Abstract
Background
While vancomycin’s exposure-dependent efficacy thresholds have been probed, less is known regarding acute kidney injury (AKI) thresholds for vancomycin. Sensitive urinary biomarkers such as Kidney injury molecule 1 (KIM-1) have shown high sensitivity and specificity for vancomycin-associated AKI. We sought to determine if dose-response curves existed with urinary KIM-1. Secondarily, we sought to evaluate the impact of therapy duration and gender status on observed relationships.
Methods
A systematic review was conducted via PubMed/MEDLINE. Data were compiled from pre-clinical studies that reported individual subject data for urinary KIM-1 concentrations, vancomycin dose (mg/kg), duration of treatment, and gender status. Sigmoidal Hill-type models were fit to the individual dose-response data.
Results
In total, 15 studies were identified and six reported vancomycin dose and KIM-1 data. Of these, three included individual animal-level data suitable for analysis. For all pooled rats, increasing total daily vancomycin doses displayed a dose-response curve with urinary KIM-1 concentrations (50% maximal toxic response=130.4 mg/kg/day). Dose-response curves were shifted left for females vs. males (p=0.05) and for long (i.e. ≥7 days) vs. short (i.e. <4 days) of vancomycin therapy (p=0.02).
Conclusions
The collective findings demonstrate a clear dose response relationship between vancomycin dose and AKI. As these analyses focused exclusively on dose-response relationships, additional pre-clinical data are needed to more clearly define vancomycin exposures that predict the onset of AKI.
Keywords: vancomycin, biological markers, nephrotoxicity, pharmacokinetics, pharmacology
1. Introduction
Vancomycin is one of the most commonly used antibiotics within acute care hospitals in the United States [1]. Current consensus guidelines recommend maintaining trough concentrations between 15 and 20 mg/L for deep seated infections such as pneumonia, meningitis, endocarditis and osteomyelitis [2]. While area under the curve (AUC) to minimum inhibitory concentration (MIC) has been linked to bactericidal activity of vancomycin, trough concentrations are used as a surrogate [2]. Despite trough monitoring recommendations, limited clinical data demonstrate improvements in efficacy by targeting these higher troughs for more severe infections [3]. However, widespread targeting of higher average vancomycin concentrations has been linked with an increased incidence of vancomycin-associated acute kidney injury (AKI) [4]. Though often reversible, vancomycin-associated AKI may impact up to 40% of vancomycin-treated patients, with higher rates of AKI reported among patients receiving more intense dosing [4]. Understanding and preventing vancomycin-associated AKI is important because even moderate degrees of AKI have been linked with increased mortality in critically ill patients [5].
While vancomycin’s pharmacokinetic/pharmacodynamic (PK/PD) efficacy relationship profile has been intensively studied, much less is known regarding vancomycin exposures that provoke vancomycin-associated AKI. Currently, vancomycin exposures that define the toxicity ceiling remain unclear. One barrier to identifying the pharmacokinetic/toxicodynamic (PK/TD) relationship for vancomycin lies in the relative insensitivity of standard clinical methods for identifying AKI. Older methods of defining AKI relied on markers such as serum creatinine (SCr), blood urea nitrogen (BUN), and urine output. Biomarkers such as SCr and BUN do not reach diagnostic levels until GFR has been substantially reduced [6]. Complicating matters further, percent changes in SCr in acute kidney injury are dependent on the baseline status of renal function in the patient [7]. Kidney Injury Molecule-1 (KIM-1) is a urinary biomarker that is highly sensitive and specific for proximal tubular injury in human and animal studies [8]. KIM-1 expression is induced when renal proximal tubular cell injury occurs [9], the exact location where vancomycin-induced cellular damage is thought to occur [10]. Additionally, KIM-1 urinary concentrations are sensitive for cellular injury [11] and peak within hours after an acute injury [8, 11]. Since KIM-1 has shown high sensitivity and specificity for vancomycin induced kidney injury, we sought to determine if vancomycin dose-response curves existed with urinary KIM-1. Secondarily, we sought to evaluate the impact of therapy duration and gender status on observed relationships. To complete our study objectives, a systematic review via PubMed/MEDLINE was performed and data from pre-clinical studies that reported individual associations between urinary KIM-1 concentrations and vancomycin dose (mg/kg), duration of treatment, and gender status were compiled for individual dose-response analysis.
2. Methods
2.1 Literature search and data extraction
PubMed/MEDLINE was searched for pre-clinical studies of vancomycin and KIM-1 using medical subject heading (MeSH) terms from inception to March 1, 2017. MeSH terms included: ("Vancomycin"[All fields] OR “Vancomycin/toxicity"[Mesh] OR “Drug-Related Side Effects and Adverse Reactions"[Mesh]) AND (“kidney injury molecule 1”[all fields] OR “KIM 1” [all fields]). Only studies written in English were considered for inclusion. Additionally, to be considered for inclusion, studies had to report animal gender, vancomycin doses used, route of administration, and raw unstandardized KIM-1 urinary concentrations. Studies reporting only gene based measurements of KIM-1 were excluded because of difficulty in standardization between studies. When raw KIM-1 urinary concentrations were reported only in graphical form, GraphGrabber 1.5.5 (Quintessa, Oxforshire, UK) was used to extract the data from the image.
2.2 Statistical analysis
Individual animal-level data was pooled to determine the dose-response relationships. Sigmoidal Emax curves (e.g., four-parameter logistic curves) were fit using GraphPad Prism (version 7.01, La Jolla, CA) with fits described with R2 and compared using the extra sum of squares F-test. For sigmoidal curve fitting, KIM-1 concentrations were transformed to natural logarithms to satisfy parametric assumptions. Doses that produced half-maximal KIM-1 responses [i.e. 50% toxic dose (TD50)] were calculated from the equation. Data points from each study were pooled to create a toxicodynamic dose: KIM-1 response analysis. Stratifications were created for naturally occurring categorical splits in: 1) treatment duration [short (e.g. ≤ 4 days of therapy) vs. long (e.g. 7–14 days)] and 2) animal gender (e.g. male vs. female). Comparison of TD50 values across treatment durations and gender groups was facilitated by estimating the shared bottom, top, and Hill slope parameters between the categorical groups (i.e. estimated parameters were required to be shared between the groups when TD50 comparisons were desired). An alpha level of ≤ 0.05 was used to determine the presence of non-overlapping TD50 values between categorical groups.
3. Results
3.1 Systematic review results
The systematic literature search identified a total of fifteen articles. Of the initial fifteen studies identified, six were pre-clinical studies that reported urinary KIM-1 expression following vancomycin exposure [8, 12–16]. Two of the six studies that reported KIM-1 expression after vancomycin exposure only reported gene expression (i.e. RNA) and were therefore not included in the analysis [14, 16]. One study reported urinary KIM-1 concentrations after vancomycin treatment but did not report the doses utilized and urinary KIM-1 concentrations were not reported at the individual animal level [15]. A total of three trials met inclusion criteria [8, 12, 13], comprising 182 rats for individual data extraction (Table 1).
Table 1.
Number of Animals per study
| Number of Animals per Study | ||||
|---|---|---|---|---|
| Vancomycin Dose |
Days of Therapy |
Vaidya et al. | Rhodes et al. | Fuchs et al. |
| 0 | 1 | 5 | ||
| 0 | 3 | 6 | 5 | |
| 0 | 4 | 10 | ||
| 0 | 7 | 6 | ||
| 0 | 8 | 10 | ||
| 0 | 14 | 6 | ||
| 50 | 4 | 10 | ||
| 50 | 8 | 10 | ||
| 70 | 3 | 6 | ||
| 70 | 7 | 6 | ||
| 70 | 14 | 6 | ||
| 140 | 3 | 6 | ||
| 140 | 7 | 6 | ||
| 140 | 14 | 6 | ||
| 150 | 1 | 10 | ||
| 150 | 3 | 10 | ||
| 200 | 1 | 12 | ||
| 200 | 3 | 10 | ||
| 210 | 3 | 6 | ||
| 210 | 7 | 6 | ||
| 210 | 14 | 4 | ||
| 300 | 4 | 10 | ||
| 300 | 8 | 10 | ||
3.2 Individual study data abstracted
Vaidya and colleagues conducted a comprehensive analysis of male Wistar Han rats, treated with 0 (saline), 70, 140, and 210 mg/kg/day of vancomycin via intraperitoneal (IP) injection for 1, 3, 7, or 14 days [8]. The authors demonstrated that baseline urinary KIM-1 protein concentrations were very low (from control animals), and only detectable among rats experiencing injury. The authors found that increasing doses of vancomycin were associated with increases in urinary KIM-1. The authors reported individual-level toxicodynamic data for vancomycin dose and KIM-1 concentrations which were extracted from the supplement.
Fuchs and colleagues evaluated protein concentrations in the urine after administration of vancomycin at 50, 150 or 450 mg/kg/day for 7 days, then weekly until day 28 in male and female Wistar rats [13]. Urinary KIM-1 was measured on day 4, 8, 15, and 29. The authors found that doses of vancomycin ≥150 mg/kg/day resulted in dose-dependent increases in urinary KIM-1 concentrations. Tubular degeneration was noted in rats receiving 150 mg/kg/day, while tubular necrosis was seen at higher doses. As the weekly dosing scheme was distinctly different from other included trials, KIM-1 concentrations following daily administration were extracted from a white paper which reported data from the same animals [17]. Only dose-response data from the initial week of daily dosing were included for analysis, with KIM-1 data examined on day 8.
Rhodes and colleagues conducted a PK/TD analysis of vancomycin exposure and urinary KIM-1 concentrations [12]. Male Sprague-Dawley rats (n=52) received vancomycin as an IP injection dosed at 0, 150 or 200 mg/kg/day for 1 to 3 days. The authors observed significant correlations between increasing vancomycin exposures and urinary KIM-1 concentrations. Individual animal-level raw data including the vancomycin doses given and KIM-1 concentrations was available to the authors (data on file).
3.3 Dose response curves
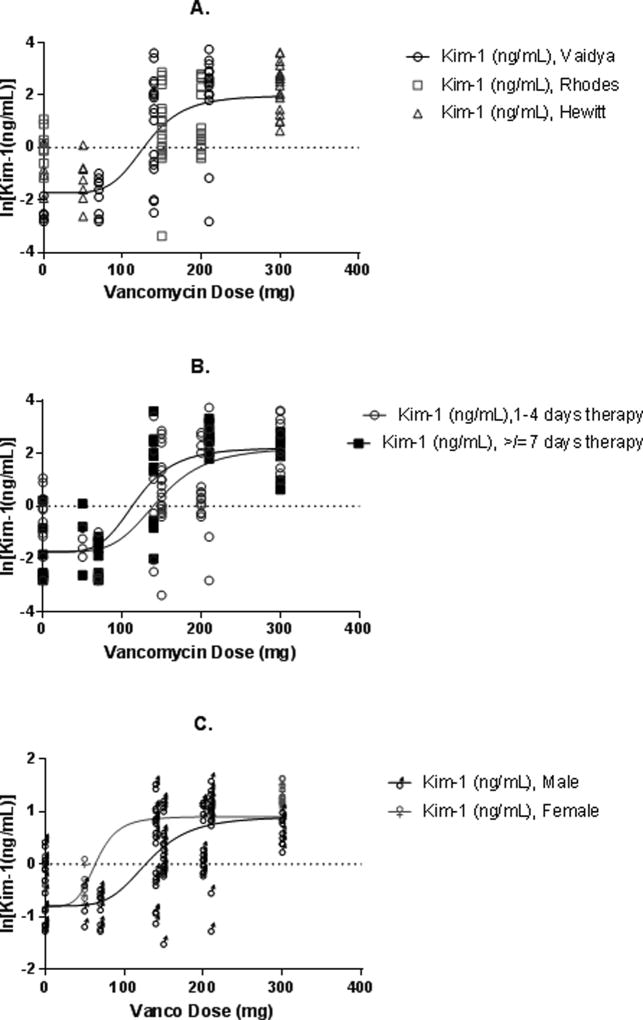
We further analyzed the individual-level data that were extracted during the systematic review from 3 separate manuscripts [8, 12, 17]. A total of 182 subjects were extracted from these studies, with 176 providing paired vancomycin dose and urinary KIM-1 data (six animals were administered vancomycin without KIM-1 reported). Paired dose-response data from all studies were pooled for all analyses (n=176). A four parameter Hill curve was fit for the full dataset (Figure 1), showing a correlation between increasing total daily vancomycin doses and urinary KIM-1 concentrations (R2=0.589; TD50=130.4 mg/kg/day).
Figure 1.
Dose:Response curves of vancomycin daily dose vs. the natural log of urinary KIM-1 for (A) the entire population, (B) stratified by duration of vancomycin therapy, and (C) stratified by gender
3.4 Vancomycin duration stratifications
Treatment groups were dichotomized into short (< 4 day) and long (≥ 7 day) durations (Figure 1) as a function of a naturally occurring split between the studies. Dose:KIM-1 response curves differed based on short vs. long vancomycin therapy (R2: 0.431 vs. R2: 0.766, respectively; p=0.02). Similarly, the TD50 value was higher for short (146.8 mg/kg/day) vs. long (118.8 mg/kg/day) vancomycin therapy.
Gender stratifications
Curve fits for animal gender stratifications are shown in Figure 1. Dose:KIM-1 response curves differed based on female vs. male gender (R2: 0.744 vs. R2: 0.567, respectively; p=0.05). TD50 values in females were lower than those for males (65.3 vs. 130.7 mg/kg/day).
4. Discussion
The analyses presented herein suggest the existence of a dose-response relationship between vancomycin and urinary KIM-1 in rats. The TD50 values for KIM-1 urinary concentration also appears to be modified by duration of treatment and animal gender. Longer durations of vancomycin therapy produced lower TD50 values (118.8 vs. 146.8 mg/kg/day, respectively), indicating that toxicity can still occur when lower daily doses are given for a longer period of time. Interestingly, TD50 values were significantly decreased in female compared to male rats, though more data are needed to evaluate the exact magnitude of this difference. While the present study has helped to further clarify this dose-response curve, elucidation of the underlying exposures (vis-a-vis PK exposure) that drives renal toxicity will ultimately lead to improved dosing regimens that will benefit patients receiving vancomycin.
The exact mechanism by which vancomycin induces kidney damage remains unclear. However, oxidative stress and mitochondrial dysfunction have been observed in vancomycin treated animals and in cell culture studies. Dietrich and colleagues studied the impact of vancomycin exposure on the transcriptomic response in BALB/c mice. They observed significant downregulation of antioxidant gene expression and upregulation of markers of oxidative stress and organelle dysfunction [10]. It remains unclear whether oxidative stress is a cause or merely a consequence of vancomycin-associated AKI. Importantly, urinary KIM-1 provides a physiological correlate for vancomycin-associated AKI irrespective of the mechanism of damage.
The findings from this study have important clinical implications. Allometric scaling of the relevant TD50 values identified in this analysis reveals that commonly utilized daily doses of vancomycin in humans can potentially induce kidney injury. Consensus treatment guidelines currently recommend empiric vancomycin daily doses of 30–40 mg/kg/day, equivalent to a dose of 180–240 mg/kg/day in rats. The observed TD50 value in the pooled analysis was 130.4 mg/kg/day (a humanized-equivalent dose of 21.7 mg/kg/day human dose) [18]. On first glance, one would surmise that pre-clinical findings appear to overcall AKI in patients since the doses needed to produce pre-clinical elevations in KIM-1 correlate with very commonly used clinical doses of vancomycin. However, an apparent relationship between higher vancomycin doses and increased rates of vancomycin-associated AKI has been seen in prospective, randomized, human, clinical studies. Wunderink and colleagues observed an 18.2% incidence of vancomycin-associated AKI among subjects randomized to receive vancomycin in a study of hospital-acquired or healthcare-associated MRSA pneumonia. The absolute difference in AKI incidence was 10% higher among patients receiving vancomycin (average dose 31 mg/kg/day; equivalent to 186 mg/kg/day in rats) versus those who were receiving a non-nephrotoxic comparator [19]. Concordance between animal and human data support the existence of an exposure-related PK/TD threshold that can ultimately be translated between rats and humans [20].
Limitations to the current analysis must be considered. First, the evaluated studies were comprised of Wistar and Sprague Dawley rats where vancomycin was dosed between 1 and 14 days. However, only data that could be reasonably combined were analyzed as such. All included studies evaluated IP dosing. It is well known that IP dosing results in more variable exposure compared to intravenous dosing, which may lead to loss of parity according to dosing group. Exposure related studies will be necessary to identify exact exposures associated with AKI. Second, the authors of two of the studies included here [8, 17] did not conduct pharmacokinetic sampling, and therefore the current data are insufficient to determine the exposure profile that predicts toxicity. Third, the toxicodynamic analysis identified a TD50 threshold dose for vancomycin-associated AKI of 127.1 mg/kg/day in the rat, which allometrically scaled to 21.2 mg/kg/day in the human. These doses are below the total daily recommended empiric doses in humans and not often considered nephrotoxic; thus, additional work is needed to clarify the true distribution of discrete damage induced by vancomycin in humans. Additionally, only three studies were identified for use in these analyses, limiting broad extrapolation of these findings. These results suggest signals associated with increased risk and warrant further investigation. It is possible that the simple allometric scaling approach between rats and humans does not fully capture the parameter scaling necessary to understand the PK/TD relationship in vancomycin. More comprehensive models may be needed.
5. Conclusion
Until mechanistic models of vancomycin-associated AKI are developed and their predictions validated, empiric models that relate vancomycin exposure to expression of urinary biomarkers (e.g. KIM-1) may allow investigation of alternative dosing regimens designed to minimize renal injury. Overall, our analysis suggests that increases in urinary KIM-1 correspond to objective evidence of vancomycin-associated AKI in an animal model. More data are needed to identify the most predictive PK/TD threshold as well as the effect of gender and therapy duration on the defined threshold for vancomycin-associated AKI.
Highlights.
We analyzed pre-clinical studies on vancomycin induced kidney injury (VIKI)
Dose-response data were compiled at the individual animal level to assess VIKI
Daily dose of vancomycin and urinary Kim-1, a sensitive VIKI biomarker, was analyzed
Longer study durations and female gender were associated with greater toxicity
Acknowledgments
We would like to acknowledge the following individuals who contributed to the data described in this manuscript: Peter Lamar, Zhong Zhang, and Seema Briyal.
Declarations
Funding: Research reported in this publication was supported in part by National Institute of Allergy and Infectious Diseases of the National Institutes of Health [award number R15-AI105742]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: None
Ethical Approval: Not required
References
- 1.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among us hospitals from 2006 to 2012. JAMA Internal Medicine. 2016;176:1639–48. doi: 10.1001/jamainternmed.2016.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–7. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 3.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 4.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrobial agents and chemotherapy. 2013;57:734–44. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney international. 2008;73:538–46. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 6.Duarte CG, Preuss HG. Assessment of renal function--glomerular and tubular. Clinics in laboratory medicine. 1993;13:33–52. [PubMed] [Google Scholar]
- 7.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–9. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nature Biotechnology. 2010;28:478–85. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–8. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich C, Puey A, Lin S, Swezey R, Furimsky A, Fairchild D, et al. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicological Sciences. 2009;107:258–69. doi: 10.1093/toxsci/kfn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. American journal of physiology Renal physiology. 2006;290:F517–29. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, et al. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicologic pathology. 2012;40:1031–48. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 14.Dieterich C, Puey A, Lin S, Swezey R, Furimsky A, Fairchild D, et al. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol Sci. 2009;107:258–69. doi: 10.1093/toxsci/kfn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang EJ, Snyder RD, Fielden MR, Smith RJ, Gu YZ. Validation of putative genomic biomarkers of nephrotoxicity in rats. Toxicology. 2008;246:91–100. doi: 10.1016/j.tox.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Kondo C, Minowa Y, Uehara T, Okuno Y, Nakatsu N, Ono A, et al. Identification of genomic biomarkers for concurrent diagnosis of drug-induced renal tubular injury using a large-scale toxicogenomics database. Toxicology. 2009;265:15–26. doi: 10.1016/j.tox.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Hewitt P, Fuchs TC, Serono EJM, Zheng W. White Paper: Detection of vancomycin-inducted subacute nephrotoxicity using MILLIPLEX® MAP rat kidney toxicity multiplex planels [Internet] Massachusetts: EMD Millipore Corporation; 2013. p. 10. Available from: https://www.emdmillipore.com/US/en/product/MILLIPLEX-MAP-Rat-Kidney-Toxicity-Magnetic-Bead-Panel-1---Toxicity-Multiplex-Assay,MM_NF-RKTX1MAG-37K#documentation. 2013. p. 1–10. [Google Scholar]
- 18.Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER) 2005
- 19.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–9. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 20.Melanie Blank ADFFG, Patricia H, Elizabeth H, David J-K, William T, Aliza T, Douglas T, Shen X. Review of Qualification Data for Biomarkers of Nephrotoxicity Submitted by the Predictive Safety Testing Consortium. Center for Drug Evaluation and Research U.S. Food and Drug Administration; 2009. [Google Scholar]