Abstract
Background
Initial declines in bone mineral density (BMD) following antiretroviral therapy (ART) initiation in HIV are well described, but data on long-term changes and risk factors for decline, particularly among women, are limited.
Methods
HIV-infected men and women in the Modena Metabolic Clinic underwent dual-energy X-ray absorptiometry (DXA) scans every 6-12 months for up to 10 years (median 4.6 years). Mixed effect regression model in combined and sex-stratified models determined annual rates of decline and clinical factors associated with BMD. Models included demographics, HIV-specific factors, and bone-specific factors; a final model added a sex*time interaction term.
Results
839 women and 1759 men contributed ≥2 DXA scans. The majority (82%) were ≤ 50 years old; 49% had HIV-1 RNA <50 copies/mL at baseline; 15% of women were post-menopausal and 7% of men had hypogonadism; 30% and 27%, respectively, had hepatitis C virus co-infection. The adjusted slopes in BMD among women and men were significantly different at both the femoral neck (women −0.00897 versus men −0.00422 g/cm2/year; p<0.001) and L-spine (women −0.0127 versus men −0.00763 g/cm2/year; p<0.001). Modifiable risks associated with BMD decline included ART exposure (greater decline with tenofovir disoproxil fumarate, less decline with integrase strand transfer inhibitor therapy), HCV, physical activity, and vitamin D insufficiency.
Conclusions
Among HIV-infected individuals, bone density at the femoral neck, a significant predictor of fracture risk, declined twice as quickly among women compared to men. Female sex was independently associated with both lower femoral neck and lumbar BMD over time in adjusted models.
Keywords: bone mineral density, HIV, aging, osteoporosis, hepatitis C virus, women
INTRODUCTION
Early and universal initiation of antiretroviral therapy (ART) among human immunodeficiency (HIV)-infected adults has resulted in near normalization of the life expectancy of people living with HIV. Consequently, there has been a marked reduction in AIDS-associated deaths, as well as in AIDS-related malignancies, among other AIDS-associated conditions1,2 Furthermore, the development of safer ART has markedly reduced the complications of lipoatrophy, hyperlipidemia, lactic acidosis, and renal disease that often complicated treatment in the early ART era. Despite these overall improvements, nearly all ART initiation trials demonstrated a decline in bone mineral density (BMD), most pronounced in the first 48–96 weeks after starting ART3–5. HIV-infected men and women continue to remain at increased risk for fractures during long-term follow-up, due in part to continued low BMD, low bone quality6,7, and a high rate of falls8,9.
Data on long-term changes in BMD and clinical factors that impact such changes, particularly among women, are limited. Among 97 HIV-infected participants (86% men) on ART for at least 96 weeks, we found a greater decline in lumbar spine BMD but not hip BMD compared to uninfected controls. Lean mass and concomitant BMD-lowering medications but not HIV-associated characteristics or ART were associated with BMD decline in this cohort, an average of 7.5 years following ART initiation10. A separate small study of 44 HIV-infected men with at least 3 months of ART, found a significantly greater increase in lumbar spine BMD over two years among HIV-infected men compared to HIV-uninfected controls (5.3 vs 0.3%) and, again, no differences in BMD change at the total hip (−0.6% among HIV-infected vs −1% among HIV-uninfected men). In this cohort, BMD changes did not differ between participants with or without tenofovir disoproxil fumarate (TDF) use11. A meta-analysis of studies with BMD measurements at least 48 weeks apart found that BMD tended to stabilize or even increase after the first year of ART initiation3. The European UPBEAT Study of 384 participants (46% HIV+, 61% men) contributed two or three BMD measurements over 3 years. The HIV-infected participants had significant declines in femoral neck and total hip BMD, but these changes did not differ significantly from HIV-uninfected controls. Among HIV-infected participants older than 30 years, being Caucasian, and ART initiation within 3 months or no ART were associated with greater BMD declines in multivariate models12. Thus, the limited existing data suggests stable rates of BMD change. However, these studies are small and include few women. Therefore, the goals of the present study were to compare long-term changes in BMD in a large cohort of HIV-infected men and women and to determine sex-specific risk factors for BMD decline.
METHODS
The multidisciplinary Modena HIV Metabolic Clinic (MHMC), at the University of Modena and Reggio Emilia, Italy, was initiated in 2004 to assess metabolic changes among people with HIV and has been described elsewhere13. Participants from the MHMC underwent dual-energy X-ray absorptiometry (DXA) scans approximately every 6 to 12 months, beginning in 2004. The current study is a longitudinal secondary analysis of existing data. All participants with at least 2 DXA scans were included. In participants starting a bisphosphonate, data was censored at the time of bisphosphonate initiation. All procedures followed were in accordance with the ethical standards of Comitato Etico Provinciale di Modena and with the Helsinki Declaration of 1975, as revised in 2000; all participants provided written, informed consent.
Data were collected from the MHMC electronic database. Data on age; sex; smoking (number of cigarettes/day); alcohol consumption (grams of alcohol/day); physical activity (none, mild [<4 hours weekly], intensive [≥4 hours weekly]); diabetes mellitus (based on self-reported physician’s diagnosis and/or use of anti-diabetic medications); metabolic syndrome (diagnosed according to the clinical criteria proposed by the NCEP-Adult Treatment Panel III14, use of testosterone therapy; hypogonadism (defined as serum total testosterone <300 ng/dL)15, menopausal (defined as estradiol <30 pg/mL + follicular stimulating hormone persistently>30 UI/mL); HIV diagnosis date; risk factors for HIV transmission; nadir CD4 T lymphocyte count; and antiretroviral medications were collected from subjects at enrollment using a structured questionnaire. Body weight was measured using a digital scale to the nearest 0.1 kg with subjects wearing light clothes without shoes. Height was measured using a wall-mounted stadiometer to the nearest 0.1 cm. Measurements for body weight and height were completed in triplicate and the mean recorded. Body mass index (BMI) was defined as the weight (kilograms) divided by the height (meters) squared.
Blood was drawn from all subjects for determination of hepatitis C virus (HCV) antibody (anti-HCV; Abbott HCV EIA 3.0 enzyme immunoassay; Abbott Laboratories), HIV RNA (Abbott RealTime™ HIV-1 assay; Abbott Laboratories; lower limit of detection: 50 copies/mL), CD4+ T lymphocyte count, and 25-hydroxyvitamin D (DiaSorin 25-hydroxyvitamin D chemiluminescence immunoassay; Stillwater, MN). Vitamin D insufficiency was defined as 25-OH vitamin D level < 30 ng/mL. BMD was measured by DXA at the lumbar spine (L1–L4) and femoral neck. All participants were scanned using the same single densitometer (Hologic Discovery W, Inc., Waltham, MA). The instrument was calibrated daily with a hydroxyapatite phantom. The in vivo coefficients of variation of the DXA measures at the lumbar and femoral sites were less than 2%.
Statistical Analyses
Descriptive statistics were used to characterize the sample. To account for correlation within patients in outcome measures, mixed effect regression models were created assuming compound symmetry variance-covariance structure for combined men and women to determine the sex-adjusted effect, and then in sex-stratified models to determine if factors that are associated with lower BMD differ between men and women. Regression models with random intercept and slope were adjusted for sex (for combined model), and the following time-updated variables: time on study, BMI, total duration of integrase strand transfer inhibitor therapy (INSTI), total duration of TDF, age group by five year increments compared to <35 years, self-reported physical activity level as none, moderate, or vigorous, hypogonadism or post-menopausal status, history of AIDS wasting, vitamin D insufficiency, HCV. Lastly, we tested the addition of a sex*time interaction term to derive slopes of change by sex. Additional variables were considered for inclusion, but either excluded due to extent of missing data or lack of significance in univariable models (p>0.10) and included duration of ART, duration of HIV, CD4+ T lymphocyte nadir, current CD4+ T lymphocyte count, total exposure to protease inhibitors (PI), nucleoside/nucleotide reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), risk for HIV acquisition, smoking (pack/year), metabolic syndrome, and diabetes. Stepwise (backward and forward) model selection method was used in building final models. The covariates identified in the combined model were used in the sex stratified models to examine their association to the outcomes in female and male separately. P-value<0.05 was considered statistically significant and all analyses were conducted using SAS 9.4 (Cary, NC).
RESULTS
At least 2 DXA scans were contributed by 839 women and 1759 men (median number of scans 5 [IQR 3,7], with a median of 4.68 [IQR 2.13, 7.71] years of follow-up. All participants were Caucasian, the majority were ≤ 50 years old (82%) and had HIV-1 RNA ≤ 50 copies/mL (76%) at the initial assessment; 30% of women and 27% of men had HCV co-infection. At baseline, 7% of men had hypogonadism and 15% of women were post-menopausal, with 24% categorized as post-menopausal during any point after baseline. Additional baseline characteristics by sex are shown in Table 1. Baseline mean BMD was 1.138 (SD 0.120) for total body, 0.833 (SD 0.153) at the femoral neck, and 1.055 (SD 0.168) at the lumbar spine; sex-specific measurements are shown in Table 1.
Table 1.
Characteristics of the Study Population at Time of First Bone Density Assessment (Baseline)
| Women (N=839) | Men (N=1759) | |
|---|---|---|
| Baseline Characteristics | N (%) or Median (IQR) | |
| Age >55 years | 37 (4) | 166 (9) |
| 51–55 years | 71 (9) | 197 (11) |
| 46–50 years | 183 (22) | 450 (26) |
| 41–45 years | 303 (36) | 536 (31) |
| 35–40 years | 182 (22) | 265 (15) |
| <35 years | 63 (8) | 145 (8) |
| Body mass index (median, IQR) | 21.6 (20.0, 24.1) | 23.5 (21.6, 25.5) |
| Smoking (pack years median, IQR) | 10.0, (1.1, 20.0) | 12.5 (0, 25.0) |
| Physical activity | ||
| None | 577 (69) | 1020 (58) |
| Moderate | 184 (22) | 438 (25) |
| Vigorous | 44 (5) | 224 (13) |
| Post-Menopausal | 124 (15) | – |
| Hypogonadism | – | 124 (7) |
| Metabolic Syndrome | 84 (10) | 144 (8) |
| Hepatitis C Virus | 250 (30) | 468 (27) |
| Vitamin D Insufficiency | 414 (49) | 831 (47) |
| History of AIDS Wasting | 113 (13) | 81 (5) |
| CD4 Nadir <200 cells/μL | 448 (53) | 856 (49) |
| HIV-1 VL ≤50 | 646 (77) | 1319 (75) |
| ART duration (years) | 9.6 (5.6, 13.1) | 8.3 (3.4, 12.0) |
| TDF use | 538 (64) | 1144 (65) |
| INSTI use | 70 (8) | 129 (7) |
| Total BMD | 1.009 (0.116) | 1.162 (0.115) |
| Femoral Neck BMD | 0.816 (0.154) | 0.842 (0.152) |
| Lumbar Spine BMD | 1.057 (0.170) | 1.054 (0.167) |
BMD, bone mineral density; Vitamin D insufficiency <30 ng/mL; TDF, tenofovir disoproxil fumarate; INSTI, integrase strand transfer inhibitor
In combined mixed effect models, femoral but not lumbar spine BMD was significantly lower among women than men (Table 2). Lower femoral neck BMD was also associated with longer TDF exposure, older age (ages 46–50, 51–55, or >55 vs <35 years), no self-reported physical activity, hypogonadism or post-menopausal status, vitamin D insufficiency, and HCV. Longer duration of INSTI, greater BMI, and a higher viral load were associated with higher BMD. Associations with lower lumbar spine BMD were similar, however, only ages 51–55 (vs < 35 years) were statistically significant, and both no and moderate (vs intense) physical activity were associated with low BMD (Table 2).
Table 2.
Effect of Clinical Characteristics on Femoral Neck or Lumbar Spine Bone Mineral Density (g/cm2)
| Baseline Characteristics | Femoral Neck | Lumbar Spine | ||||
|---|---|---|---|---|---|---|
| Estimate | SD | P value | Estimate | SD | P value | |
| Women (vs Men) | −0.0353 | 0.0052 | <0.001 | NS | NS | NS |
| Body mass index | 0.00487 | 4.7×10−4 | <0.001 | 0.00257 | 4.8×10−4 | <0.001 |
| INSTI exposure (years) | 0.00003 | 5.4×10−6 | <0.001 | 0.000027 | 5.3×10−6 | <0.001 |
| TDF exposure (years) | −0.00284 | 0.00047 | <0.001 | −0.00295 | 0.00048 | <0.001 |
| Age > 55 years (vs <35) | −0.0522 | 0.012 | <0.001 | −0.0187 | 0.015 | 0.21 |
| 51–55 years (vs <35) | −0.0481 | 0.012 | <0.001 | −0.0354 | 0.014 | 0.012 |
| 46–50 year (vs <35) | −0.0330 | 0.010 | 0.001 | −0.0175 | 0.012 | 0.15 |
| 41–45 year (vs <35) | −0.0078 | 0.0098 | 0.42 | 0.00664 | 0.012 | 0.57 |
| 35–40 year (vs <35) | −0.00336 | 0.0103 | 0.75 | 0.0165 | 0.013 | 0.19 |
| No PA (vs intense) | −0.00632 | 0.0029 | 0.031 | −0.00893 | 0.0029 | 0.002 |
| Moderate PA (vs intense) | −0.00071 | 0.0028 | 0.80 | −0.00556 | 0.0028 | 0.046 |
| Hypogonadism (M) or Post-Menopausal (F) | −0.0322 | 0.0036 | <0.001 | −0.0460 | 0.0036 | <0.001 |
| Hx of AIDS Wasting | −0.0285 | 0.0047 | <0.001 | −0.0189 | 0.0048 | <0.001 |
| HIV-1 VL ≥ 50 copies | 0.0579 | 0.0020 | <0.001 | 0.0581 | 0.0019 | <0.001 |
| Vitamin D Insufficiency | −0.0152 | 0.0025 | <0.001 | −0.0119 | 0.0025 | <0.001 |
| Hepatitis C | −0.0130 | 0.0052 | 0.012 | −0.0174 | 0.0063 | 0.006 |
SD, standard deviation; INSTI, integrase strand transfer inhibitor; TDF, tenofovir disoproxil fumarate; PA, physical activity; VL, viral load
When we introduced a sex*time interaction term to the model, the adjusted slopes in BMD among women and men were significantly different at both the femoral neck (women −0.00897 [SE 8.85×10−4] versus men −0.00422 [SE 8.88×10−4] g/cm2/year; p<0.001) and L-spine (women −0.0127 [SE 7.60×10−4] versus men −0.00763 (SE 9.01×10−4) g/cm2/year; p<0.001); Figure 1.
Figure 1.
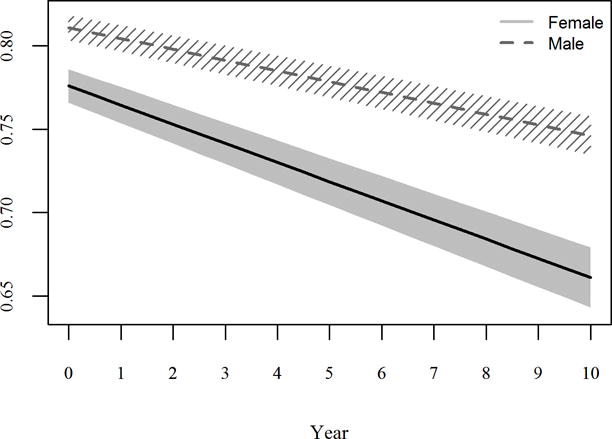
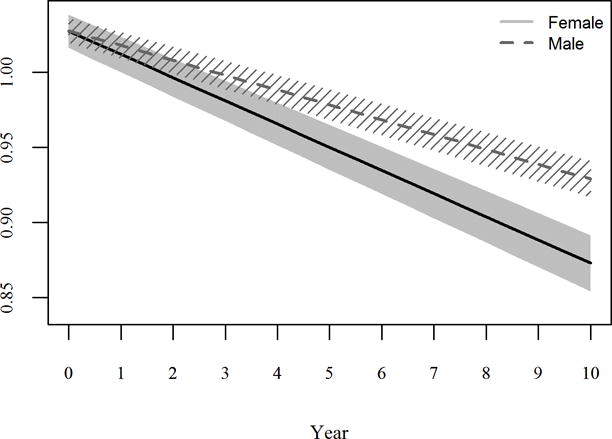
Estimated change in femoral neck BMD (A) and lumbar spine BMD (B) among females (solid line) and males (dashed line) from linear mixed models.
Sex-stratified models (Tables 3 and 4) yielded similar results with a few exceptions: among women, metabolic syndrome was associated with lower lumbar spine BMD (estimate −0.0193, SE 0.0061, p=0.0025); physical activity was not associated with lumbar spine or femoral neck BMD. Among men, neither lumbar spine nor femoral neck BMD was associated with HCV.
Table 3.
Effect of Clinical Characteristics on Femoral Neck Bone Mineral Density (g/cm2), in Sex-Stratified Models
| Baseline Characteristics | Women: Femoral Neck | Men: Femoral Neck | ||||
|---|---|---|---|---|---|---|
| Estimate | SD | P value | Estimate | SD | P value | |
| Body mass index | 0.0054 | 7.8×10−4 | <0.001 | 0.0055 | 5.5×10−4 | <0.001 |
| INSTI exposure (years) | 0.00003 | 1.1×10−6 | 0.012 | 0.00003 | 6.0×10−6 | <0.001 |
| TDF exposure (years) | −0.0032 | 0.0084 | <0.001 | −0.0026 | 0.00054 | <0.001 |
| Age > 55 years (vs <35) | −0.116 | 0.026 | <0.001 | −0.045 | 0.015 | <0.001 |
| 51–55 years (vs <35) | −0.088 | 0.022 | <0.001 | −0.037 | 0.013 | 0.004 |
| 46–50 year (vs <35) | −0.049 | 0.018 | 0.006 | −0.028 | 0.011 | 0.009 |
| 41–45 year (vs <35) | −0.011 | 0.017 | 0.53 | 0.012 | 0.011 | 0.30 |
| 35–40 year (vs <35) | −0.0049 | 0.018 | 0.78 | 0.0072 | 0.012 | 0.54 |
| No PA (vs intense) | −0.0089 | 0.003 | 0.0027 | |||
| Moderate PA (vs intense) | −0.0023 | 0.003 | 0.45 | |||
| Hypogonadism (M) or Post-Menopausal (F) | −0.0288 | 0.0050 | <0.001 | −0.024 | 0.005 | <0.001 |
| Hx of AIDS Wasting | −0.0242 | 0.0063 | <0.001 | −0.023 | 0.007 | 0.001 |
| HIV-1 VL ≥ 50 copies | 0.065 | 0.0034 | <0.001 | 0.055 | 0.002 | <0.001 |
| Vitamin D Insufficiency | −0.011 | 0.0044 | 0.014 | −0.0119 | 0.002 | <0.001 |
| Hepatitis C | −0.029 | 0.0087 | <0.001 | |||
SD, standard deviation; INSTI, integrase strand transfer inhibitor; TDF, tenofovir disoproxil fumarate; PA, physical activity; VL, viral load
Table 4.
Effect of Clinical Characteristics on Lumbar Spine Bone Mineral Density (g/cm2), in Sex-Stratified Models
| Baseline Characteristics | Women: Lumbar Spine | Men: Lumbar Spine | ||||
|---|---|---|---|---|---|---|
| Estimate | SD | P value | Estimate | SD | P value | |
| Body mass index | 0.0048 | 0.0008 | <0.001 | 0.0019 | 5.6×10−4 | <0.001 |
| INSTI exposure (years) | 0.00004 | 1.0×10−5 | <0.001 | 0.000018 | 5.8×10−6 | 0.002 |
| TDF exposure (years) | −0.0031 | 0.0009 | <0.001 | −0.0031 | 0.0005 | <0.001 |
| Age > 55 years (vs <35) | −0.139 | 0.031 | <0.001 | |||
| 51–55 years (vs <35) | −0.090 | 0.026 | <0.001 | |||
| 46–50 year (vs <35) | −0.051 | 0.021 | 0.016 | |||
| 41–45 year (vs <35) | −0.0009 | 0.020 | 0.97 | |||
| 35–40 year (vs <35) | 0.013 | 0.021 | 0.54 | |||
| Metabolic Syndrome | −0.019 | 0.006 | 0.0025 | |||
| No PA (vs intense) | −0.011 | 0.003 | <0.0001 | |||
| Moderate PA (vs intense) | −0.0068 | 0.003 | 0.026 | |||
| Hypogonadism (M) or Post-Menopausal (F) | −0.033 | 0.005 | <0.001 | −0.044 | 0.005 | <0.001 |
| Hx of AIDS Wasting | −0.028 | 0.007 | <0.001 | |||
| HIV-1 VL ≥ 50 copies | 0.063 | 0.003 | <0.001 | 0.055 | 0.002 | <0.001 |
| Vitamin D Insufficiency | −0.020 | 0.005 | <0.001 | −0.014 | 0.003 | <0.001 |
| Hepatitis C | −0.032 | 0.010 | 0.0022 | |||
SD, standard deviation; INSTI, integrase strand transfer inhibitor; TDF, tenofovir disoproxil fumarate; PA, physical activity; VL, viral load
DISCUSSION
In a large cohort of HIV-infected men and women on long-term ART, BMD at both the femoral neck and lumbar spine, a significant predictor of fracture risk, declined twice as quickly among HIV-infected women compared to men, even after adjusting for other covariates. Notably, the majority of our study population were less than 50 years old, and only 15% of female participants were menopausal at baseline and 24% during follow-up. Thus, with aging and menopause, the rate of BMD decline among HIV-infected women is expected to be even more pronounced, as suggested by our sex*time differences.
Few studies report long-term changes in BMD among HIV-infected persons, regardless of sex. Compared to both HIV-infected and -uninfected populations, our participants tended to have lower baseline BMD, but a similar rate of annual decline. The UPBEAT study of 384 HIV-infected and 474 HIV-uninfected (176 and 210 with multiple DXA scans; median age 39 years, 39% women) reported a median baseline BMD of 1.024 and 1.055 g/cm2 at the femoral neck and 1.164 and 1.238 g/cm2 at the lumbar spine16. Annual decreases of −0.0063 g/cm2 at the femoral neck and 0.0024 g/cm2 at the lumbar spine for HIV-infected participants were not significantly different from HIV-uninfected controls. Sex-specific rates of decline were not reported, although adjustment for sex minimally affected rates of decline16. In the ACTG study A5318, 83 men and 14 women had a DXA scan at ART initiation, 96 weeks and approximately 7.5 years following ART initiation17. From baseline to week 96, hip BMD decreased by 1.56%/year and lumbar spine by 0.76%/year, slowing to −0.31%/year and −0.25%/year, respectively, after week 96. With a small proportion of women, this study lacked statistical power to compare sex-specific rate of BMD change in a meaningful way. Among participants in the Women’s Interagency HIV Study (WIHS), BMD decline was similar among pre-menopausal women regardless of HIV status, and accelerated among HIV-infected vs -uninfected post-menopausal women18. Importantly, a markedly increased fracture risk was also described among post-versus pre-menopausal HIV-infected women19–21. Rates of decline among post-menopausal women were similar but slightly smaller than changes observed in females in our cohort (LS: −0.010 and FN −0.007)19.
Our large sample size allowed us to explore the impact of risk factors on BMD changes, and contrast these effects by sex. Several findings with the potential to impact clinical care should be emphasized: first, age-associated changes in BMD were most pronounced following age 45, even after adjusting for post-menopausal or hypogonadal state, supporting the current HIV guidelines to screen for BMD starting at age 5022. Next, a protective effect of INSTI therapy (nearly entirely raltegravir use in this cohort) was apparent among both men and women, and has been demonstrated in multiple prior studies with raltegravir initiation or switch4,23,24 and in limited data with other INSTIs25–27. Third, the effect of HCV on BMD was similar to that of 5 additional years of aging, and was most significant among the women, as has been reported in prior studies28,29. HCV is increasingly recognized as an independent risk factor for low BMD, with rates of osteoporosis among HCV-monoinfected individuals ranging 14–28%, and greater losses in BMD seen with increasing liver disease severity30,31. A recent meta-analysis32 found a 1.63 greater odds of osteoporosis with HCV/HIV co-infected vs HIV infection alone, and a 1.77 greater odds for fracture with HCV/HIV co-infection vs HIV infection alone. The impact of direct-acting HCV therapies on BMD after eradication of HCV are currently not known, but may prove to be an effective (albeit costly) treatment intervention to preserve BMD with aging, particularly among HIV/HCV co-infected women30.
The protective effects of increased BMI and detrimental effect of AIDS wasting were expected33,34, and, in our cohort, were independent of a protective effect of moderate or vigorous physical activity. Several prior studies among HIV-infected adults have demonstrated that lean body mass is the BMI component with the most protective effect on BMD17,35. Notably, although physical activity is a well-established intervention to attenuate bone loss with aging, regular physical activity (with the exceptions of high-intensity loading or resistance exercise) seldom reverses BMD loss, especially among post-menopausal women or hypogonadal men.36 We do suspect that the association with metabolic syndrome but not physical activity among the women and the opposite among men may reflect co-linearity with these variables, such that metabolic syndrome effects were attenuated by exercise among the men. Our finding of greater BMD with higher HIV-1 RNA likely reflects greater BMD seen prior to ART initiation. Lastly, the loss of an age-association among men at the lumbar spine and loss against some age categories at the femoral spine is notable, and emphasizes that much of the age-associated declines in this population is explained by changes that occur in the women, likely driven, in part, by the menopausal transition period.
Several important strengths and limitations of the analysis should be recognized. Foremost, the large number of participants, with nearly 50% women, in addition to the extent of DXA follow-up, surpasses any prior published data on BMD trajectories among HIV-infected adults. The Modena Cohort is, however, a large clinical database, and is subject to missing data and variability in timing and frequency of DXA ascertainment. The cohort reflects the racial/ethnic background of Italy, and may not be generalizable to more diverse populations. Surprisingly low rates of alcohol use may suggest underreporting of some substances, which may have limited our ability to detect additional important associations with BMD. Lastly, without an HIV-uninfected comparison group, we cannot determine whether the slopes that we found are accelerated or consistent with normal aging.
In the largest and longest study of BMD changes among HIV-infected men and women to date, we have found nearly double the rate of BMD decline among HIV-infected women compared to HIV-infected men, with rates of BMD decline among HIV-infected men mirroring findings of smaller, previously published cohorts16,17. Our results highlight BMD losses among women, independent of menopause, effects that require future consideration with ART selection, particularly when prioritizing switch from TDF to tenofovir alafenamide. Several modifiable risk factors provide potential targets for intervention to slow decline including treatment of HCV, treatment of vitamin D deficiency, consideration for hormone replacement if the benefits outweigh the risks, augmenting a low BMI, and moderate to vigorous activity, likely including resistance training. Importantly, low BMD is one of several risk factors for fracture. Therefore, the interventions likely to have the greatest impact in this aging population are those that both attenuate BMD losses and minimize fracture risk through reduced falls37.
Acknowledgments
Sources of Funding: This work was supported by the National Institute of Aging of the National Institutes of Health (K23AG050260; R01AG054366 to KME) and the National Institute of Allergy and Infectious Diseases (K24 AI120834 to TTB and K23 AI110532 to JEL).
Footnotes
Conflicts of Interest TB has served as a consultant to Gilead Sciences, Merck, BMS, Theratechnologies, and EMD-Serono. JEL has served as a consultant to Merck and receives research funding from Gilead Sciences. GG has served as a consultant to Gilead Sciences, Merck, and ViiV. KME has received research funding (paid to the University of Colorado) from Gilead Sciences, and has served as a consultant to Gilead Sciences and Theratechnologies.
Meetings: The data from this manuscript were presented in part at the 9th Conference of the International AIDS Society; Paris, France, July 2017
References
- 1.Lima VD, Lourenco L, Yip B, Hogg RS, Phillips P, Montaner JS. AIDS incidence and AIDS-related mortality in British Columbia, Canada, between 1981 and 2013: a retrospective study. The lancet HIV. 2015;2(3):e92–97. doi: 10.1016/S2352-3018(15)00017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 3.Bolland MJ, Wang TK, Grey A, Gamble GD, Reid IR. Stable bone density in HAART-treated individuals with HIV: a meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2721–2731. doi: 10.1210/jc.2011-0591. [DOI] [PubMed] [Google Scholar]
- 4.Brown TT, Moser C, Currier JS, et al. Changes in Bone Mineral Density After Initiation of Antiretroviral Treatment With Tenofovir Disoproxil Fumarate/Emtricitabine Plus Atazanavir/Ritonavir, Darunavir/Ritonavir, or Raltegravir. J Infect Dis. 2015;212(8):1241–1249. doi: 10.1093/infdis/jiv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arribas JR, Thompson M, Sax PE, et al. Brief Report: Randomized, Double-Blind Comparison of Tenofovir Alafenamide (TAF) vs Tenofovir Disoproxil Fumarate (TDF), Each Coformulated With Elvitegravir, Cobicistat, and Emtricitabine (E/C/F) for Initial HIV-1 Treatment: Week 144 Results. Journal of acquired immune deficiency syndromes. 2017;75(2):211–218. doi: 10.1097/QAI.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 6.Guerri-Fernandez R, Molina-Morant D, Villar-Garcia J, et al. Bone density, microarchitecture and tissue quality following long-term treatment with tenofovir/emtricitabine or abacavir/lamivudine. Journal of acquired immune deficiency syndromes. 2017 doi: 10.1097/QAI.0000000000001396. [DOI] [PubMed] [Google Scholar]
- 7.Sellier P, Ostertag A, Collet C, et al. Disrupted trabecular bone micro-architecture in middle-aged male HIV-infected treated patients. HIV Med. 2016;17(7):550–556. doi: 10.1111/hiv.12380. [DOI] [PubMed] [Google Scholar]
- 8.Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. Journal of acquired immune deficiency syndromes. 2012;61(4):484–489. doi: 10.1097/QAI.0b013e3182716e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlandson KM, Plankey MW, Springer G, et al. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Med. 2016;17(10):740–748. doi: 10.1111/hiv.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant PM, Kitch D, McComsey GA, et al. Long-term Bone Mineral Density Changes in Antiretroviral-Treated HIV-Infected Individuals. J Infect Dis. 2016;214(4):607–611. doi: 10.1093/infdis/jiw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolland MJ, Grey A, Horne AM, et al. Stable bone mineral density over 6 years in HIV-infected men treated with highly active antiretroviral therapy (HAART) Clinical endocrinology. 2012;76(5):643–648. doi: 10.1111/j.1365-2265.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 12.Tinago W, Cotter AG, Sabin CA, et al. Predictors of longitudinal change in bone mineral density in a cohort of HIV-positive and negative patients. AIDS. 2017;31(5):643–652. doi: 10.1097/QAD.0000000000001372. [DOI] [PubMed] [Google Scholar]
- 13.Guaraldi G, Orlando G, Squillace N, et al. Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials. 2006;7(3):97–106. doi: 10.1310/EYWJ-8B5K-X7VQ-9CPE. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Rochira V, Guaraldi G. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am. 2014;43(3):709–730. doi: 10.1016/j.ecl.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Tinago W, Cotter AG, Sabin CA, et al. Predictors of longitudinal change in bone mineral density in a cohort of HIV-positive and negative patients. Aids. 2017;31(5):643–652. doi: 10.1097/QAD.0000000000001372. [DOI] [PubMed] [Google Scholar]
- 17.Grant PM, Kitch D, McComsey GA, et al. Long-term Bone Mineral Density Changes in Antiretroviral-Treated HIV-Infected Individuals. J Infect Dis. 2016 doi: 10.1093/infdis/jiw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes YI, Yin MT, Reame NK. Bone Density and Fractures in HIV-infected Postmenopausal Women: A Systematic Review. The Journal of the Association of Nurses in AIDS Care: JANAC. 2015;26(4):387–398. doi: 10.1016/j.jana.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A, Cohen HW, Freeman R, Santoro N, Schoenbaum EE. Prospective evaluation of bone mineral density among middle-aged HIV-infected and uninfected women: Association between methadone use and bone loss. Maturitas. 2011;70(3):295–301. doi: 10.1016/j.maturitas.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A, Shi Q, Hoover DR, et al. Increased Fracture Incidence in Middle-Aged HIV-Infected and HIV-Uninfected Women: Updated Results From the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2015;70(1):54–61. doi: 10.1097/QAI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49(3):298–308. doi: 10.1097/QAI.0b013e3181893e8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardino JI, Mocroft A, Mallon PW, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. The lancet HIV. 2015;2(11):e464–473. doi: 10.1016/S2352-3018(15)00181-2. [DOI] [PubMed] [Google Scholar]
- 24.Curran A, Martinez E, Saumoy M, et al. Body composition changes after switching from protease inhibitors to raltegravir: SPIRAL-LIP substudy. AIDS. 2012;26(4):475–481. doi: 10.1097/QAD.0b013e32834f3507. [DOI] [PubMed] [Google Scholar]
- 25.Negredo E, Estrada V, Domingo P, et al. Switching from a ritonavir-boosted PI to dolutegravir as an alternative strategy in virologically suppressed HIV-infected individuals. J Antimicrob Chemother. 2017;72(3):844–849. doi: 10.1093/jac/dkw504. [DOI] [PubMed] [Google Scholar]
- 26.Tebas P, Kumar P, Hicks C, et al. Greater change in bone turnover markers for efavirenz/emtricitabine/tenofovir disoproxil fumarate versus dolutegravir + abacavir/lamivudine in antiretroviral therapy-naive adults over 144 weeks. AIDS. 2015;29(18):2459–2464. doi: 10.1097/QAD.0000000000000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, Phase IIIb study. Antivir Ther. 2017 doi: 10.3851/IMP3166. [DOI] [PubMed] [Google Scholar]
- 28.Lawson-Ayayi S, Cazanave C, Kpozehouen A, et al. Chronic viral hepatitis is associated with low bone mineral density in HIV-infected patients, ANRS CO 3 Aquitaine Cohort. J Acquir Immune Defic Syndr. 2013;62(4):430–435. doi: 10.1097/QAI.0b013e3182845d88. [DOI] [PubMed] [Google Scholar]
- 29.Lo Re V, 3rd, Guaraldi G, Leonard MB, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. Aids. 2009;23(16):2191–2198. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin MT, Brown TT. HIV and Bone Complications: Understudied Populations and New Management Strategies. Current HIV/AIDS reports. 2016;13(6):349–358. doi: 10.1007/s11904-016-0341-9. [DOI] [PubMed] [Google Scholar]
- 31.Bedimo R, Maalouf NM, Lo Re V., 3rd Hepatitis C virus coinfection as a risk factor for osteoporosis and fracture. Current opinion in HIV and AIDS. 2016;11(3):285–293. doi: 10.1097/COH.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong HV, Cortes YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: a systematic review and meta-analysis. Aids. 2014;28(14):2119–2131. doi: 10.1097/QAD.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairfield WP, Finkelstein JS, Klibanski A, Grinspoon SK. Osteopenia in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 2001;86(5):2020–2026. doi: 10.1210/jcem.86.5.7515. [DOI] [PubMed] [Google Scholar]
- 34.Huang JS, Wilkie SJ, Sullivan MP, Grinspoon S. Reduced bone density in androgen-deficient women with acquired immune deficiency syndrome wasting. J Clin Endocrinol Metab. 2001;86(8):3533–3539. doi: 10.1210/jcem.86.8.7728. [DOI] [PubMed] [Google Scholar]
- 35.Erlandson KM, Kitch D, Tierney C, et al. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. Aids. 2013;27(13):2069–2079. doi: 10.1097/QAD.0b013e328361d25d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR, American College of Sports M American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 37.Erlandson KM, Guaraldi G, Falutz J. More than osteoporosis: age-specific issues in bone health. Current opinion in HIV and AIDS. 2016;11(3):343–350. doi: 10.1097/COH.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]


