Abstract
Pseudomonas aeruginosa is an important human pathogen, the physiology and virulence of which are under the control of quorum sensing signals. These signals often have dual roles, functioning as toxins to some cells and as oxidative-stress protectors for their producer cells. Hence, their internal and external concentrations should be tightly controlled. In this study, we analyzed the interplay between the multidrug efflux transporters MexEF-OprN and MexG/HI-OpmD in quorum sensing of P. aeruginosa. We found that the two transporters have overlapping substrate specificities but different efficiencies. When overproduced, both MexEF-OprN and MexG/HI-OpmD provide clinical levels of resistance to diverse fluoroquinolones and protect P. aeruginosa against toxic phenazines. However, this similarity is enabled by synergistic interactions with the outer membrane. In hyperporinated cells, MexG/HI-OpmD is saturated by much lower concentrations of fluoroquinolones but is more efficient than MexEF-OprN in efflux of phenazines. Unlike MexEF-OprN, mutational inactivation of MexG/HI-OpmD reduces the levels of pyocyanin and makes P. aeruginosa cells hypersusceptible to phenazines. Our results further show that MexG binds pyocyanin, physically associates with MexHI and represses the activity of the transporter, revealing a negative regulatory role of this protein. We conclude that differences in kinetic properties of transporters are critical to maintain proper intra- and extra- cellular concentrations of phenazines and other signaling molecules, and that MexG/HI-OpmD controls the steady-state in the synthesis and secretion of phenazines.
Keywords: Antibiotic Resistance, Outer Membrane Barrier, Permeation, Efflux Constant, Phenazines, Hyperporination
Graphical Abstract
Pseudomonas aeruginosa is a gram-negative, opportunistic human pathogen that is most commonly associated with nosocomial diseases and infections in cystic fibrosis patients 1–2. The pathogen achieves its high level of drug resistance mainly through the interplay of low outer membrane permeability and active drug efflux across the cell envelope 3–4. In gram-negative bacteria, the most prominent efflux transporters involved in resistance are RND type transporters, largely because of their ability to translocate substrates across the outer membrane and their broad range of substrates 5. These transporters associate as tripartite complexes consisting of the inner membrane RND transporter, a membrane fusion protein (MFP), and an outer membrane channel (OMF). P. aeruginosa has at least twelve RND transporters encoded on its chromosome, which differ from each other in their level of expression and substrate specificity 6–7.
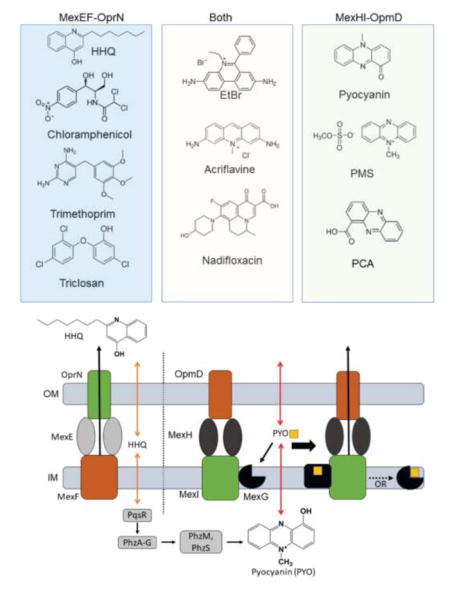
The most clinically relevant of these are MexAB-OprM 8, MexCD-OprJ 9, MexEF-OprN 10–11, and MexXY 11. The role these transporters play in resistance is well-documented and their broad substrate specificity highlights some redundancy between them. What is less understood is their role in cell physiology and virulence and why different transporters are overproduced and selected under different environmental conditions. It has been shown that MexAB-OprM translocates 3-oxo-acyl-homoserine lactones and, thus, exerts some control over the quorum sensing network of P. aeruginosa 12. HHQ (4-hydroxy-2-heptylquinoline), a direct precursor for the quorum sensing regulator PQS (Pseudomonas Quinolone Signal), was found to be a substrate of MexEF-OprN 13 (Figure S1). In fact, overexpression of this RND transporter was shown to decrease the production of PQS, as well as several PQS regulated virulence factors like pyocyanin and rhamnolipids 13–15. In addition, MexHI-OpmD was also shown to be involved in virulence of P. aeruginosa 16–17. A mutation in either mexI or opmD, the RND transporter or the OMF respectively, resulted in the loss of virulence and a reduction of quorum sensing signaling molecules 16. These phenotypes were suggested to result from MexHI-OpmD transport of a PQS precursor 16. Another study found that a deletion of MexHI-OpmD results in different colony morphology when compared to WT colonies and that the transporter exports the endogenously produced phenazine 5-methylphenazine-1-carboxylate (5-Me-PCA) 18 (Figure S1).
Homology and clustering analyses showed that MexG/HI-OpmD is the most closely related to MexVW-OpmM (70% sequence similarity between MexI and MexW) and MexEF-OprN (55% sequence similarity between MexI and MexF). Among various RND pumps, MexHI-OpmD is somewhat unusual because of the fourth protein MexG encoded by the first gene in the operon. Sequence analysis indicates that MexG is an inner membrane protein with four transmembrane α-helices along with one long, and one short periplasmic loop. Other transporters containing additional genes in the operons, like MdtABC of E. coli 19 or TriABC from P. aeruginosa 20, contain either an additional RND or MFP subunit, whereas MexG does not share any homology to those components. It is also distinctly different from small peptides like AcrZ of E. coli, which changes the substrate specificity of the transporter 21. Previous studies found that MexG is not required for the antibiotic efflux activity of MexHI-OpmD 22, and that MexG binds to PQS 23. However, the function of MexG and whether or not it is a component of the complex is still unknown.
All RND transporters function in the context of the two-membrane cell envelope and are believed to translocate their substrates through the specific outer membrane channel and across the outer membrane. The synergistic interactions with the low permeability barrier of the outer membrane masks the activities of efflux pumps and complicates the assessment of their kinetic properties and substrate specificities 24–25. In kinetic terms, this interaction is described by two constants: the barrier factor (B-factor) that compares maximum fluxes across the outer membrane and via efflux pumps (B = F/V, where F is the flux across the barrier and V is the maximal efflux velocity) and the efflux constant (KE) that relates efficiency of efflux pumps and the diffusion rate across the membrane (KE = V/(Km 0.5k2) , where Km is a Michaelis constant and k2 is the rate constant for drug diffusion across the outer membrane)25. Importantly, for some drugs the outer membrane barrier becomes saturated before efflux pumps do (B>1), creating a situation when efflux pumps never reach saturation and appear to be highly efficient, even with very poor substrates. We previously developed a hyperporination approach that enables influx of various compounds across the Gram-negative outer membranes, allowing for the separation of the contributions of active efflux and of the outer membrane in intracellular accumulation of compounds and in antibacterial activities 24, 25 , 26. In this study, we used this approach to gain insight into the role of RND pumps in quorum sensing.
We analyzed substrate specificities and efflux capacities of MexG/HI-OpmD and MexEF-OprN, the two pumps implicated in efflux of quorum sensing signals. Unexpectedly, the overexpression of MexHI-OpmD provides strong resistance to fluoroquinolones (FQ), at levels comparable to those provided by MexEF-OprN. This MexHI-OpmD efficiency, however, is achieved only if the outer membrane is intact. In hyperporinated cells, MexEF-OprN retains its efflux efficiency, whereas MexG/HI-OpmD is oversaturated by substrates and loses almost all of its ability to protect cells from FQ. In contrast, MexHI-OpmD is more efficient then MexEF-OprN in protection of P. aeruginosa against toxic phenazines, as seen not only in strains overexpressing the pumps but also in mutants lacking MexHI-OpmD. The transporter allows for the extracellular accumulation of pyocyanin, while providing resistance from its toxic effects. Our results also show that MexG interacts with the MexHI-OpmD complex and negatively affects its efflux activity. We conclude that the endogenous activity of MexG/HI-OpmD establishes the steady-state concentration of phenazines inside and outside of cells.
Results and Discussion
Physiological responses of P. aeruginosa cells to efflux pump inactivation are exaggerated by hyperporination
P. aeruginosa contains a large array of efflux pumps responsible for the intricate interplay of controlling the intracellular concentrations of endo- and exo- toxins. MexAB-OprM is the only efflux pump of this bacterium whose deletion makes cells highly susceptible to antibiotics, suggesting that the expression of other pumps is too low to significantly contribute to intrinsic antibiotic resistance 27. We previously showed that hyperporination of P. aeruginosa cells allows characterization of efflux pump functions without the contribution of the outer membrane barrier 24. To analyze the role of efflux in P. aeruginosa physiology, we integrated the gene encoding the pore under control of an IPTG-inducible promoter onto the chromosomes of strains with progressive deletions of operons encoding the efflux pumps: PΔ4-Pore lacking mexAB-OprM, mexCD-OprJ, mexXY and mexJKL, and PΔ6-Pore, a derivative of PΔ4-Pore further lacking mexEF-oprN and triABC pumps (Table S1). The properties of the constructed strains were compared to those of PAO1-Pore with full efflux capacity and PΔ3-Pore with mexAB, mexCD and mexXY deletions 24.
Efflux-proficient and -deficient strains without pores did not have significant differences in growth rates in LB medium (Figure S2). However, the optical densities of stationary PΔ4 and PΔ6 cultures were ~30–40% lower compared to those of PAO1 and PΔ3 strains. Increasing concentrations of the inducer IPTG did not have a notable effect on growth parameters (Figure S2).
In contrast, the growth of the Pore-producing (“Pore”) strains was sensitive to the presence of inducer. Without IPTG, the cell cultures of “Pore” strains were visually similar to their parental strains, including the difference in pigmentation (Figure 1A and 1E). PAO1-Pore produced the largest amounts of the blue-green pigment pyocyanin, whereas PΔ3-Pore, PΔ4-Pore, and PΔ6-Pore cells produced 5–10 times less of this pigment, the amount of which decreased with increasing number of inactivated efflux pumps (Figure 1E). In the presence of IPTG, both the cell densities and the amounts of pyocyanin decreased progressively with the number of inactivated efflux pumps (Figure 1B). The PΔ6-Pore cells were the most sensitive to the expression of the pore. Based on OD600 measurements, even at low concentrations of the inducer these cells grew slower and entered stationary phase at cell densities 30–50% lower than PAO1-Pore cells (Figure 1B–D). At 0.1 mM IPTG, growth rates and OD600 of stationary phase cells decreased for all efflux-deficient constructs suggesting that inactivation of efflux and hyperporination of the outer membrane have a cumulative effect on growth physiology of P. aeruginosa.
Figure 1. The effect of hyperporination on growth of P. aeruginosa cells with different efflux capacities.
A. Growth curves of the indicated P. aeruginosa strains grown in the absence of the inducer. Overnight cultures were diluted 1:100 into a fresh LB medium; the cells were grown for 18 hours and OD600 measured every 30 min. Data shown are the averages of three repeats and the error bars are SD (n=3). B. The same as in A but cultures were grown in the presence of 0.1 mM IPTG. C. After the growth curves were analyzed as described in A and B, cell aliquots were plated onto LB agar plates and CFUs counted after 24 hrs incubation at 37ºC. Averages of three repeats are shown with SD (n=3) as the error bars. D. Growth rates of indicated P. aeruginosa strains at increasing concentrations of IPTG. Averages of six repeats are shown with SD (n=3) as the error bars. E. Amounts of pyocyanin produced by indicated strains in the absence and presence of 0.1 mM IPTG. Test tubes containing overnight cultures of the indicated strains and their OD600 readings.
We noticed that overnight cultures of PΔ4 and PΔ6 strains and their hyperporinated variants contained large cell clumps and biofilms (Figure 1E), suggesting that the decreased cell density in growth measurements could be caused by cell aggregation and surface adhesion. Such aggregation of P. aeruginosa cells is usually caused by quorum response signaling and a transition from a planktonic to a sessile cell growth 28. Indeed, we found that decreased cell densities of the induced “Pore” cultures did not correlate with decreased number of colony forming units (CFUs). In fact, for both efflux-proficient and –deficient cells, the CFUs/ml/OD600 remained the same in the presence and absence of the inducer (Figure 1C). This result suggested that the observed differences in cell densities and apparent growth rates are due to cell aggregation and not caused by cell division defects. Since such aggregation is already measurable in PΔ4 and PΔ6 cultures (Figure S2), these results further suggest that hyperporination of the outer membrane does not lead to cell death or cell division defects, but exaggerates cell aggregation caused by the loss of active efflux, presumably because of the hypersensitivity of cells to quorum sensing signals.
MexHI-OpmD and MexEF-OprN have overlapping substrate specificities
Pyocyanin is one of the most important virulence factors in P. aeruginosa and is required to establish full virulence of the pathogen 29–30. It leads to oxidative stress by the formation of reactive oxygen species and reduces ATP levels through the oxidation of NADH in host cells 30–31. This phenazine has significant antimicrobial and antifungal activity 32–34, but P. aeruginosa is intrinsically resistant to its antibiotic activity. Furthermore, pyocyanin was shown to assist in the adaptation of P. aeruginosa to anaerobic conditions by acting as an electron mediator between NADH and oxygen 35 (Figure S1). Thus, the intracellular and extracellular levels of this compound have to be tightly regulated to ensure that it can act as a signaling molecule and electron mediator without becoming toxic to the cell.
The amounts of extracellular pyocyanin were previously linked to the expression of MexEF-OprN (negative correlation) and MexG/HI-OpmD (positive correlation) efflux pumps 15–16. To analyze the efficiency and substrate specificity of MexHI-OpmD and MexEF-OprN, we created three constructs that constitutively overexpress either MexGHI-OpmD, MexHI-OpmD, or MexEF-OprN. The overexpression of these efflux pumps is expected to increase significantly the efflux efficiency for their substrates. We confirmed similar expression levels of these constructs in PΔ4 cells (Figure 2A) and tested susceptibilities of these cells against selected antibacterials (Table 1). In agreement with previous results, all three constructs provided resistance against the FQ ciprofloxacin and levofloxacin, ethidium bromide, and acriflavine but only the overexpression of MexEF-OprN drastically increased the MICs of chloramphenicol, tetracycline, triclosan, and trimethoprim. Hence, all three pumps are overproduced and functional. Interestingly, we were unable to find an antibacterial agent that is a substrate of MexG/HI-OpmD but not MexEF-OprN. Thus, in agreement with previous studies 10, 22, MexEF-OprN has a broader substrate specificity than MexG/HI-OpmD.
Figure 2. Fluoroquinolone susceptibility testing of cells overexpressing MexHI-OpmD, MexGHI-OpmD, and MexEF-OprN.
A. Immunoblotting of membrane fractions from cells harboring the plasmids overproducing the indicated transporters. The respective outer membrane channels (OMF) are tagged with a C-terminal His-tag and visualized with anti-His monoclonal antibody. B and C. Effect of IPTG inducer concentration on MICs of ciprofloxacin in PΔ4 (B) and PΔ4-Pore (C) cells expressing indicated transporters. D and E. PΔ4 (D) or PΔ4-Pore (E) cells harboring either pMexHI-OpmD, pMexGHI-OpmD, or pMexEF-OprN are tested against a library of FQ. Fold changes in MICs are shown for cells carrying different plasmid constructs are shown. FQ for each ratio are listed from bottom to top: Ciprofloxacin, Enrofloxacin, Levofloxacin, Gatifloxacin, Moxifloxacin, Prulifloxacin, Sparfloxacin, Difloxacin, Lomefloxacin, Ofloxacin, Pazufloxacin, Norfloxacin, Pefloxacin, Sarafloxacin, and Nadifloxacin.
Table 1.
Minimal inhibitory concentrations of antibiotics in P. aeruginosa cells with intact and hyperporinated outer membranes carrying the plasmid borne MexEF-OprN and MexG/HI-OpmD.
| Drugs | MICs (μg ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PAO1 | PΔ4 | PΔ4-Pore | |||||||
|
| |||||||||
| - | - | MexEF-OprN | MexHI-OpmD | MexGHI-OpmD | - | MexEF-OprN | MexHI-OpmD | MexGHI-OpmD | |
| CIP | 0.031 | 0.007 | 0.41 | 0.41 | 0.21 | 0.002 | 0.052 | 0.026 | 0.013 |
| LEV | 0.125 | 0.007 | 0.45 | 0.9 | 0.45 | 0.002 | 0.056 | 0.014 | 0.014 |
| EtBr | ND | 128 | 2048 | 2048 | 2048 | 8 | 16 | 32 | 32 |
| ACR | >64 | 32 | >1024 | >1024 | >1024 | 4 | 8 | 16 | 16 |
| TET | 4 | 0.25 | 1 | 0.25 | 0.25 | 0.031 | 0.063 | 0.031 | 0.031 |
| CF | 8 | 0.5 | >128 | 0.5 | 0.5 | 0.25 | 2 | 0.25 | 0.25 |
| TRI | 1024 | 128 | >256 | 64 | 64 | 16 | 128 | 16 | 16 |
| TRM | >256 | 1 | >256 | 1 | 1 | 1 | 32 | 1 | 1 |
CIP, ciprofloxacin; LEV, levofloxacin; EtBr, ethidium bromide; ACR, acriflavine; TET,
tetracycline; CF, chloramphenicol; TRI, triclosan; TRM, trimethoprim. ND, no data.
To gain insight into the underlying differences in substrate specificities of the two pumps, we next analyzed MICs of a library of 15 structurally diverse FQ in PΔ4 cells carrying the three efflux pump constructs (Table 2). In PΔ4, both MexEF-OprN and MexHI-OpmD transporters provided strong resistance to all FQ tested, indicating that overexpression of either one of the pumps could lead to clinical levels of fluoroquinolone resistance (Figure 2D). Surprisingly, for the majority of tested FQ there was no difference in MICs in cells producing either MexEF-OprN or MexHI-OpmD. In a few cases, the MICs differed only by two-fold (Table 2 and Figure 2D). Furthermore, for most fluoroquinolones the fold MIC change in PΔ4(pump)/PΔ4(empty vector) was 64–128 with a few exceptions of 32 fold change in MICs (Figure 2D). Thus, despite the different substrate specificities, MexEF-OprN and MexHI-OpmD seem unable to recognize differences in structures of FQ.
Table 2.
Antibacterial activities of fluoroquinolones in P. aeruginosa cells with intact and hyperporinated outer membranes carrying the plasmid borne MexEF-OprN and MexG/HI-OpmD.
| FQs | MICs, μM
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PAO1 | PΔ4 | PΔ4MexEF-OprN | PΔ4 MexHI-OpmD | PΔ4 MexGHI-OpmD | PΔ4-Pore | PΔ4-Pore MexEF-OprN | PΔ4-Pore MexHI-OpmD | PΔ4-Pore MexGHI-OpmD | |
| Ciprofloxacin | 0.39 | 0.02 | 1.25 | 1.25 | 0.625 | 0.005 | 0.16 | 0.08 | 0.04 |
| Enrofloxacin | 1 | 0.04 | 5 | 5 | 1.25 | 0.005 | 0.31 | 0.16 | 0.04 |
| Levofloxacin | 1 | 0.02 | 1.25 | 2.5 | 1.25 | 0.005 | 0.16 | 0.04 | 0.04 |
| Gatifloxacin | 1 | 0.04 | 2.5 | 2.5 | 1.25 | 0.002 | 0.16 | 0.04 | 0.02 |
| Moxifloxacin | 6.25 | 0.04 | 5 | 2.5 | 0.313 | 0.002 | 0.31 | 0.04 | 0.005 |
| Prulifloxacin | 0.25 | 0.01 | 0.313 | 0.313 | 0.156 | 0.003 | 0.08 | 0.02 | 0.01 |
| Sparfloxacin | 1 | 0.04 | 2.5 | 5 | 2.5 | 0.001 | 0.08 | 0.08 | 0.02 |
| Difloxacin | 1.56 | 0.04 | 5 | 2.5 | 0.625 | 0.002 | 0.16 | 0.04 | 0.02 |
| Lomefloxacin | 1.56 | 0.08 | 5 | 5 | 2.5 | 0.01 | 0.31 | 0.31 | 0.08 |
| Ofloxacin | 1.56 | 0.08 | 5 | 5 | 2.5 | 0.01 | 0.31 | 0.16 | 0.08 |
| Pazufloxacin | 1 | 0.04 | 2.5 | 1.25 | 0.625 | 0.01 | 0.16 | 0.08 | 0.04 |
| Norfloxacin | 1 | 0.04 | 2.5 | 1.25 | 1.25 | 0.02 | 0.63 | 0.08 | 0.08 |
| Pefloxacin | 1 | 0.09 | 5 | 5 | 2.5 | 0.02 | 1.25 | 0.16 | 0.16 |
| Sarafloxacin | 0.39 | 0.02 | 1.25 | 0.625 | 0.313 | 0.005 | 0.08 | 0.02 | 0.02 |
| Nadifloxacin | 6.25 | 0.08 | 5 | 5 | 2.5 | 0.001 | 0.16 | 0.08 | 0.08 |
As was previously reported 22, MexG was not required for the functionality of MexHI-OpmD. In fact, we observed a reduction of the activity of MexHI-OpmD when MexG is co-expressed (Table 2 and Figure 2D). However, this difference was not the same across all compounds. For example, most of the compounds tested showed 2–4 fold decrease in MIC, whereas the presence of MexG potentiated the activity of moxifloxacin by 8 fold, decreasing the MIC from 2.5 μM in PΔ4(pMexHI-OpmD) to an MIC of 0.3 μM in PΔ4(pMexGHI-OpmD). Since we did not detect notable differences in expression between the MexHI-OpmD and MexGHI-OpmD constructs (Figure 2A), this result suggests that MexG negatively affects the activity of MexHI-OpmD in a substrate-dependent manner.
Hyperporination of the outer membrane differentially affects activities of MexEF-OprN and MexHI-OpmD
We next analyzed the change in MICs of FQ in the hyperporinated PΔ4-Pore overproducing efflux pumps, thus reducing the contribution of the outer membrane barrier in antibacterial activities. We first titrated IPTG, the inducer of the pore, to select its optimal concentration (Figure 2B and C). As expected, a decrease in MICs of ciprofloxacin with increasing concentration of inducer was seen in PΔ4-Pore strains, but not in PΔ4 strains. We measured a 4-fold decrease in the MIC of ciprofloxacin in PΔ4-Pore(pBSPII) in the presence of 2 mM IPTG. Similarly, we also observed a decrease in the MIC by 4 fold and 8 fold in the induced PΔ4-Pore(pMexEF-OprN) and PΔ4-Pore(pMexHI-OpmD), respectively.
We then tested the remaining FQ in PΔ4-Pore strains induced by 2 mM IPTG (Table 2 and Figure 2E). In agreement with previous studies 24, hyperporinated cells were much more susceptible to the antibiotics as seen by a decrease of the MIC up to 64-fold when comparing PΔ4 and PΔ4-Pore(pBSPII). Hyperporination also increased susceptibilities of strains overexpressing the efflux pump constructs, albeit to a different extent. Surprisingly, the hyperporination seemed to affect the activity of MexG/HI-OpmD much stronger than MexEF-OprN. For most of the FQ, the overexpression of MexEF-OprN in PΔ4-Pore generated the same 64–128 fold increase in MICs. This result suggests that even with the reduced barrier factor and increased influx of FQ, this pump still operates below saturation. However, some substrate specificity could be deduced from the fold MIC change in MexEF-OprN overproducers with and without the Pore. Norfloxacin, prulifloxacin and perfloxacin are excellent substrates of MexEF-OprN and the efficiency of their efflux is only weakly (4 fold) affected by hyperporination (Table 2). Thus, even in the hyperporinated cells, MexEF-OprN is far from saturation with these FQ.
In contrast, nadifloxacin, sparfloxacin and difloxacin are poor substrates of MexEF-OprN and the pump is effective against these FQ only if the influx is very slow (Table 2). In contrast, in hyperporinated cells MexHI-OpmD could provide the same 64-fold MIC change only for sparfloxacin and nadifloxacin, whereas for all other antibiotics the fold MIC changes were significantly lower with most in the 4–16 fold range. As a result, for such antibiotics as moxifloxacin, norfloxacin, or pefloxacin, the difference in susceptibility between MexHI-OpmD and MexEF-OprN in hyperporinated PΔ4-Pore was up to 8-fold (Table 2 and Figure 2E). In addition to nadifloxacin, sparfloxacin and difloxacin for which MexEF-OprN is not as effective, MexHI-OpmD also lost its efficiency against moxifloxacin, gatifloxacin and levofloxacin (64-fold MIC change). Thus, MexHI-OpmD is not only less efficient in efflux of FQs than MexEF-OprN, but also less specific to these antibiotics. This result explains why the overproduction of MexEF-OprN but not MexHI-OpmD is selected in clinical isolates exposed to high concentrations of FQs 10, 36.
Interestingly, for other non-FQ substrates of MexEF-OprN and MexHI-OpmD, hyperporination dramatically reduced the efficiency of both pumps (Table 1), suggesting that they are effective against these drugs only because of the low permeability barrier of the outer membrane.
The presence of MexG further diminished the ability of MexHI-OpmD to protect against FQ as seen from only a 4–8 fold change in MICs for most of the compounds in PΔ4-Pore(MexGHI-OpmD) cells. Surprisingly, the efflux of nadifloxacin remained unaffected by hyperporination and MexG, further supporting the conclusion that the negative effect of MexG depends on specific substrates of MexHI-OpmD.
MexEF-OprN and MexHI-OpmD have different efflux efficiencies
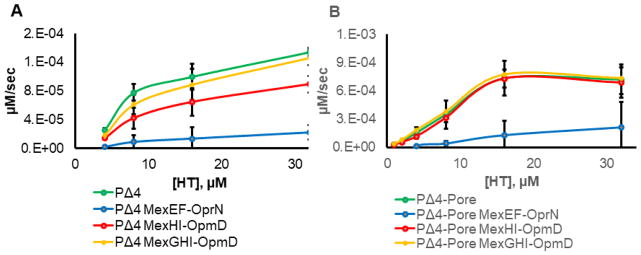
To establish that the changes in MICs observed in hyperporinated cells are due to the differences in efflux activities of the overproduced pumps, we utilized a real-time fluorescence uptake assay. For this purpose, we used a fluorescent probe Hoechst 33342 (HT) to carry out the uptake experiment. Like fluoroquinolones, HT is an inhibitor of DNA topoisomerases 37. However, HT is also a fluorescent probe, the emission of which is significantly enhanced when it binds to lipids or DNA. This enhancement allows us to monitor its uptake into cells 25. The uptake data were fitted to an exponential equation to extract initial rates of uptake (Figure 3 and Figure S3). We observed a clear difference in the initial rates of HT uptake with and without the transporters present, indicating that HT is a substrate of both MexEF-OprN and MexHI-OpmD efflux pumps. Overexpression of MexEF-OprN dramatically (up to 10-fold) decreased the rates of uptake of HT both in the absence and presence of the pore, suggesting that HT is an excellent substrate of this efflux pump. Thus, as with the MICs of FQ, hyperporination does not affect the efficiency of this pump against HT. The overexpression of MexHI-OpmD also decreased the rates of HT uptake, but only in cells with an intact outer membrane. In PΔ4(pMexHI-OpmD) cells, the rate of HT uptake decreased by ~2 fold, indicating that HT is also a substrate of MexHI-OpmD, but is expelled from the cells more slowly than from cells overexpressing MexEF-OprN. The co-expression of MexG with MexHI-OpmD further reduced its activity, supporting the conclusion that MexG negatively affects the activity of the efflux pump. Furthermore, neither MexHI-OpmD nor MexGHI-OpmD overexpression was able to reduce the rate of HT uptake in hyperporinated cells, indicating that slow diffusion across the outer membrane is critical for activity of MexG/HI-OpmD.
Figure 3. Kinetic uptake measurements.
A. The plot of initial rates of HT accumulation in the indicated cells as a function of extracellular HT concentration. B. Graph of the initial rates of HT accumulation in pore expressing cells plotted against the extracellular HT concentration. All kinetic measurements were done in triplicate. Error bars are SD (n=3).
Taken together with MIC measurements, these results suggest that MexHI-OpmD is a weak efflux pump that heavily relies on the outer membrane barrier for its activity against fluoroquinolones and HT. Alternatively, in hyperporinated cells, MexHI-OpmD is presented with an elevated concentration of an endogenously produced substrate that outcompetes the antibiotics, resulting in increased susceptibility.
Deletion of mexGHI-opmD leads to a decrease in the extracellular levels of pyocyanin
We next investigated a possibility that endogenous substrates outcompete FQ in the hyperporinated cells overproducing MexG/HI-OpmD. For this purpose, we constructed a series of strains lacking mexGHI-opmD. As described above, the progressive deletion of efflux pumps leads to the progressive loss of pyocyanin secretion due to changes in quorum sensing signaling (Figure 1E). Therefore, the mexGHI-opmD operon was deleted from chromosomes of PAO1, PΔ3, PΔ4 and PΔ6 strains. For simplicity reasons, the ΔmexGHI-opmD variants will be referred to as PAO1ΔGHID, PΔ3ΔGHID, PΔ4ΔGHID, and PΔ6ΔGHID respectively.
The constructed mutants did not show an extended lag phase (Figure S4) that was previously reported in mexI and opmD mutants 16. Similarly, we were unable to see a decrease in the production of PQS (data not shown), but we did notice that the ΔmexGHI-opmD mutants secreted significantly less pyocyanin even in already pyocyanin-deficient efflux mutants (Figure 4A). We measured the amounts of this phenazine in supernatants of bacterial cultures and found a noticeable (more than 2-fold) decrease in the amounts of pyocyanin in the cultures of all ΔmexGHI-opmD strains when compared to their respective parental strains (Figure 4A).
Figure 4. Expression MexG/HI-OpmD correlates with amounts of extracellular pyocyanin and is required for P. aeruginosa self-protection against its toxicity.
A. Extracellular concentrations of pyocyanin in PAO1, PΔ3, PΔ4, and PΔ6 parental strains (blue) and their derivatives lacking mexGHI-opmD (ΔGHID) mutants (orange). B. Extracellular concentrations of pyocyanin in cultures of PAO1 strains overexpressing MexEF-OprN or different combinations of MexG/HI-OpmD components. All measurements were done on cultures grown to stationary phase and in triplicate. Error bars are SD (n=3). C. Spot assays with pyocyanin showing zones of inhibition in strains with and without mexGHI-opmD. D. The same as C but with PMS. E and F. Quantification of zones of inhibition of pyocyanin (E) and PMS (F) in indicated strains. Error bars are SD (n=3).
To confirm that MexGHI-OpmD is linked to the synthesis and excretion of pyocyanin, we measured amounts of this pigment in the cultures of the PAO1 strains overproducing different components of the transporter (Figure 4B). When compared to the wild type, PAO1 overexpressing MexGHI-OpmD or MexHI-OpmD produced twice the amount of pyocyanin. Expression of MexG alone did not change the amounts of pyocyanin in the culture medium, but expression of MexGHI without the outer membrane channel OpmD resulted in a 50% increase. This suggests that MexHI might be able to associate with another outer membrane channel to achieve at least partial activity. MexG does not appear to be required for this functionality of the transporter. In agreement with previous results, overexpression of MexEF-OprN resulted in decreased production of pyocyanin 14. Thus, the amount of secreted pyocyanin positively correlates with the expression of MexG/HI-OpmD in P. aeruginosa cells and this correlation is independent of the presence or absence of other RND pumps.
MexGHI-OpmD provides a self-protection of P. aeruginosa to phenazines
Pyocyanin is a powerful toxin that inhibits the electron transport chain and several other pathways 29, 35. To test whether pyocyanin is a substrate of MexG/HI-OpmD, we performed growth spot inhibition assays with PAO1 and its efflux deficient mutants (Figure 4C and E). All strains that contained a chromosomal copy of mexGHI-opmD were fully resistant to extracellular pyocyanin. On the other hand, the efflux deficient mutants that lacked mexGHI-opmD were all hypersusceptible, as seen from large zones of inhibition. At the tested concentrations, PAO1ΔGHID was still resistant to pyocyanin, likely indicating that the constitutively expressed MexAB-OprM can provide some level of resistance to extracellular phenazines.
Consistent with the above results and previous studies 18, deletion of mexGHI-opmD resulted in hypersusceptibility to PMS, a close analog of 5-Me-PCA, which is an endogenous precursor of pyocyanin (Figure 4D and F). Compared to that of pyocyanin, the zone of inhibition for PAO1ΔGHID was roughly 2-fold larger than for PAO1, indicating that PMS is more toxic than pyocyanin and that the MexG/HI-OpmD is indispensable for immunity against PMS even when MexAB-OprM is expressed.
We next inserted the Pore onto the chromosomes of PΔ4ΔGHID and PΔ6ΔGHID strains, as well as of PΔ5, a PΔ4 derivative lacking MexEF-OprN, and analyzed MICs of pyocyanin and PMS in these strains (Table 3). Deletion of mexGHI-opmD from PΔ4 resulted in a 4 fold and a 16 fold decrease of the MICs of exogenous pyocyanin and PMS, respectively. Interestingly, PΔ4ΔGHID strain is four times more resistant to chloramphenicol and ciprofloxacin than PΔ4. This result suggests that upon deletion of mexGHI-opmD, another transporter, likely MexEF-OprN as based on the resistance profile, is overexpressed. Indeed, no increase in MICs of these antibiotics could be seen in strains lacking MexEF-OprN (Table 3). Interestingly, hyperporination reduced the MICs of pyocyanin, chloramphenicol and ciprofloxacin, but not the MIC of PMS in PΔ4ΔGHID-Pore. This result shows that the expression of MexEF-OprN can provide partial protection from the toxic effects of pyocyanin but not from PMS.
Table 3.
The contributions of MexEF-OprN and MexG/HI-OpmD in P. aeruginosa susceptibility to phenazines and antibiotics.
| MIC, μM | Pyocyanin | PMS | Chloramphenicol | Ciprofloxacin | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Genotype | - | Pore | - | Pore | - | Pore | - | Pore | |
| PΔ4 | EF+, GHI+ | 1000 | 250 | 500 | 62.5 | 3.1 | 1.6 | 0.001 | 0.0002 |
| PΔ4ΔG | EF+, GHI− | 250 | 31.25 | 31.3 | 31.3 | 12.4 | 1.6 | 0.004 | 0.0002 |
| PΔ5 | EF−, GHI+ | >1000 | 250 | 500 | 25 | 3.1 | 1.6 | 0.001 | 0.0002 |
| PΔ6 | EF−, GHI+ | >1000 | 125 | 500 | 125 | 1.6 | 1.6 | 0.001 | 0.0002 |
| PΔ6ΔG | EF−, GHI− | 31.3 | 31.3 | 31.3 | 15.6 | 1.6 | 1.6 | 0.0002 | 0.0001 |
| PΔ6ΔG(pMexEF-OprN) | EF↑, GHI− | >500 | 125 | 500 | 31.25 | >256 | 3.1 | 0.4 | 0.012 |
| PΔ6ΔG(pMexHI-OpmD) | EF−, HI↑ | >500 | 1000 | 2000 | 125 | 1.6 | 0.8 | 0.4 | 0.012 |
| PΔ6ΔG(pMexGHI-OpmD) | EF−, GHI↑ | >500 | 500 | 1000 | 62.5 | 1.6 | 0.8 | 0.2 | 0.012 |
Both PΔ5 and PΔ6 strains were still completely resistant to exogenous pyocyanin and PMS, indicating that MexGHI-OpmD alone is sufficient to provide protection against toxic effects of phenazines. In both strains, the activities of phenazines were potentiated by hyperporination, indicating that the protection is mediated by efflux. In contrast, the MIC of pyocyanin decreased by a staggering 64 fold in PΔ6ΔGHID and no further decrease was observed in the hyperporinated cells, suggesting that PΔ6ΔGHID is depleted of phenazine efflux. Furthermore, MICs of both phenazines were the same in PΔ4ΔGHID-Pore, PΔ6ΔGHID, and PΔ6ΔGHID-Pore cells, suggesting that the MexEF-OprN mediated efflux of pyocyanin in PΔ4ΔGHID cells is weak and highly dependent on the outer membrane barrier. On the other hand, hyperporination of cells producing MexGHI-OpmD decreased the MICs of phenazines by only 4–8 fold, indicating that MexGHI-OpmD is more efficient in efflux of both phenazines and can compensate for the increased influx across the outer membrane. In agreement, even when MexEF-OprN or MexG/HI-OpmD were strongly overproduced from plasmids in strains with and without the Pore, MexG/HI-OpmD was more efficient in protection against phenazines (Table 3).
Taken together, these results show that MexG/HI-OpmD is the major and highly efficient efflux pump of phenazines whereas inactivation of MexEF-OprN has no effect on the MIC of phenazines. The absence of MexG/HI-OpmD and MexAB-OprM upregulates the expression of MexEF-OprN that can provide partial protection against pyocyanin but not PMS.
Antibacterial activities of phenazines and fluoroquinolones are additive
To test whether phenazines are responsible for the decreased activity of MexHI-OpmD against fluoroquinolones in hyperporinated strains, we set up checkerboard assays to analyze possible interactions between ciprofloxacin and extracellular pyocyanin with PΔ4-Pore cells containing different plasmids (Figure S5). For each strain, we calculated an average fractional inhibitory concentration (FIC) index. The FIC is the fraction of the MIC in combination with a second drug and the MIC by itself. The FIC index is the sum of the two FIC from each drug and is commonly used to describe interactions of two antimicrobials 38–39. Generally, an FIC index of 0.5 represents synergy, an FIC index of 1 represents an additive/inconclusive effect, and an FIC of 2 is defined as antagonism between the two antimicrobials 40–41. We found the FIC index of ~2.3 for the control PΔ4-Pore with empty vector, whereas the FIC indices measured for PΔ4-Pore cells expressing MexEF-OprN, MexHI-OpmD, and MexG/HI-OpmD were 1.6, 1.1, and 1.0 respectively. This suggests that there is no significant interaction between pyocyanin and ciprofloxacin in the hyperporinated strains overproducing efflux pumps, however an antagonistic interaction is possible in the absence of these efflux pumps. The higher FIC indicies for MexEF-OprN and the empty vector strain could, presumably, be due to physiological effects of exogenous pyocyanin. Low, non-inhibitory concentrations of pyocyanin could stimulate cell growth in these strains by maintaining redox homeostatis35. These results also show that in the strains overproducing MexG/HI-OpmD, ciprofloxacin and pyocyanin do not interact with each other and the antibacterial activities are simply additive.
Thus, we conclude that the increase in the susceptibility to FQ in hyperporinated cells highlights the low efficiency of the MexG/HI-OpmD transporter for these substrates and the high dependency on synergistic interactions with the outer membrane barrier. In contrast, phenazine efflux by MexG/HI-OpmD is specific and efficient even with increased influx of these toxins in hyperporinated cells.
MexG binds MexHI-OpmD and pyocyanin
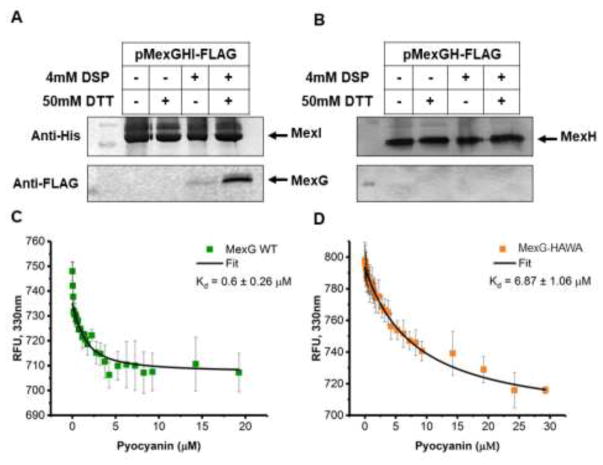
The results described above show that MexG/HI-OpmD is highly specific for phenazines and that the presence of MexG has a negative effect on the activity of the transporter. Hence, we tested whether MexG physically associates with MexHI. For this purpose, we created a construct expressing MexG with an N-terminal FLAG tag, and MexH and MexI with a C-terminal His tag. Cells producing the tagged MexGHI were split into two aliquots and the 12 Å crosslinker dithiobis(succinimidylpropionate) (DSP) was added to one of them, before purification of MexI using His Bind resin. After purification, the MexI-containing fractions were analyzed by immunoblotting with anti-His and anti-FLAG antibodies to detect MexI and MexG, respectively. No MexG was detected by immunoblotting in MexI fractions purified from cells untreated with the cross-linker. In contrast, we could clearly detect MexG in elution fractions purified from the cross-linked cells, even after extensive washes with imidazole. Reduction of the cross-linker yielded a 15 kDa band reacting with anti-FLAG antibody and corresponding by size to MexG (Figure 5A). We repeated the experiment with a plasmid expressing MexG with N-terminal FLAG tag and MexH with a C-terminal His tag, however we were unable to detect any crosslinking of MexG and MexH (Figure 5B). This result suggests that there is a physical interaction between MexG and MexHI-OpmD, and that MexI is required for this interaction.
Figure 5. MexG binds MexHI and pyocyanin.
A and B. Immunoblotting analyses of MexIHis (A) and MexHHis (B) elution fractions purified from PΔ3 cells harboring pMexGHI-FLAG and pMexGH-Flag respectively. Cells were treated with crosslinker prior to lysis as indicated. The lower panel shows the release of FLAG-tagged MexG from crosslinked samples when elution fractions are treated with a reducing agent (DTT). DSP, dithiobis(succinimidylpropionate); DTT, dithiothreitol. Top panels show the development with anti-His and the lower panels with anti-FLAG primary antibodies. C and D. Fluorescence emission at 330 nm (excitation at 290 nm) of the wild type MexG (C) and MexG-HAWA (D) incubated with increasing concentration of pyocyanin was measured and normalized as described in Methods and is plotted as a function pyocyanin concentration. Fitted line shown in black. Error bars are SD (n=3).
We next analyzed whether MexG will bind to pyocyanin. To test this, we purified MexG using metal affinity chromatography and analyzed binding to pyocyanin by fluorescence spectroscopy. MexG contains five tryptophan residues that are predicted to be in the transmembrane helices. As tryptophan residues were excited, the emission of MexG fluorescence peaked at around 330 nm. In contrast, pyocyanin has a very low fluorescence at the same excitation and emission wavelengths (Figure S6). However, addition of increasing concentrations of pyocyanin to MexG significantly quenches the fluorescence of MexG and causes a slight red shift in the emission spectra (Figure S6), suggesting that pyocyanin binds MexG and causes a change in the environments of the tryptophan residues. We calculated a KD of pyocyanin to MexG to be about 0.6 μM (Figure 5C). This suggests that the binding is quite strong, with the KD is similar to what was previously reported for MexG and PQS 23.
To determine whether pyocyanin binding is specific, we constructed the MexG mutant MexG–HAWA, which contains two substitutions His95Ala and Trp98Ala at the C-terminal end of the transmembrane domain 3 facing the periplasm. We repeated the fluorescence binding experiment with this mutant and found that the affinity of MexG–HAWA to pyocyanin decreased by at least 10 fold when compared to MexG WT to a KD of about 7 μM (Figure 5D and Figure S7). The lower binding affinity for MexG–HAWA shows that pyocyanin binds specifically to MexG, suggesting a functional relationship between the protein and the MexHI-OpmD transporter.
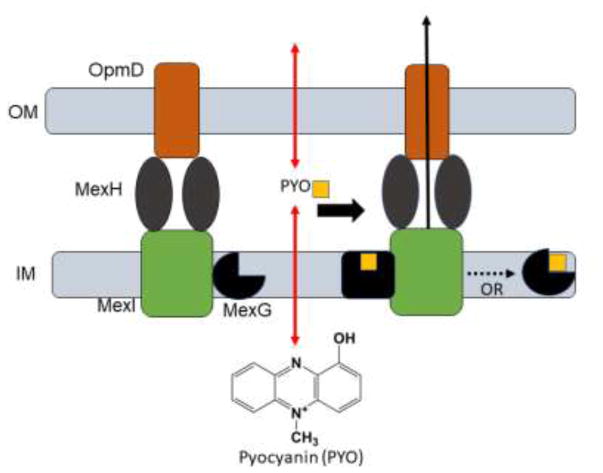
Based on the described results, we propose that MexG plays a role as a negative regulator of the efflux activity of MexHI-OpmD. We found that it physically associates with the transporter in vivo, establishing that at least a fraction of this pump exists as a four component complex MexGHI-OpmD. Antibiotic susceptibility testing showed a decrease in activity of the transporter in respect to some substrates when MexG is co-expressed (Figure 2D and E, Tables 2 and 3). This decrease seems to vary depending on the substrate tested. Norfloxacin, ethidium bromide, and acriflavine did not show any changes in activity, whereas moxifloxacin showed the biggest reduction of the MIC of 8 fold. Some of these compounds might more closely resemble the native binding partner of MexG and can activate the small membrane protein. This could point to a regulatory function of MexG with respect to the activity of MexHI-OpmD. The fluorescence assays showed that MexG binds pyocyanin and that this binding is specific with a KD of about 0.6 μM. It is possible that MexG could act as a sensor of phenazines and other signaling molecules in the inner membrane and modulate the activity of MexHI-OpmD by changing the conformation or by dissociating from the complex in the ligand-bound state (Figure 6).
Figure 6. A proposed mechanism of MexG/HI-OpmD efflux pump.
The presence of MexG negatively affects the activity of MexHI-OpmD efflux pump. MexG physically interacts with the pump and its substrates such as pyocyanin. In the ligand-bound state MexG could be in a different conformation or could dissociate from the pump, which in turn could lead to increased efflux of compounds.
Conclusions
P. aeruginosa is an important human pathogen that utilizes an array of regulatory networks and virulence factors to successfully establish itself and proliferate in the human host. Numerous efflux pumps play an important role in this process but often the assigned role is non-specific. Using different levels of expression of efflux pumps and hyperporination of the outer membrane, we manipulated the barrier factors and efflux constants for antibiotics and phenazines in P. aeruginosa. We uncovered a specific role of MexG/HI-OpmD in maintaining the non-toxic intra- and extra-cellular concentrations of pyocyanin, an important virulence factor. Our results show that the low expression of efflux pumps is essential for their specificities, so that only the substrates with high affinities are expelled from cells. Furthermore, MexG provides an additional level of control over the activity of MexHI-OpmD.
Supplementary Material
Acknowledgments
These studies are sponsored by the Department of the Defense, Defense Threat Reduction Agency and by the NIH grant AI132836. The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred. We would like to thank Drs. Olga Lomovskaya (Rempex Pharmaceuticals) and Herbert Schweizer for strains and plasmids used in this study. We would also like to thank Dr. Zbigniew Darzynkiewicz for help with fluorescence binding experiments and data analysis and Bryan Nguyen for his help with strain creation.
Footnotes
Detailed description of Materials and Methods used in the study with references; Tables S1 and S2 containing strains/plasmids and primers constructed in this study, respectively; Supporting Figures S1–S7.
References
- 1.Mulcahy LR, Isabella VM, Lewis K. Pseudomonas aeruginosa Biofilms in Disease. Microb Ecol. 2013;68(1):1–12. doi: 10.1007/s00248-013-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health, O. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. [Google Scholar]
- 3.Zgurskaya HI, Lopez CA, Gnanakaran S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches To Bypass It. ACS Infect Dis. 2015;1(11):512–522. doi: 10.1021/acsinfecdis.5b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 5.Zgurskaya HI, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol Microbiol. 2000;37(2):219–25. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]
- 6.Dreier J, Ruggerone P. Interaction of antibacterial compounds with RND e ux pumps in Pseudomonas aeruginosa. Frontiers in Microbiology. 2015;6:660. doi: 10.3389/fmicb.2015.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Schweizer HP. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Advanced Drug Delivery Reviews. 2005;57(10):1486–1513. doi: 10.1016/j.addr.2005.04.004. https://doi.org/10.1016/j.addr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175(22):7363–72. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeannot K, Elsen S, Kohler T, Attree I, van Delden C, Plesiat P. Resistance and virulence of Pseudomonas aeruginosa clinical strains overproducing the MexCD-OprJ efflux pump. Antimicrob Agents Chemother. 2008;52(7):2455–62. doi: 10.1128/AAC.01107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobel ML, Neshat S, Poole K. Mutations in PA2491 (mexS) promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J Bacteriol. 2005;187(4):1246–53. doi: 10.1128/jb.187.4.1246-1253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolter DJ, Smith-Moland E, Goering RV, Hanson ND, Lister PD. Multidrug resistance associated with mexXY expression in clinical isolates of Pseudomonas aeruginosa from a Texas hospital. Diagnostic microbiology and infectious disease. 2004;50(1):43–50. doi: 10.1016/j.diagmicrobio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Minagawa S, Inami H, Kato T, Sawada S, Yasuki T, Miyairi S, Horikawa M, Okuda J, Gotoh N. RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol. 2012;12:70. doi: 10.1186/1471-2180-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamarche MG, Deziel E. MexEF-OprN efflux pump exports the Pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline) PLoS One. 2011;6(9):e24310. doi: 10.1371/journal.pone.0024310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty LK, Pechere JC. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23(2):345–54. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 15.Kohler T, van Delden C, Curty LK, Hamzehpour MM, Pechere JC. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J Bacteriol. 2001;183(18):5213–22. doi: 10.1128/JB.183.18.5213-5222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aendekerk S, Diggle SP, Song Z, Hoiby N, Cornelis P, Williams P, Camara M, Høiby N, Cornelis P, Williams P, Cámara M. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology (Reading, U K) 2005;151(4):1113–1125. doi: 10.1099/mic.0.27631-0. [DOI] [PubMed] [Google Scholar]
- 17.Aendekerk S, Ghysels B, Cornelis P, Baysse C. Characterization of a new efflux pump, MexGHI-OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology. 2002;148(Pt 8):2371–81. doi: 10.1099/00221287-148-8-2371. [DOI] [PubMed] [Google Scholar]
- 18.Sakhtah H, Koyama L, Zhang Y, Morales DK, Fields BL, Price-Whelan A, Hogan DA, Shepard K, Dietrich LEP. The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(25):E3538–47. doi: 10.1073/pnas.1600424113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J Bacteriol. 2002;184(15):4161–7. doi: 10.1128/JB.184.15.4161-4167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol. 2007;189(21):7600–9. doi: 10.1128/JB.00850-07. JB.00850-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs EC, Yin X, Paul BJ, Astarita JL, Storz G. Conserved small protein associates with the multidrug efflux pump AcrB and differentially affects antibiotic resistance. Proc Natl Acad Sci U S A. 2012;109(41):16696–16701. S16696/1–S16696/9. doi: 10.1073/pnas.1210093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekiya H, Mima T, Morita Y, Kuroda T, Mizushima T, Tsuchiya T. Functional Cloning and Characterization of a Multidrug Efflux Pump , MexHI-OpmD , from a Pseudomonas aeruginosa Mutant Functional Cloning and Characterization of a Multidrug Efflux Pump , MexHI-OpmD , from a Pseudomonas aeruginosa Mutant. Antimicrobial Agents and Chemotherapy. 2003;47(9):2990–2992. doi: 10.1128/AAC.47.9.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgkinson JT, Gross J, Baker YR, Spring DR, Welch M. A new Pseudomonas quinolone signal (PQS) binding partner: MexG. Chem Sci. 2016;7(4):2553–2562. doi: 10.1039/C5SC04197J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamoorthy G, Leus IV, Weeks JW, Wolloscheck D, Rybenkov VV, Zgurskaya HI. Synergy between Active Efflux and Outer Membrane Diffusion Defines Rules of Antibiotic Permeation into Gram-Negative Bacteria. MBio. 2017;8(5) doi: 10.1128/mBio.01172-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westfall DA, Krishnamoorthy G, Wolloscheck D, Sarkar R, Zgurskaya HI, Rybenkov VV. Bifurcation kinetics of drug uptake by Gram-negative bacteria. PLoS One. 2017;12(9):e0184671. doi: 10.1371/journal.pone.0184671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnamoorthy G, Wolloscheck D, Weeks JW, Croft C, Rybenkov VV, Zgurskaya HI. Breaking the Permeability Barrier of Escherichia coli by Controlled Hyperporination of the Outer Membrane. Antimicrob Agents Chemother. 2016;60(12):7372–7381. doi: 10.1128/AAC.01882-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomovskaya O, Lee A, Hoshino K, Ishida H, Mistry A, Warren MS, Boyer E, Chamberland S, Lee VJ. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43(6):1340–6. doi: 10.1128/aac.43.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13(1):27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Rada B, Leto TL. Pyocyanin effects on respiratory epithelium: relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013;21(2):73–81. doi: 10.1016/j.tim.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rada B, Leto TL. Redox warfare between airway epithelial cells and Pseudomonas: dual oxidase versus pyocyanin. Immunologic research. 2009;43(1–3):198–209. doi: 10.1007/s12026-008-8071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl(-) transport in human bronchial epithelial cells. Free radical biology & medicine. 2008;45(12):1653–62. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends in Molecular Medicine. 2004;10(12):599–606. doi: 10.1016/j.molmed.2004.10.002. http://dx.doi.org/10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Baron SS, Rowe JJ. Antibiotic action of pyocyanin. Antimicrob Agents Chemother. 1981;20(6):814–20. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr JR, Taylor GW, Rutman A, Høiby N, Cole PJ, Wilson R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. Journal of Clinical Pathology. 1999;52(5):385. doi: 10.1136/jcp.52.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price-Whelan A, Dietrich LE, Newman DK. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189(17):6372–81. doi: 10.1128/jb.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita Y, Tomida J, Kawamura Y. Efflux-mediated fluoroquinolone resistance in the multidrug-resistant Pseudomonas aeruginosa clinical isolate PA7: identification of a novel MexS variant involved in upregulation of the mexEF-oprN multidrug efflux operon. Front Microbiol. 2015;6:8. doi: 10.3389/fmicb.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen AY, Yu C, Gatto B, Liu LF. DNA minor groove-binding ligands: a different class of mammalian DNA topoisomerase I inhibitors. Proc Natl Acad Sci U S A. 1993;90(17):8131–5. doi: 10.1073/pnas.90.17.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meletiadis J, Pournaras S, Roilides E, Walsh TJ. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother. 2010;54(2):602–9. doi: 10.1128/aac.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Te Dorsthorst DT, Verweij PE, Meis JF, Punt NC, Mouton JW. Comparison of fractional inhibitory concentration index with response surface modeling for characterization of in vitro interaction of antifungals against itraconazole-susceptible and -resistant Aspergillus fumigatus isolates. Antimicrob Agents Chemother. 2002;46(3):702–7. doi: 10.1128/AAC.46.3.702-707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botelho MG. Fractional inhibitory concentration index of combinations of antibacterial agents against cariogenic organisms. Journal of Dentistry. 2000;28(8):565–570. doi: 10.1016/s0300-5712(00)00039-7. http://dx.doi.org/10.1016/S0300-5712(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 41.Chaturvedi V, Ramani R, Andes D, Diekema DJ, Pfaller MA, Ghannoum MA, Knapp C, Lockhart SR, Ostrosky-Zeichner L, Walsh TJ, Marchillo K, Messer S, Welshenbaugh AR, Bastulli C, Iqbal N, Paetznick VL, Rodriguez J, Sein T. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob Agents Chemother. 2011;55(4):1543–8. doi: 10.1128/aac.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.