Abstract
Background
Bipolar disorder (BD) is associated with reductions in the P3b event-related potential (ERP) to target auditory stimuli, which suggests deficits in context updating. Previous studies have typically examined these responses in the temporal domain, which may not capture alterations in specific frequencies of phase-locked or induced electrophysiological activity. Therefore, the present study examined early and late ERPs in temporal and frequency domains in a bipolar sample with and without current psychotic features.
Methods
The electroencephalogram (EEG) was recorded during an auditory oddball task. 75 BD and 98 healthy controls (HC) discriminated between standard and target tones. N1 ERPs to standards and P3b to targets were analyzed in the temporal domain. Event-related spectral perturbation (ERSP) and inter-trial coherence (ITC) were analyzed in the frequency domain.
Results
The early N1 response to standard tones was not significantly different between the total HC and BD samples irrespective of psychotic features. However, N1 amplitude was reduced in BD with psychotic features (BDP) compared to HC and BD without psychotic features. P3b was reduced in BD versus HC, with the BDP sample having the most reduced amplitude. In the time-frequency analysis, delta and theta ERSP and ITC were reduced across the time window for both standard and target stimuli in BD compared to HC, but did not differ in the psychotic features analysis.
Conclusions
The results provide neural evidence that BD is associated with disrupted sensory, attentional, and cognitive processing of auditory stimuli, which may be worsened with the presence of psychotic features.
Keywords: psychotic features, oddball, P3b, N1, event-related spectral perturbation, inter-trial coherence
Bipolar disorder (BD) often presents with psychotic features (1), which is consistent with shared genetic risk factors between BD and psychotic illness (2, 3). BD is associated with event-related brain potential (ERP) amplitude reductions during the auditory oddball paradigm, similar to those seen in schizophrenia (SZ) (4, 5). Given these ERP findings among others, it has been argued that BD and SZ lie on the same psychopathological spectrum (6, 7). Importantly, these ERP reductions during the auditory oddball paradigm have been suggested as endophenotypes (8, 9), or heritable biobehavioral characteristics intermediate between the genotype and phenotype of an illness (10). ERP abnormalities in conjunction with other endophenotypes may lead to improved understanding of the complex etiologies of psychopathology and to the discoveries of more efficacious treatments. The present study evaluated the impact of psychosis on ERP endophenotypes in BD.
One potential endophenotype of BD and SZ is a reduction in the P3b ERP, thought to index working memory and context updating of the sensory systems (11). The P3b (P3) is a centroparietally maximal ERP peaking approximately 300 milliseconds (ms) post-target stimuli in the auditory oddball paradigm in which participants discriminate between frequently presented (standard) and rarely presented (target) tones. P3 deficits are often investigated in SZ (12), and less so BD; however, studies have found reductions in P3 amplitude (4, 5) and/or prolonged latencies (13) in BD compared to healthy controls (HC). P3 deficits have been detected in probands in a euthymic state (14) and in remission (15), as well as in unaffected first-degree relatives (9, 13). The P3 is likely not a specific marker of BD or SZ but perhaps a marker of cognitive vulnerability or impairment (16).
Another potential ERP endophenotype in the auditory oddball paradigm called the N100 (N1) is elicited 100 ms post-stimulus and may index early sensory registration and attention (11). Findings of N1 amplitude in response to standard stimuli in BD are mixed, so it is unclear whether basic electrophysiological responses to typical sounds earlier in the information processing sequence are intact in this disorder. Studies more often report N1 amplitude reductions in SZ (5, 17, 18) than BD (19), although others do not find deficits in BD (14) or SZ (20). N1 has been found to differentiate BD from SZ in a prior report due to a greater reduction in SZ, whereas P3 amplitude did not differentiate the two (21). One potential explanation for the mixed results in BD could be the heterogeneity of the illness that is often not explicitly studied; it is possible that early electrophysiological responses to standard stimuli differ between BDP and BD individuals who are not experiencing psychotic features (BDNP).
Most studies have only examined these oddball responses in the temporal domain, wherein ERPs are informative for detailing the millisecond-level stimulus-locked sequence of the post-synaptic activity of assemblies of cortical pyramidal cells (11). However, temporal domain analyses are limited in that ERPs are averages of trials and hence do not capture neural activity in the frequency domain that is not reliably synchronized with stimulus onset (22). Time-frequency (TF) analysis can capture additional information such as the amplitude of activity in particular frequency bands (event-related spectral perturbation, ERSP) and the degree to which activity is phase-locked across individual trials (inter-trial coherence, ITC) in sensory and cognitive neural responses.
In TF analyses of auditory oddball responses, deficits in activity have been found in low and occasionally high frequency ranges in BD and SZ. Numerous studies have reported associations between the target P3 and delta and theta, low frequencies posited to be involved in focused attention, novelty, signal matching/detection, and decision-making (23, 24). Reduced delta and theta band activity to standards and targets have been reported in BD (25–27) and SZ (20). Reduced theta/alpha activity to standards differentiated both BDP and SZ from HC (28). Reductions in early high frequency gamma activity in response to standard tones have been found in SZ (29, 30), whereas minimal available evidence suggests an intact response in BD (31). While oddball TF findings are varied, studies overall report reduced activity in low frequencies in BD and SZ, which could indicate decreased sustained attention and allocation of processing resources in stimulus discrimination (20).
In the present study, auditory oddball electroencephalographic (EEG) responses were investigated in temporal and frequency domains to standard and target stimuli in BD and HC. In the ERP analyses, the N1 to standard stimuli and the P3 to target stimuli were analyzed. In the TF analyses, ERSP and ITC were analyzed from delta to low gamma. ERP and TF analyses were performed on BDP and BDNP subsets of the BD group. Lastly, factor analyses were performed in HC and BD to elucidate the interrelations between EEG measures, cognitive measures, and clinical symptomatology. N1 amplitude was predicted to be reduced in BDP but not BDNP. Reduced P3 amplitude and diminished delta and theta ERSP and ITC to target stimuli were predicted in BD, with more severe reductions in BDP. In the factor analysis, later target-related activity was expected to be more associated with cognitive measures than early standard-related activity.
Methods
Participants
The present study included data from 173 participants, including 75 individuals with bipolar disorder (BD) and 98 healthy controls (HC). Participants were 21–63 years old (M = 41.08, SD = 11.21), including 76 males and 97 females. One hundred sixteen participants identified as white, 52 as black or African-American, 1 as Hispanic/Latino, and 4 identified as other. Participants were recruited via flyers in the community, electronic newsletters, and from the Indiana University School of Medicine Neuroscience Clinical Research Center in Indianapolis, IN, USA. Data from 69 BD and 52 HC were published previously in an ERP-focused study (21). The present study was approved by a local institutional review board, and all participants underwent the process of informed consent prior to study participation.
Participants were interviewed by trained research assistants with the Structured Clinical Interview for DSM-IV for Axis I Disorders (SCID) (32), and diagnoses were made on the basis of SCID, symptom rating measures, and chart review by the research group. All participants in our patient sample were diagnosed with BD type I. Many BD participants underwent the M.I.N.I. International Neuropsychiatric Interview (33), the Positive And Negative Syndrome Scale (PANSS) (34), the Brief Psychiatric Rating Scale (BPRS) (35), the Montgomery-Asberg Depression Scale (MADRS) (36) and the Young Mania Rating Scale (YMRS) (37). Additional assessments conducted in both groups were the Wechsler Abbreviated Scale of Intelligence (WASI-II) IQ test (38) and the Wechsler Adult Intelligence Scale (WAIS) digit symbol coding test (39), thought to measure processing speed. The presence of current psychotic features in BD was determined based on rating scale information available within a month of the EEG, including a diagnosis of BD with psychotic features on the M.I.N.I./SCID or endorsement of hallucinations on the BPRS/PANSS. The BD sample included 28 individuals with (BDP) and 42 without (BDNP) current psychotic features, as well as 5 individuals with insufficient data to determine. Psychotic features analyses were performed on age- (+/− 3 years) and gender-matched samples of HC, BDP, and BDNP, with 25 individuals per group.
Almost the entire sample (~94%) had a high school degree, and the majority (~65%) had completed some college or higher. Twenty-three participants in the BD sample were unmedicated, including 13 off medications for two weeks or less. Fifty-two participants were currently on medication, including 29 on atypical antipsychotics, 5 on typical antipsychotics, 20 on antidepressants, 23 on anticonvulsants or mood stabilizers, 13 on benzodiazepines, 11 on lithium, 2 on anticholinergic medications, and 1 on an anxiolytic. Thirty-eight of the medicated patients were on multiple medications simultaneously.
Exclusion criteria were serious head injury, loss of consciousness exceeding five minutes, severe neurological or medical illness, and current substance dependence. HC were additionally excluded for meeting criteria for a current or past Axis I disorder or having a first-degree relative with SZ or BD. The initial dataset included 199 participants. Nineteen participants were excluded for excessive artifacts in their EEG data, and 7 participants were excluded for poor task performance (see EEG Data Collection and Processing for specific methods), leaving 173 participants.
Independent samples T-tests and chi-square tests of independence were performed to test for significant differences in demographic and cognitive measures between HC and BD. Lastly, these tests were performed on demographic and ERP measures between the subset of age- and gender-matched HC in the psychotic features analyses and the total HC sample to determine whether the smaller group was a representative subset.
EEG Data Collection and Processing
The auditory oddball paradigm consisted of 75 target tones at 1500 Hz randomly occurring amongst 425 standard tones of 1000 Hz. Tones were 50 ms in duration with a 1200 ms interstimulus interval and were presented to participants at 86 dB SPL through Etymotic insert earphones. Participants were instructed to respond to target tones with a button press using their left hand.
EEG data were collected using a 31-channel cap (Falk-Minow Services, Munich, Germany) with Ag/AgCl electrodes. EEG was recorded with a Neuroscan SYNAMPS I recording system (Neuroscan, Inc., El Paso, TX, USA). The electrodes used for recording EEG activity according to the 10/20 standard system were: FP1, FP2, AFZ, FZ, F4, F8, F3, F7, FCZ, FC4, FT8, FC3, FT7, CZ, C4, T8, C3, T7, CPZ, CP4, CP3, PZ, P4, P8, P3, P7, OZ, O2, O1. VEOG and HEOG electrodes recorded eye movements and blinks. A nose reference was used, and the ground electrode was embedded in the cap. The online bioamplifier filters were 0.1–200 Hz, and impedances were maintained below 10 kΩs. The majority of datasets were sampled at a rate of 1000 Hz with 18 datasets sampled at 500 Hz. Participants sat in a recliner chair in a dimly lit electrically-shielded Faraday room.
Data were preprocessed and analyzed using custom-made made scripts based on functions from EEGLAB version 13.5.4 (40) in the MATLAB environment (MATLAB and Statistics Toolbox Release 2015a, The MathWorks, Inc., Natick, MA, USA). Datasets sampled at 1000 Hz were downsampled to 500 Hz to make all datasets uniform in sampling rate. A 100 Hz low-pass Finite Infinite Response (FIR) filter was applied to the continuous data. Data were then segmented into 1150 ms epochs with a 400 ms pre-stimulus baseline period.
To remove ocular blink artifacts from the EEG data, independent component analysis (ICA) was implemented. Principal component analysis (PCA) was performed initially to reduce data dimensionality to 24 components for processing efficiency. The component most accounting for ocular blink activity was removed after being identified based on frontal scalp topography, power spectra, and aligning activity temporally from the VEOG channel with the component activations. Author NBL and research assistant JM separately identified the eyeblink IC with 94.8% interrater agreement. Data then underwent baseline correction of 400 ms.
Automatic artifact rejection was next performed on the data, which removed segments containing voltage that exceeded +/−100μV in Fz, Cz, and Pz. These midline electrodes were selected for analysis on the basis of previous oddball studies (41). Additional trials with signal flat-lining or noisy data across channels were removed after visual inspection. Participants were excluded if less than 70% of trials remained, resulting in the exclusion of 19 participants (13 BD, 6 HC). Seven participants were excluded due to a less than chance rate of response to target stimuli in trials remaining post-artifact rejection (4 BD, 3 HC). Preprocessed datasets at this stage were entered into TF analyses. For ERP analyses, datasets underwent an additional 100 ms baseline correction and a low-pass 24 Hz FIR filter. Trials were then averaged separately for standard and target stimuli.
Event-Related Potentials
In the present study, the N1 wave in response to standard stimuli and the P3 wave in response to target stimuli were analyzed. The midline electrode (Fz, Cz, Pz) with maximal mean amplitude voltage for N1 and P3 was determined by performing repeated measures analyses of variance (ANOVAs). N1 mean amplitude was maximal at Fz, and P3 was maximal at Pz (p < .05). Mean amplitudes and peak latencies were measured in the time window of 80–150 ms for N1 and 280–600 ms for P3. Peak latency was measured as the time point at which voltage was minimum for N1 and maximum for P3.
Time-Frequency Decomposition
A Morlet wavelet spectral decomposition was performed to extract event-related spectral perturbation (ERSP) in the form of total power (evoked and induced activity) and inter-trial coherence (ITC), i.e., the variability of single trials in phase-locking with respect to the stimulus. In order to analyze lower frequencies within the constraints of the epoch size, data were reflected onto either side of each individual epoch. This technique is explained in-depth in other sources (22) and has been utilized previously (42). The wavelet transform was performed on epochs of 1725 sample points utilizing the 400 ms period preceding stimulus onset for baseline normalization. The wavelet increased from 3 cycles at 2 Hz up to 37.5 cycles at 50 Hz. Two hundred time points and 100 log-spaced frequencies were generated, and the resulting window size was 835 samples wide. Epochs were trimmed to their original non-reflected length for subsequent statistical analysis.
Statistical Analysis
In the ERP analyses in HC and BD, one-way analyses of covariance were performed for mean amplitudes and peak latencies of the N1 and P3 waves utilizing SPSS for Windows software (v24). The model included a fixed factor of group and a covariate of age, given associations with P3 and age (43, 44). Correlations between ERP amplitudes and latencies and age were performed to investigate these relationships in our dataset. Psychotic features ANOVAs for N1 and P3 were performed on subsamples of HC, BDP, and BDNP. Bonferroni-corrected post-hoc T-tests were performed to investigate the direction of group differences in significant effects. Differences were considered statistically significant at a threshold of p < .05. Effect sizes reported are partial eta squared values.
In a data-driven approach toward the TF analysis, non-parametric permutation testing was implemented using EEGLAB’s statcond function on ERSP and ITC data from 2–50 Hz from stimulus onset to 750 ms. ERSP and ITC were analyzed at channel Pz for target stimuli and Fz for frequent stimuli, coinciding with ERP analyses. Unpaired group T-tests with 10,000 permutations were performed. A false discovery rate (FDR) correction was applied to the data, and group differences were then considered significant at a threshold of p < .05. Permutation tests were then performed in the subgroups of HC, BDP and BDNP.
Lastly, PCAs with non-orthogonal oblique Promax rotations were performed separately for HC and BD. The number of components was determined with a Kaiser normalization in which components emerge if they explain a sufficient portion of the data’s variance defined by having an Eigenvalue greater than 1. Variables were considered meaningful representations of the components in the pattern matrix when they had factor loadings greater than 0.4. Age was included as a variable for both groups. EEG variables included were mean ERSP and ITC to standard and target stimuli in regions of peak activity, N1 amplitude, and P3 amplitude and latency. Cognitive measures included were WASI IQ score and raw digit symbol score. Raw rather than scaled digit symbol scores were included in the model as to not remove notable associations with age. The BD PCA included depressive and manic symptoms as measured by total MADRS and YMRS scores, respectively.
Results
Demographics
In the total sample, HC and BD did not differ by age, sex, or ethnicity (see Table 1). The groups differed in years of education, with HC having achieved higher levels of education than BD participants. The groups did not significantly differ on WASI IQ scores, but HC did have significantly higher scores in the WAIS digit symbol coding test than BD (see Table 1). The HC subset in the psychotic features analysis (n=25) did not significantly differ from the total HC sample (n=98) in age, gender, education level, or ERP measures of interest. An equal number of participants were unmedicated in the BD participants with (BDP) and without (BDNP) psychotic features (n=7 in each group).
Table 1.
Subject demographics and clinical data
| HC | BD | T-test/Chi-square | |
|---|---|---|---|
| N | 98 | 75 | -- |
|
| |||
| Age, years | 40.1(12) | 42.4(10) | t = −1.37, p = .173 |
|
| |||
| Sex (F/M) | 51/47 | 46/29 | X2 = 1.49, p = .279 |
|
| |||
| Ethnicity | 36 black, | 16 black, | X2 = 5.8, p = .123 |
| 59 white, | 57 white, | ||
| 1 Hispanic/Latino, | 2 other | ||
| 2 other | |||
|
| |||
| Education | 1 junior high, | 10 junior high, | X2 = 17.39, p = .004* |
| 24 high school, | 27 high school, | ||
| 40 some college, | 18 some college, | ||
| 25 bachelors, | 14 bachelors, | ||
| 7 masters, | 4 masters, | ||
| 1 doctoral | 2 doctoral | ||
|
| |||
| WASI IQ | 107(12.7) (n=57) | 102.8(15.9) (n=57) | t = 1.56, p = .122 |
|
| |||
| Digit symbol | 10.1(2.5) (n=44) | 8.5(2.9) (n=58) | t = 3, p = .003* |
|
| |||
| Medicated (Y/N) | -- | 52/23 | -- |
|
| |||
| MADRS | -- | 11.9(10.7) | -- |
|
| |||
| YMRS | -- | 15.6(11.5) | -- |
p < .05.
Values are presented as mean (SD). HC = healthy controls; BD = bipolar disorder; WASI = Wechsler Abbreviated Scale of Intelligence; YMRS = Young Mania Rating Scale; MADRS = Montgomery Asberg Depression Rating Scale. Digit symbol values are scaled scores.
Behavioral Performance
The mean percentage of correctly identified target stimuli for usable trials was 93.64 (SD = 12.34) for HC and 90.75 (SD = 12.2) for BD. This difference was not statistically significant.
Event-Related Potentials
N1 did not significantly correlate with age in HC or BD. P3 amplitude had a negative association with age in HC (r = −.265, p = .008) but not BD (r = −.062, p = .597). P3 latency had a positive association with age in HC (r = .394, p < .001) but not BD (r = .202, p = .082). In the following results, amplitude values are in units of microvolts, and latency values are in units of milliseconds (ms). Results did not differ when analyzed with peak instead of mean amplitude measurements.
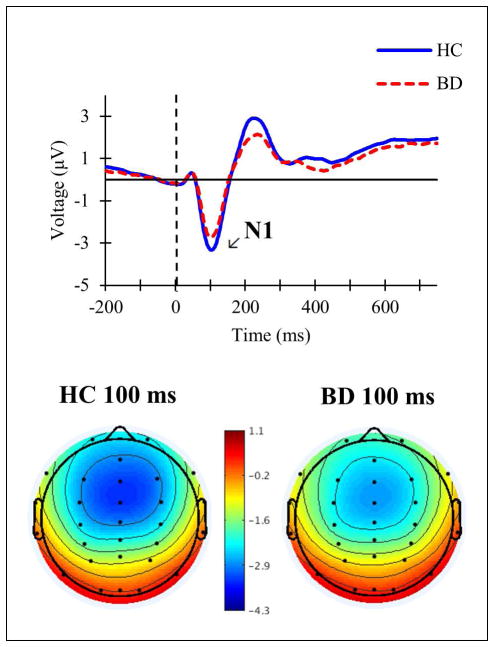
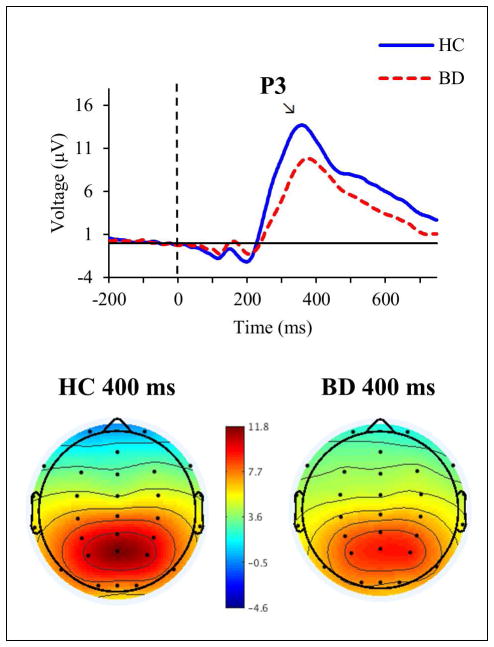
In the total sample analysis, the N1 was not significantly different between the groups, whereas the P3 was significantly altered in BD compared to HC. Specifically, for the N1, significant differences were not found between HC (M = −2.41, SD = 1.67) and BD (M = −1.92, SD = 1.8) in mean amplitude (F(1,170) = 3.717, p = .056, ) or peak latency (F(1,170) = 0.019, p = .89, ) (see Fig. 1). For the P3, amplitude was significantly decreased in BD (M = 6.55, SD = 4.88) compared to HC (M = 9.59, SD = 5.77) (F(1,170) = 11.833, p = .001, ), but there was no significant difference in P3 latency between BD (M = 406.08, SD = 74.73) and HC (M = 381.4, SD = 67.2) (F(1,170) = 3.787, p = .053, ) (see Fig. 2).
Fig. 1.
Top: grand averaged event-related potentials at Fz in response to standard tones in healthy controls (HC; blue solid line) and individuals with bipolar disorder (BD; red dashed line). Bottom: topographic maps of voltage across all scalp channels in HC (left) and BD (right) at 100 milliseconds (ms) post-stimulus.
Fig. 2.
Top: grand averaged event-related potentials at Pz in response to target tones in healthy controls (HC; blue solid line) and individuals with bipolar disorder (BD; red dashed line). Bottom: topographic maps of voltage across all scalp channels in HC (left) and BD (right) at 400 milliseconds (ms) post-stimulus.
In the psychotic features analysis, graded patterns of amplitudes were found for both N1 and P3 in which HC had the highest amplitude, BDP had the lowest amplitude, and BDNP had an intermediate value. N1 amplitude was significantly decreased (p = .005) in BDP (M = −1.52, SD = 1.39) compared to HC (M = −2.89, SD = 1.28) (F(2,72) = 5.317, p = .007, ) (see Fig. 3). There were no significant differences in N1 amplitude between the two BD groups (p = .17) or BDNP (M = −2.34, SD = 1.79) and HC (p = .589). N1 latency group differences were not significant. P3 amplitude was significantly decreased (p = .011) in BDP (M = 5.91, SD = 4.14) compared to HC (M = 10.52, SD = 6.44) (F(2,72) = 4.646, p = .013, ) (see Fig. 4). There were no significant differences in P3 amplitude between the BD groups (p = .958) or BDNP (M = 7.46, SD = 5.51) and HC (p = .151). P3 latency did not significantly differ between the groups.
Fig. 3.
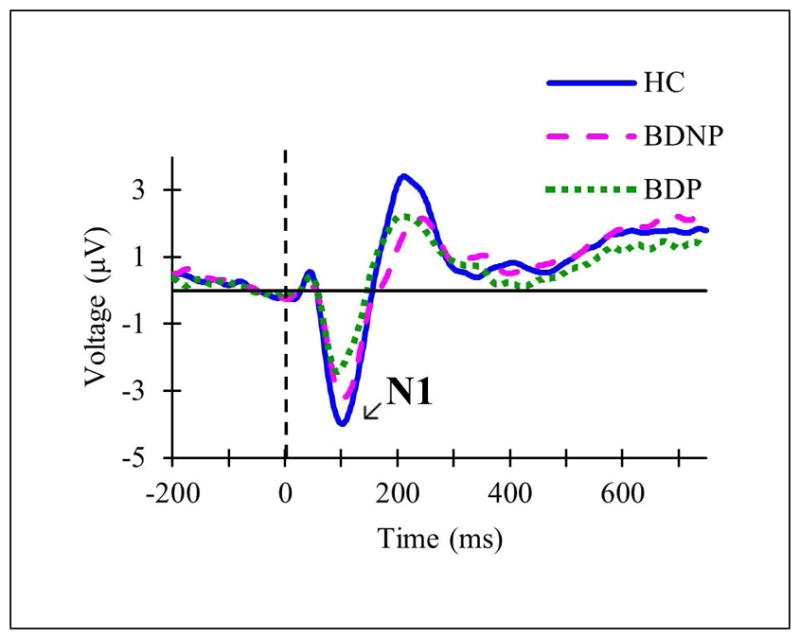
Grand averaged event-related potentials at Fz in response to standard tones in subsets of the total sample including healthy controls (HC; solid blue line), individuals with bipolar disorder without current psychotic features (BDNP; pink dashed line), and individuals with bipolar disorder with current psychotic features (BDP; green dotted line).
Fig. 4.
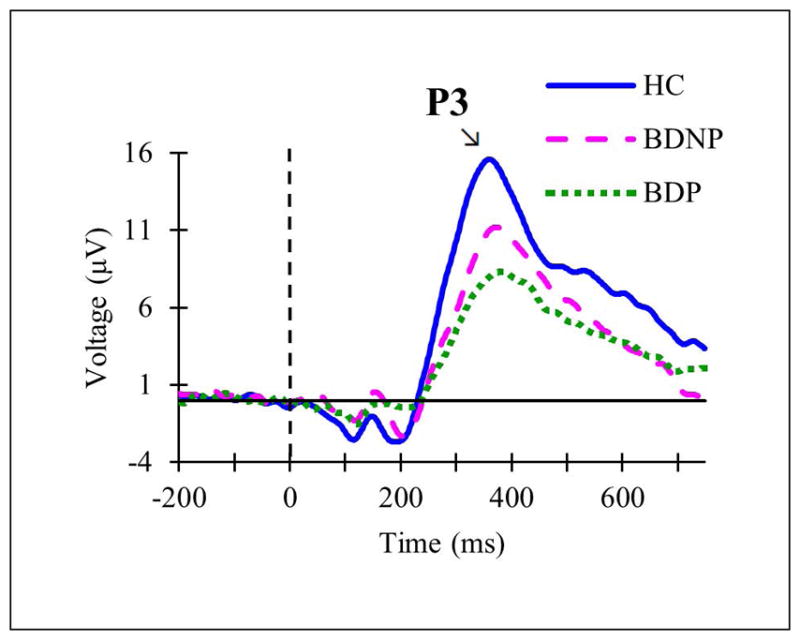
Grand averaged event-related potentials at Pz in response to target tones in subsets of the total sample including healthy controls (HC; solid blue line), individuals with bipolar disorder without current psychotic features (BDNP; pink dashed line), and individuals with bipolar disorder with current psychotic features (BDP; green dotted line).
Event-Related Spectral Perturbation (ERSP) and Inter-Trial Coherence (ITC)
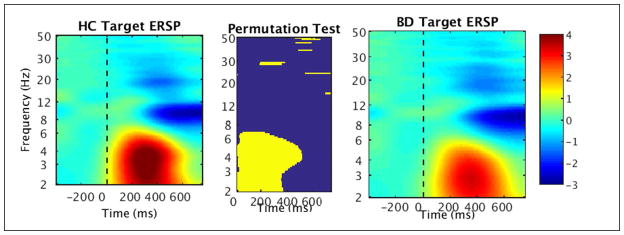
Overall in the full sample time-frequency (TF) analyses, reductions were found in ERSP and ITC to standard and target stimuli in BD compared to HC. These reductions in activity spanned the epoch time window and were primarily in the lower frequencies delta and theta.
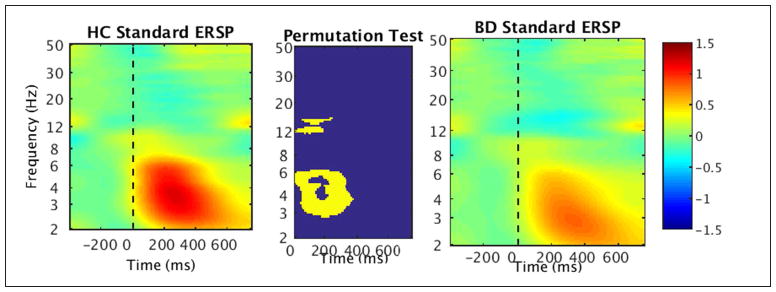
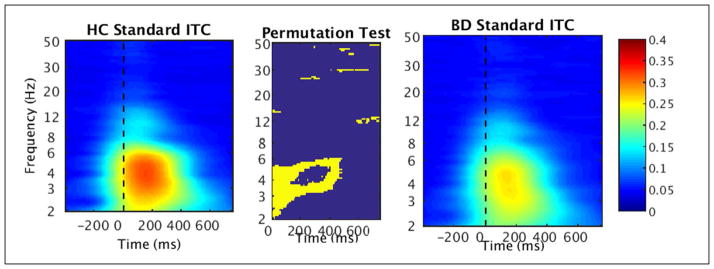
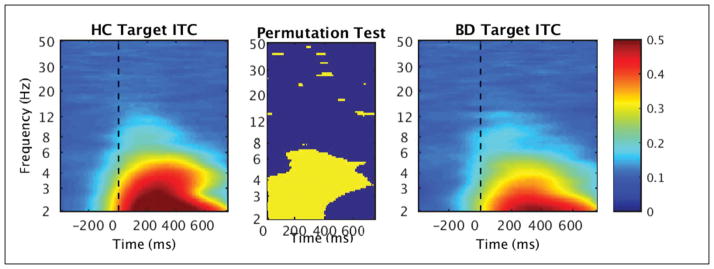
In particular, in the total sample analysis of standard stimuli ERSP, decreased power was found in BD in the high delta/low theta range (3–6 Hz) in the first half of time range from stimulus onset to 400 ms (FDR corrected, p < .05) (see Fig. 5). There was also a brief early reduction in activity in the low beta range (12–16 Hz) in BD from 0 to 200 ms. In addition to reduced ERSP in BD, reduced ITC to standards compared to HC was found in a similar low frequency range of 3–6 Hz, from stimulus onset to 500 ms (FDR corrected, p < .05) (see Fig. 6). Results of the permutation tests for standards additionally showed brief segments of high frequency ITC (e.g., 13 Hz, 30 Hz) to be significantly reduced in BD throughout in the time window (see Fig. 6).
Fig. 5.
Time-frequency plots of event-related spectral perturbation (ERSP) in response to standard stimuli measured at Fz in healthy controls (HC; left) and individuals with bipolar disorder (BD; right). The non-parametric permutation test (center) shows significant group differences (FDR corrected, p < .05).
Fig. 6.
Time-frequency plots of inter-trial coherence (ITC) in response to standard stimuli measured at Fz in healthy controls (HC; left) and individuals with bipolar disorder (BD; right). The non-parametric permutation test (center) shows significant group differences (FDR corrected, p < .05).
The permutation test for target ERSP found decreased high delta/low theta power (2–6 Hz) from stimulus onset until 400 ms and additional decreased theta from 400–500 ms in BD (FDR corrected, p < .05) (see Fig. 7). Alpha (8–12 Hz) suppression from 400 ms (approximately aligning with the peak of the P3 wave) until the end of the epoch (750 ms) was evident in HC and BD without significant group differences. As in the standard stimuli results, there were brief segments of decreased high frequency activity in BD across the time window, such as approximately 23 Hz at 550–750 ms, 30 Hz at 200–400 ms, and 40–50 Hz at 450–600 ms (see Fig. 7). Lastly, decreased target ITC was found in the delta and theta ranges (2–6 Hz) in BD compared to HC spanning the majority of the epoch starting at stimulus onset, with additional brief intervals of decreased beta and gamma scattered throughout the time window (FDR corrected, p < .05) (see Fig. 8).
Fig. 7.
Time-frequency plots of event-related spectral perturbation (ERSP) in response to target stimuli measured at Pz in healthy controls (HC; left) and individuals with bipolar disorder (BD; right). The non-parametric permutation test (center) shows significant group differences (FDR corrected, p < .05).
Fig. 8.
Time-frequency plots of inter-trial coherence (ITC) in response to target stimuli measured at Pz in healthy controls (HC; left) and individuals with bipolar disorder (BD; right). The non-parametric permutation test (center) shows significant group differences (FDR corrected, p < .05).
In the psychotic features analysis, none of the permutation tests reached significance for standard or target ERSP or ITC. However, the same graded pattern seen in the ERPs was evident for the TF results such that BDNP had an intermediate magnitude of neural response between HC and BDP.
Principal Component Analysis of Electrophysiological, Cognitive, and Symptom Measures
Regions of mean ERSP and ITC included in the PCA based on group-level peak activity were 0–400 ms at 3–6 Hz for standard stimulus response and 0–500 ms at 2–6 Hz for target stimulus response because these were found to statistically distinguish groups. Mean alpha ERSP (8–12 Hz) at 400–750 ms to target stimuli was included as well due to the prominent alpha suppression evident in both HC and BD.
The PCA for HC converged in five iterations. Four components emerged, which explained 71.13% of the variance. The PCA for BD converged in nine iterations. Five components emerged, which explained 69.46% of the variance in the data. Components resulting from the oblique rotation PCA pattern matrices were interpreted and are described below.
In HC, component 1 encompassed EEG variables to standard stimuli (see Table 2). It contained high positive factor loadings of delta/theta ERSP and ITC to standard stimuli and a high negative loading of N1 (stronger N1 amplitude). Component 2 appeared to represent target stimulus processing with positive loadings of delta/theta ERSP and ITC to targets, and negative loadings of P3 latency and age. Component 3 contained positive loadings of cognitive measures WASI IQ and digit symbol along with P3 amplitude. Lastly, component 4 had a negative loading of age and a positive loading of late alpha ERSP to targets.
Table 2.
PCA Pattern Matrix for Healthy Controls
| Components | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Standard ITC 3–6 Hz | .880* | −.025 | .042 | .060 |
| Standard ERSP 3–6 Hz | .825* | −.025 | .138 | .164 |
| Standard N1 amplitude | −.690* | −.155 | .233 | .042 |
| Target P3 latency | .040 | −.982* | .377 | −.093 |
| Target ITC 2–6 Hz | .091 | .753* | .249 | −.174 |
| Target ERSP 2–6 Hz | .327 | .531* | .227 | −.050 |
| Age | .224 | −.460* | −.140 | −.555* |
| WASI IQ | .044 | −.254 | .853* | −.134 |
| Digit symbol | −.084 | −.073 | .748* | .390 |
| Target P3 amplitude | −.040 | .269 | .667* | −.158 |
| Target ERSP 8–12 Hz | .203 | −.117 | −.136 | .945* |
Asterisks indicate factor loadings > 0.4. WASI = Wechsler Abbreviated Scale of Intelligence; ERSP = event-related spectral perturbation; ITC = inter-trial coherence. Digit symbol values are raw scores.
In BD, component 1 similarly had high loadings related to standard stimulus processing (see Table 3). It had positive loadings of delta/theta ERSP and ITC to standards and a negative loading of N1 amplitude. Additionally, component 1 had a positive loading of digit symbol and a negative loading of P3 amplitude. Component 2 encompassed target stimulus processing similar to in HC, with positive loadings of delta/theta ERSP and ITC to targets and P3 amplitude. Component 3 had a negative factor loading of IQ and positive loadings of depressive and manic symptom scores. Component 4 had a positive loading of age and negative loading of late alpha ERSP to targets, similar to the fourth component for HC. A fifth component emerged in the BD PCA, with positive loadings of P3 latency and late alpha ERSP to targets.
Table 3.
PCA Pattern Matrix for Bipolar Disorder
| Components | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Standard N1 amplitude | −.854* | .251 | .173 | −.147 | .119 |
| Standard ITC 3–6 Hz | .792* | .026 | .155 | .162 | −.225 |
| Standard ERSP 3–6 Hz | .739* | .154 | .245 | −.229 | −.118 |
| Digit symbol | .578* | .129 | −.244 | −.238 | .263 |
| Target P3 amplitude | −.500* | .720* | −.132 | .023 | −.122 |
| Target ITC 2–6 Hz | .131 | .806* | −.010 | .106 | −.140 |
| Target ERSP 2–6 Hz | .149 | .806* | .003 | −.124 | −.014 |
| YMRS | .064 | −.160 | .813* | −.092 | .002 |
| MADRS | .056 | .219 | .743* | .276 | .302 |
| WASI IQ | .304 | .122 | −.514* | .262 | .199 |
| Age | −.013 | −.025 | .013 | .804* | .285 |
| Target ERSP 8–12 Hz | −.023 | −.019 | .037 | −.695* | .433* |
| Target P3 latency | −.204 | −.167 | .090 | .067 | .819* |
Asterisks indicate factor loadings > 0.4. WASI = Wechsler Abbreviated Scale of Intelligence; ERSP = event-related spectral perturbation; ITC = inter-trial coherence; YMRS = Young Mania Rating Scale; MADRS = Montgomery Asberg Depression Rating Scale. Digit symbol values are raw scores.
Discussion
The present study was novel in its concurrent examination of early sensory and late cognitive EEG responses in the auditory oddball paradigm in bipolar disorder with (BDP) and without (BDNP) psychotic features across temporal and frequency domains. Reduced neural responses were found in BD in the temporal and frequency domains to frequent standard and rare target auditory stimuli indicating deficits in basic sensory, attentional, and cognitive processes. Additionally, greater reductions in ERPs were found to both types of auditory stimuli in BDP.
In the auditory oddball paradigm, the N1 ERP to standard stimuli may index basic sensory and attentional processes, and the P3 to target stimuli may index later context updating of the sensory systems (11). The present finding of a non-significant difference in N1 amplitude in response to standard stimuli in BD compared to HC is similar to a number of previous studies (5, 14, 21). Reduced N1 amplitude is more commonly reported in SZ (5, 17, 18), which may align with the finding of reduced N1 in BDP. This reduction in N1 in BDP and not BDNP suggests either a shared underlying endophenotype between BDP and SZ (7–9) or simply a more severe form of psychopathology with more prominent cognitive deficits (45) that could affect early information processing. Of note, N1 amplitude reductions were previously found in BDP and BDNP compared to HC (19). However, this prior study analyzed a history of psychotic features whereas the present study analyzed current psychotic features. Findings of reduced P3 amplitude to target stimuli in the total BD sample replicated previous findings (4, 5, 14), and BDP was found to have the most severe P3 reduction. Reduction in target-related neural responses in BD overall may reflect disrupted circuitry in neural regions thought to be generators of the P3b, such as the medial temporal cortex, temporal-parietal junction, and lateral prefrontal cortex, as well as alterations in glutamatergic and cholinergic neurotransmitter systems (46).
In the present time-frequency analyses, the most robust deficits were found in the slower frequencies delta and theta in BD in response to standard and target stimuli, coinciding with previous research (25–27). These deficits were evident in both event-related spectral perturbation (ERSP) and inter-trial coherence (ITC) analyses, indicating decreased total power as well as increased signal variability. Late alpha suppression or desynchronization to target stimuli found in both groups has been reported previously (47). Brief periods of reduced beta and gamma ERSP and ITC in BD survived rigorous permutation tests, although it is unclear if these differences are reliable due to their fleeting durations and distribution across the time window. While the psychological phenomena associated with particular frequency bands are complex and not well understood, reductions in delta and theta frequencies in BD during stimulus discrimination have been suggested to represent deficits in cognitive load, sustained attention, and signal detection (48). Theta activity is thought to have a role in the synchronization of activity across frontal, medial temporal, and hippocampal regions to form networks relevant to cognitive processes and information coding (49). Therefore, decreased theta activity under cognitive load could be a biomarker for cognitive deficits in psychopathology (27). Future work is encouraged to investigate effects of pre-stimulus power and phase angle on post-stimulus EEG activity.
Factor analyses aimed at parsimoniously depicting EEG, clinical symptom, and cognitive measures were useful in modeling the complex interrelations in this multivariate data. The PCAs yielded four components in HC and five components in BD. Inspection of the factor loadings revealed three highly similar components across the groups (i.e., components 1, 2, and 4). Specifically, the first component that emerged in both HC and BD loaded with variables assessing early sensory processing in response to standard stimuli in temporal and frequency domains. This component was slightly more complex in BD given that it additionally pulled in loadings of digit symbol score and target P3 amplitude. The second component loaded with variables assessing late cognitive processing in response to target stimuli in temporal and frequency domains. Target P3 latency negatively loaded on this component in HC whereas P3 amplitude positively loaded on this component in BD. Additionally, age loaded negatively onto this second component in HC, consistent with reported associations between increased age and decreased target stimulus processing (26, 43, 44), but this association did not appear in BD. The third component that emerged from both PCAs contained high loadings of cognitive measures but also differed slightly between the groups. This component in HC had positive loadings of WASI IQ, digit symbol, and P3 amplitude, aligning with previous reports of IQ and P3 (50). In BD, this component had a negative loading of WASI IQ with positive loadings of depression and mania scores, suggesting greater cognitive deficits with higher bipolar symptom severity. The fourth component that emerged in PCAs of both groups had high loadings of late target ERSP and age, suggesting greater target alpha suppression with aging. Lastly, a fifth component emerged in BD that captured additional positive loadings of late target ERSP and P3 latency, of which an interpretation was unclear.
The present study has limitations. A fine-grained characterization of lifetime experience of psychotic features was not possible, and future studies would benefit from more continuous measures of psychotic symptoms rather than dichotomous groupings. In general, we saw a graded response across subgroups, with BDP showing the most blunted neural response and BDNP showing an intermediary response between the BDP and HC groups. Given the lack of continuous measures of psychotic symptom severity, this graded difference between the groups is open to interpretation. It is possible that neural responses to BDP are more similar to SZ given their close proximity on the psychotic spectrum, but it is also possible that those individuals with BD experiencing psychotic features simply have a more severe form of psychopathology (45). Another possibility is that the neural activity related to auditory hallucinations in individuals with BDP is associated with neural and attentional interference in discrimination between auditory tones in this paradigm. Lastly, future studies are encouraged to perform data reduction techniques to derive electrophysiological activity from all EEG sensors and more fully capture the signal across the scalp over time (9).
In summary, we found reduced neural responses in bipolar disorder to frequent and rare auditory stimuli thought to be endophenotypes for psychosis, and these reductions were more severe for those individuals with active psychotic features. These findings suggest disruptions in early sensory and attentional processes as well as late cognitive processes such as context updating. Future analyses of EEG responses in temporal and frequency domains across dimensions of clinical symptoms can broaden our understanding of the etiology and maintenance of psychopathology.
Acknowledgments
This research was supported by National Science Foundation Graduate Research Fellowship Program Award 1342962 (NBL), National Institute of Drug Abuse Grant R21 DA035493-01A1 (BFO), and National Institute of Mental Health Grant R01 MH074983 (WPH). This research is also supported in part by Lilly Endowment, Inc., through its support for the Indiana University Pervasive Technology Institute (including the Karst supercomputer) and in part by the Indiana METACyt Initiative. The Indiana METACyt Initiative at IU is also supported in part by Lilly Endowment, Inc.
We wish to thank the patients and their families for participation in this study. We additionally acknowledge Larue D. Carter Memorial Hospital staff for their assistance in data collection, Jessica Mitroi for her assistance with independent components identification, and Aina Puce and Isaiah Innis for their contributions to methodological discussions.
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Goodwin FK, Jamison KR. Manic-Depressive Illness. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 2.Lin PI, Mitchell BD. Approaches for unraveling the joint genetic determinants of schizophrenia and bipolar disorder. Schizophr Bull. 2008;34:791–797. doi: 10.1093/schbul/sbn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. Curr Opin Psychiatry. 2006;19:165–170. doi: 10.1097/01.yco.0000214342.52249.82. [DOI] [PubMed] [Google Scholar]
- 4.Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21:867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: evidence from twin and patient studies. Psychiatry Res. 2009;169:212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2015;77:127–136. doi: 10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 11.Luck SJ, Kappenman ES. The Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press, Inc; 2012. [Google Scholar]
- 12.Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35:96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- 13.Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, Bramon E. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10:377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 14.Fridberg DJ, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, Mallory FW, O’Donnell BF. Relationships between auditory event-related potentials and mood state, medication, and comorbid psychiatric illness in patients with bipolar disorder. Bipolar Disord. 2009;11:857–866. doi: 10.1111/j.1399-5618.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaya E, Aydemir O, Selcuki D. Residual symptoms in bipolar disorder: the effect of the last episode after remission. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1387–1392. doi: 10.1016/j.pnpbp.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Polich J. P300 clinical utility and control of variability. J Clin Neurophysiol. 1998;15:14–33. doi: 10.1097/00004691-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia – a critical review. Psychiatry Res. 2008;161:259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Hamm JP, Ethridge LE, Shapiro JR, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Thaker GK, Clementz BA. Family history of psychosis moderates early auditory cortical response abnormalities in non-psychotic bipolar disorder. Bipolar Disord. 2013;15:774–786. doi: 10.1111/bdi.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doege K, Bates AT, White TP, Das D, Boks MP, Liddle PF. Reduced event-related low frequency EEG activity in schizophrenia during an auditory oddball task. Psychophysiology. 2009;46:566–577. doi: 10.1111/j.1469-8986.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 21.Johannesen JK, O’Donnell BF, Shekhar A, McGrew JH, Hetrick WP. Diagnostic specificity of neurophysiological endophenotypes in schizophrenia and bipolar disorder. Schizophr Bull. 2013;39:1219–1229. doi: 10.1093/schbul/sbs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MX. Analyzing Neural Time Series Data. Cambridge, MA: The Massachusetts Institute of Technology Press; 2014. [Google Scholar]
- 23.Basar-Eroglu C, Basar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- 24.Demiralp T, Ademoglu A, Istefanopulos Y, Basar-Eroglu C, Basar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 25.Atagün MI, Güntekin B, Masali B, Tülay E, Basar E. Decrease of event-related delta oscillations in euthymic patients with bipolar disorder. Psychiatry Res. 2014;223:43–48. doi: 10.1016/j.pscychresns.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Atagün MI, Güntekin B, Özerdem A, Tülay E, Basar E. Decrease of theta response in euthymic bipolar patients during an oddball paradigm. Cogn Neurodyn. 2013;7:213–223. doi: 10.1007/s11571-012-9228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özerdem A, Güntekin B, Atagün MI, Basar E. Brain oscillations in bipolar disorder in search of new biomarkers. Suppl Clin Neurophysiol. 2013;62:207–221. doi: 10.1016/b978-0-7020-5307-8.00014-4. [DOI] [PubMed] [Google Scholar]
- 28.Ethridge LE, Hamm JP, Shapiro JR, Summerfelt AT, Keedy SK, Stevens MC, Pearlson G, Tamminga CA, Boutros NN, Sweeney JA, Keshavan MS, Thaker G, Clementz BA. Neural activations during auditory oddball processing discriminating schizophrenia and bipolar disorder. Biol Psychiatry. 2012;72:766–774. doi: 10.1016/j.biopsych.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. 2009;37:778–787. doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, Kane F, Murray RM, Bramon E, Sham P, Rijsdijk F. The genetic and environmental influences of event-related gamma oscillations on bipolar disorder. Bipolar Disord. 2011;13:260–271. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV- TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 34.Kay S, Opler L, Fiszbein A. Structured Clinical Interview for the Positive and Negative Syndrome Scale (SCI-PANSS) New York: Albert Einstein College of Medicine; 1988. [Google Scholar]
- 35.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- 36.Montgomery SA, Asberg MA. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. Wechsler Abbreviated Scale of Intelligence – Second Edition (WASI-II) San Antonio, TX: NCS Pearson; 2011. [Google Scholar]
- 39.Wechsler D. WAIS-III administration and scoring. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 40.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Ford JM, Roach BJ, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res. 2008;1235:133–142. doi: 10.1016/j.brainres.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Blasio FM, Barry RJ. Prestimulus alpha and beta determinants of ERP responses in the Go/NoGo task. Int J Psychophysiol. 2013;89:9–17. doi: 10.1016/j.ijpsycho.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA. Effects of aging on event-related brain potentials and reaction times in an auditory oddball task. Psychophysiology. 1993;30:10–22. doi: 10.1111/j.1469-8986.1993.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 44.O’Donnell BF, Friedman S, Swearer JM, Drachman DA. Active and passive P3 latency and psychometric performance: influence of age and individual differences. Int J Psychophysiol. 1992;12:187–195. doi: 10.1016/0167-8760(92)90010-9. [DOI] [PubMed] [Google Scholar]
- 45.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- 47.Yordanova J, Kolev V, Polich J. P300 and alpha event-related desynchronization (ERD) Psychophysiology. 2001;38:143–152. [PubMed] [Google Scholar]
- 48.Güntekin B, Basar E. Review of evoked and event-related delta responses in the human brain. Int J Psychophysiol. 2016;103:43–52. doi: 10.1016/j.ijpsycho.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 50.Wronka E, Kaiser J, Coenen AM. Psychometric intelligence and P3 of the event-related potentials studied with a 3-stimulus auditory oddball task. Neurosci Lett. 2013;535:110–115. doi: 10.1016/j.neulet.2012.12.012. [DOI] [PubMed] [Google Scholar]