2014, Cockcroft et al. 2001).
Currently most of the research on the mammalian PLDs has focused on PLD1 and PLD2. It is known that PLD1 has low basal activity in vitro, is activated by ADP ribosylation factor (ARF), PKC (α and β), and the small G-proteins RhoA and Rac1. It requires PIP2 as a cofactor and has been reported to be primarily localized to intracellular environments (Jones et al. 1999). PLD2 also requires PIP2 as a cofactor, but in contrast to PLD1, has high basal activity in vitro and has been reported to be localized almost exclusively to the plasma membrane. PLD2 is not activated by ARF or RhoA, but it is thought that Rac2 might regulate its activity (Gomez-Cambronero 2011).
5.1 Phospholipases D in the brain
While there is interest in elucidating the potential role of PLDs in neurons and neurological diseases, there seems to be a fundamental disagreement on the expression and localization of PLD1 and PLD2 in the mammalian brain. In 2000, Saito et al. examined the localization of PLD1 and PLD2 mRNA in the developing and mature rat brain. They concluded that PLD1 mRNA is expressed in presumptive oligodendrocytes, while PLD2 mRNA was expressed in presumptive astrocytes. They also found that PLD2 mRNA is transiently expressed in early postnatal neurons. In 2004, Zhang et al. studied expression of the two mammalian PLD isoforms during experimentally induced hippocampal mossy fiber outgrowth. Immunohistochemically, they were able to detect both isoforms in mouse hippocampal neurons and found that expression of both increased significantly after stimulation, particularly PLD2. They also detected expression of PLD1 in astrocytes and found no expression of PLD2 in astrocytes or microglia.
More recently, a few papers have reported a time course of PLD1 expression in maturing neuronal cell cultures. Zhu et al. 2012 reports that both PLD1 protein expression and activity peak at DIV5, then decrease by more than 50% and maintain this level through DIV11 in rat hippocampal cultures. In 2015, Ammar et al. reported that PLD1 protein expression peaked at DIV6, followed by a 5-fold decrease in expression to nearly negligible amounts at DIV12 in mouse cortical neuron cultures. Most recently, Zhu et al. 2016 reported that expression of PLD1 in rat neuron cultures was nearly undetectable from DIV9-15, highlighting that expression of PLD1 was 7 times greater in astrocytes of the same DIV. They also used an astrocyte-specific PLD1shRNA to show that PLD1 from astrocytes affects dendritic branching through secreted signals. This suggests that PLD1 derived from astrocytes has a functional effect on neurons as well.
As all three of these studies used the same commercially available PLD1 antibody to produce slightly to dramatically different results, we conclude that there is currently a discrepancy in the field about the expression and localization of PLD1 and PLD2 protein expression in the mammalian brain. This fundamental information requires clarification before further characterization of PLD isoforms in the mammalian central nervous system.
Apart from PLD1 protein expression, several studies have established a potential role for PLD1 in dendritic branching and even Alzheimer’s Disease (AD). Zhu et al. 2012 found that the transfection of WT PLD1 into DIV3 hippocampal neurons decreased the number of primary and secondary dendrites compared to the transfection of a control vector. In contrast, the transfection of dominant negative PLD1 increased the number of primary and secondary dendrites. In a subsequent paper, Zhu et al. transfected neuron-glial mixed cultures with a PLD1shRNA and found this decreased the number of dendrites and dendritic tips. Upon the addition of PtdOH to the knockdown cultures, they found this rescued the effect. In addition to dendritic branching, PLD1 has been linked to regulation of secretory granule exocytosis and neurite outgrowth in PC12 cells. It has also been reported that PLD1 expression increases in hippocampal neurons during seizure-induced mossy fiber sprouting. Several papers also report a link between PLD1 and Alzheimer’s Disease. Cai et al. 2006 found that PLD1 interacts with presenilin-1 (PS1). PS1 modulates the proteolysis of β-amyloid precursor protein (βAPP), plaques of which are found in the brains of AD patients. Additionally, Cai et al. found that the interaction of PLD1 with PS1 also affected the intracellular trafficking of βAPP. Jin et al. 2007 found that PLD1 expression and enzymatic activity were increased in brain tissue of AD patients. They reported that βAPP interacted with the pleckstrin homology domain in PLD1 and that the amyloid region of βAPP associated with PLD.
There are fewer studies focused on neuronal PLD2, although there are some interesting reports. It is thought that PLD2 localizes primarily to the plasma membrane and co-fractionates with caveolin, a key player in receptor-mediated endocytosis (Czarny et al. 1999). Though a slightly controversial topic, Payton et al. 2004 reported that all three naturally occurring synuclein isoforms (α, β, and γ) inhibit the enzymatic activity of PLD2. Additionally, Oliveira et al. 2010 has reported a potential link between PLD2 and Alzheimer’s Disease. The authors of the study found that in neuron cultures, the oligomeric amyloid β-peptide stimulated PLD enzymatic activity and that this affect did not occur in PLD2 KO neuron cultures. They found that the PLD activity was also increased in a transgenic mouse model of Alzheimer’s Disease compared to WT controls. Ultimately, they found that the ablation of PLD2 in the AD mouse model ameliorated memory deficits and conferred synaptic protection (Oliveira et al. 2010, Ghims et al. 2016).
6.2 Phospholipases D in neurotransmission
While much research has established potential roles for PLD1 and PLD2 in the central nervous system, our lab is most interested in whether PLD1 or PLD2 has a regulatory role in the synaptic vesicle cycle. We predict that the ablation of PLD and therefore a change in the amount in PtdOH may result in a change in the exocytic rate of synaptic vesicles. To date, there is at least one piece of evidence that PLD, specifically PLD1, may be involved in neurotransmission. In 2001, Humeau et al. examined the effect of PLD1 on inhibitory postsynaptic current (IPSC) amplitude. They injected catalytically inactive PLD1 into presynaptic cholinergic neurons in the buccal ganglion of Aplysia californica. This catalytically inactive PLD1 caused a fast and dose-dependent inhibition of acetylcholine release, suggesting that a rapid exchange of WT PLD1 for inactive PLD1 causes a dramatic decrease in the amount of exocytosis at the membrane. These results encourage the idea that PLD1 produces PtdOH-rich microdomains that recruit or activate proteins required for synaptic vesicle fusion with the membrane. These PtdOH-rich domains could also physically bend the membrane in such a way to promote the mixing of lipids in plasma and vesicle membrane during fusion. Altogether, these results support the idea that the production of PtdOH by PLD could be a point of regulation for the synaptic vesicle cycle in cortical and hippocampal neurons. Currently there is no evidence for the involvement of PLD2 in neurotransmission, but this possibility should not be ignored.
7. Conclusion
The synaptic vesicle cycle is a crucial series of events that is required for proper brain function. Proteins and lipids alike serve as important regulators of the cycle. Specifically, the production of PtdOH by the lipid-metabolizing enzymes DGKθ and PLD1 is a potential regulatory pathway. Our lab has found that the ablation of DGKθ results in reduced rates of synaptic vesicle endocytosis, but no difference in exocytosis. We predict that the metabolic action of PLD also regulates synaptic vesicle recycling rates. Ultimately, the formation of PtdOH by lipid-metabolizing enzymes and the interaction of PtdOH with other proteins is importantly implicated in membrane fusion, synaptic vesicle exo- and endocytosis, neurotransmission, and efficient brain function.
Acknowledgments
This work was supported by funding from the National Institutes of Health R01NS077923 (DMR) and RO1NS036715 (RLH). All animal used in this study were treated in accordance with the Johns Hopkins University Animal Care and Use Committee guidelines.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammar M, Kassas N, Chasserot-Golaz S, Bader MF, Vitale N. Lipids in Regulated Exocytosis: What are They Doing? Front Endocrinol. 2013;4:125. doi: 10.3389/fendo.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar M, Thahouly T, Hanauer A, Stegner D, Nieswandt B, Vitale N. PLD1 participates in BDNF-induced signalling in cortical neurons. Sci Reports. 2015;5:14778. doi: 10.1038/srep14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Südhof T, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Blatchley B, Cooper W, Coleman J. Development of auditory brainstem response to tone p stimuli in the rat. Dev Brain Res. 1987;32:75–84. doi: 10.1016/0165-3806(87)90140-4. [DOI] [PubMed] [Google Scholar]
- Burger K, Demel R, Schmid S, Kruijff B. Dynamin Is Membrane-Active: Lipid Insertion Is Induced by Phosphoinositides and Phosphatidic Acid. Biochemistry-us. 2000;39:12485–12493. doi: 10.1021/bi000971r. [DOI] [PubMed] [Google Scholar]
- Bruntz RC, Lindsley CW, Brown HA. Phospholipase D Signaling Pathways and Phosphatidic Acid as Therapeautic Targets in Cancer. Pharmacol Rev. 2014;66:1033–1079. doi: 10.1124/pr.114.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Zhong M, Wang R, Netzer W, Shields D, Zheng H, Sisodia S, Foster D, Gorelick F, Xu H, Greengard P. Phospholipase D1 corrects impaired βAPP trafficking and neurite outgrowth in familial Alzheimer’s disease-linked presenilin-1 mutant neurons. P Natl Acad Sci Usa. 2006;103:1936–1940. doi: 10.1073/pnas.0510710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzolli R, Shemon A, Fang M, Hughes W. Phospholipid signalling through phospholipase D and phosphatidic acid. Iubmb Life. 2006;58:457–461. doi: 10.1080/15216540600871142. [DOI] [PubMed] [Google Scholar]
- Chasserot-Golaz S, Coorssen JR, Meunier FA, Vitale N. Lipid dynamics in exocytosis. Cell Mol Neurobiol. 2010;30:1335–42. doi: 10.1007/s10571-010-9577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft Signalling roles of mammalian phospholipase D1 and D2. Cell Mol Life Sci. 2001;58:1674–1687. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Gaudin R, Kirchhausen T. Dynamin recruitment and membrane scission at the neck of a clathrin-coated pit. Mol Biol Cell. 2014;25:3595–3609. doi: 10.1091/mbc.E14-07-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarny M, Lavie Y, Fiucci G, Liscovitch M. Localization of Phospholipase D in Detergent-insoluble, Caveolin-rich Membrane Domains. J Biol Chem. 1999;274:2717–2724. doi: 10.1074/jbc.274.5.2717. [DOI] [PubMed] [Google Scholar]
- Davletov B, Montecucco C. Lipid function at synapses. Curr Opin Neurobiol. 2010;20:543–549. doi: 10.1016/j.conb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Garcia R, Forde C, Godwin HA. Calcium triggers an intramolecular association of the C2 domains in synaptotagmin. PNAS. 2000;97:5883–5888. doi: 10.1073/pnas.100127197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghim J, Chelakkot C, Bae Y, Suh P, Ryu S. Accumulating insights into the role of phospholipase D2 in human diseases. Adv Biol Regul. 2016;61:42–6. doi: 10.1016/j.jbior.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Goldschmidt H, Tu-Sekine B, Volk L, Anggono V, Huganir R, Raben D. DGKθ Catalytic Activity Is Required for Efficient Recycling of Presynaptic Vesicles at Excitatory Synapses. Cell Reports. 2016;14:200–207. doi: 10.1016/j.celrep.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J. The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (And a surprise discovery: PLD2 is a GEF) Cell Signal. 2011;23:1885–1895. doi: 10.1016/j.cellsig.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Vitale N, Chasserot-Golaz S, Dupont JL, Du G, Frohman M, Bader MF, Poulain B. A role for phospholipase D1 in neurotransmitter release. Proc Natl Acad Sci. 2001;98:15300–15305. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K, Willott J. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hearing Research. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Jin JK, Ahn BH, Na YJ, Kim JI, Kim YS, Choi EK, Ko YG, Chung K, Kozlowski P, Min D. Phospholipase D1 is associated with amyloid precursor protein in Alzheimer’s disease. Neurobiol Aging. 2007;28:1015–1027. doi: 10.1016/j.neurobiolaging.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Jones D, Morgan C, Cockcroft S. Phospholipase D and membrane traffic: Potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochimica et Biophysica Acta. 1999;1439:229–244. doi: 10.1016/s1388-1981(99)00097-9. [DOI] [PubMed] [Google Scholar]
- Lam A, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel E. SNARE-catalyzed Fusion Events Are Regulated by Syntaxin1A–Lipid Interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumen G, Ferber A, Klump G, Tollin D. The Physiological Basis and Clinical Use of the Binaural Interaction Component of the Auditory Brainstem Response. Ear Hearing. 2016;37:e276. doi: 10.1097/AUD.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A, De Graan P, Gispen W, Wirtz K. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992;267:7207–10. [PubMed] [Google Scholar]
- Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Galpha-O decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
- Oliveira T, Chan R, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff K, Wenk M, Arancio O, Paolo G. Phospholipase D2 Ablation Ameliorates Alzheimer’s Disease-Linked Synaptic Dysfunction and Cognitive Deficits. J Neurosci. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Shields L, Mendelsohn B, Haddad D, Lin W, Gerencser A, Kim H, Brand M, Edwards R, Nakamura K. The Role of Mitochondrially Derived ATP in Synaptic Vesicle Recycling. J Biol Chem. 2015;290:22325–22336. doi: 10.1074/jbc.M115.656405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton JE, Perrin RJ, Woods WS, George JM. Structural determinants of PLD2 inhibition by alpha-synuclein. J Mol Biol. 2004;337:1001–1009. doi: 10.1016/j.jmb.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Pinto L, Invergo B, Shimomura K, Takahashi J, Troy J. Interpretation of the mouse electroretinogram. Documenta Ophthalmologica. 2007;115:127–136. doi: 10.1007/s10633-007-9064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V. The mouse pattern electroretinogram. Documenta Opthalmologica. 2007;115:145–153. doi: 10.1007/s10633-007-9059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchkov D, Haucke V. Greasing the synaptic vesicle cycle by membrane lipids. Trends Cell Biol. 2013;23:493–503. doi: 10.1016/j.tcb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Raben D, Barber C. Phosphatidic acid and neurotransmission. Adv Biol Regul. 2017;63:15–21. doi: 10.1016/j.jbior.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T. Phosphoinositide signaling in somatosensory neurons. Adv Biol Regul. 2016;61:2–16. doi: 10.1016/j.jbior.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Lipid regulation of the synaptic vesicle cycle. Nat Rev Neurosci. 2005;6:139–150. doi: 10.1038/nrn1608. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakagami H, Kondo H. Localization of mRNAs for phospholipase D (PLD) type 1 and 2 in the brain of developing and mature rat. Dev Brain Res. 2000;120:41–47. doi: 10.1016/s0165-3806(99)00189-3. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, Abrams C, Kanaho Y, Camilli P. Phosphatidylinositol-4-Phosphate 5-Kinases and Phosphatidylinositol 4,5-Bisphosphate Synthesis in the Brain*. J Biological Chem. 2010;285:28708–28714. doi: 10.1074/jbc.M110.132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang P, Du G, Kanaho Y, Frohman M, Tsirka S. Increased expression of two phospholipase D isoforms during experimentally induced hippocampal mossy fiber outgrowth. Glia. 2004;46:74–83. doi: 10.1002/glia.10322. [DOI] [PubMed] [Google Scholar]
- Zhu YB, Gao W, Zhang Y, Jia F, Zhang HL, Liu YZ, Sun XF, Yin Y, Yin DM. Astrocyte-derived phosphatidic acid promotes dendritic branching. Sci Reports. 2016;6:21096. doi: 10.1038/srep21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YB, Kang K, Zhang Y, Qi C, Li G, Yin DM, Wang Y. PLD1 Negatively Regulates Dendritic Branching. J Neurosci. 2012;32:7960–7969. doi: 10.1523/JNEUROSCI.5378-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]