Abstract
Background
Concentrations of tenofovir (TFV) in hair and TFV-diphosphate (TFV-DP) in dried blood spots (DBS) as measures of cumulative exposure have been primarily studied in younger, HIV-uninfected individuals taking pre-exposure HIV prophylaxis. Data on these measures among older HIV-infected individuals is limited.
Methods
We evaluated longitudinal TFV and TFV-DP concentrations in hair and DBS, respectively, from HIV-infected adults. Multivariable model variables included age group (18–35 and ≥ 60 years), creatinine clearance (CrCl), hematocrit (TFV-DP), and gray hair color (TFV).
Results
Baseline hair TFV and DBS TFV-DP were moderately correlated [r= 0.5 (0.2, 0.7); p=0.001] across both age groups [younger (N=23) and older (N=22)]. In adjusted models, CrCl was associated with increases of 15.9% (7.4%, 25.0%); p=0.0006 and 5.7% (−0.2%, 11.9%); p=0.057 for TFV in hair and TFV-DP in DBS, respectively, for every 20 ml/min CrCl decrease. Although older age (versus younger age) was univariately associated with increased TFV hair levels, older age was not significantly associated with higher concentrations in hair [−1.4% (−26.7%, 32.7%); p=0.93] or DBS [4.0% (−14.1%, 25.9%); p=0.68] after adjustment. Similarly, gray color was not significantly associated with higher TFV levels in hair [27.6% (−11.1%, 83.0%; p=0.18)] in adjusted models. In both adjusted and unadjusted models of TFV-DP levels in DBS, a 1% hematocrit increase was associated with a 3.3% (0.2%, 6.5%) TFV-DP increase (p=0.04).
Conclusions
Cumulative drug exposure measures (hair and DBS) were comparable in younger and older HIV-infected individuals on TFV-based therapy after adjustment for renal function.
Introduction
Tenofovir (TFV) concentrations in hair and TFV-diphosphate (TFV-DP) in dried blood spots (DBS) are used to measure cumulative exposure and adherence in studies that generally include younger, HIV-uninfected individuals taking TFV disoproxil fumarate (TDF)/emtricitabine (FTC) for pre-exposure prophylaxis (PrEP) [1–3]. In this patient population, TFV concentrations in hair and TFV-DP in DBS are linearly related to cumulative TDF dosing [4, 5] and these measures correlate with one another, indicating similar pharmacokinetic profiles [6–8]. To date, few studies have characterized TFV in hair or TFV-DP in DBS in an HIV-infected population, especially among older adults, who represent a growing segment of patients with HIV. Older age and corresponding physiological changes, such as graying hair, lower hematocrit (Hct), and reduced renal function, may alter concentrations of TFV in hair and TFV-DP in DBS [9]. The study objective was to characterize and compare concentrations of TFV in hair and TFV-DP in DBS in virally suppressed, HIV-infected adults from younger (18–35) and older (≥60 years) age groups. The correlation between these measures, and the clinical covariates associated with each exposure measure were also determined.
Methods
This was a secondary, hypothesis-generating analysis performed in a study examining renal function over one year in virally suppressed (<48 copies/mL on two consecutive visits), HIV-infected adults aged either 18–35 years or ≥60 years with ≥1 year of consistent use of TDF-based antiretroviral therapy. All participants provided written informed consent before entry. The study was approved by the local IRB and registered with ClinicalTrials.gov (NCT02304263). Participants completed 2 study visits, separated by 1 year. Blood and hair samples were collected at each study visit. Creatinine clearance (CrCl) was calculated at each visit using the Cockcroft-Gault equation[10]. For the DBS samples, 25 μl of whole blood was spotted onto 903 Protein Saver cards (Whatman/GE Healthcare, Piscataway, NJ), allowed to dry for ≥2 hours (up to overnight), placed in plastic bags with humidity indicators and desiccants, and stored in a sample box at −80°C until analysis [11]. Approximately 100 strands of hair (≥1 inch) were cut from the occipital region of the scalp [12]; hair color was noted by the laboratory upon analysis. Validated liquid chromatography/tandem mass spectrometry (LC/MS-MS) methods were used to quantify TFV concentrations in plasma [6, 13], the proximal 1.5 centimeters of hair, and TFV-DP from a 3-mm DBS punch[4, 14].
Participants were included in the study if they were virologically-suppressed through electronic medical chart review indicating viral suppression (<48 copies/mL) on two consecutive clinic visits preceding Visit 1. Eligibility for Visit 2 required viral suppression at one clinic appointment preceding the second visit. In addition, a Visual Analog Scale (VAS) for self-reported adherence over the past 3 months, 30 days, and 3 days was completed by the participants at both visits.
Statistical Analysis
Predictors in the multivariable longitudinal regression models included age group, CrCl, completely gray hair color (yes/no) (hair model) and hematocrit (DBS model). Study visit was not considered as subjects were on stable treatment. TFV and TFV-DP concentrations were natural log-transformed prior to analysis. To reduce skewness, a constant [the lower limit of quantification for the assay (0.002 ng/ml)] was added to TFV concentrations in hair before log transformation, and analysis was performed on the outcome, [ln(0.002 + TFV in hair)], consistent with previous studies involving TFV in hair [15]. This approach is necessary as log transformations tend to exaggerate differences between very small values, which are clinically comparable [16, 17]. Results represent a percent change in TFV levels by covariate after adjusting for all other variables in the model. A repeated measures multivariable regression model using data from both study visits (when available) was used to assess relationships between drug concentrations and participant characteristics. A Pearson correlation between TFV-DP in DBS and TFV in hair was calculated at Visit 1 only, given some loss to follow up and to meet independence assumptions. All analyses were complete in R/SAS, versions 3.3.3/9.4, respectively. As this was a secondary, hypothesis-generating analysis, no adjustment was made for multiple comparisons [18, 19].
Results
Study participants
Visits 1 and 2 included 45 and 34 participants, respectively; of those not included in Visit 2: 9 were from the 18–35 year-old group [7 were no longer eligible (5 switched to non-TDF based ART, 1 moved out of state, 1 stopped taking ART), 1 lost to follow-up, 1 death] and 2 were from the ≥60 year-old group (1 lost eligibility due to medical complications, 1 lost to follow-up). Table 1 shows the baseline demographics of all study participants. The number of participants taking a pharmacokinetic “booster” (ritonavir or cobicistat) as part of their antiretroviral regimen did not differ between the cohorts. As expected, mean (SD) CrCl was significantly lower among the older participants. Baseline TFV concentrations [geometric mean (95% CI)] in plasma did not differ significantly between the younger [103 ng/ml (73, 144)] and older [92 ng/ml (40, 210)] cohorts (p=0.65). One older participant had a plasma concentration below the level of quantification (BLQ) for which one half of the lower limit of quantification (LLOQ was 10 ng/ml [13]) was imputed. Overall, mean (SD) self-reported adherence using VAS over the previous 3 months, 30 days, and 3 days was 97% (5), 98% (4), and 99% (6) for Visit 1 and 97% (4), 95% (16), and 97% (16) for Visit 2, respectively, with no significant differences between the younger and older groups (all p > 0.290). These VAS and plasma quantitation values were consistent and indicative of high adherence.
Table 1.
| N(%) or Mean(SD) | Younger (N=23) | Older (N=22) | P-value |
|---|---|---|---|
|
| |||
| Age (yrs) | 31 (3) | 64 (4) | … |
|
| |||
| Sex | |||
| Female | 2 (9%) | 2 (9%) | 1.0 |
| Male | 21 (91%) | 20 (91%) | |
|
| |||
| Race | |||
| African-American | 2 (9%) | 4 (18%) | 0.4 |
| Non African-American | 21 (91%) | 18 (82%) | |
|
| |||
| Current smoker | 12 (52%) | 6 (27%) | 0.1 |
|
| |||
| Current HCV infection | 0 (0%) | 2 (9%) | 0.2 |
|
| |||
| TDF duration (yrs) | |||
| 1 year | 2 (9%) | v | 0.5 |
| >1 year | 21 (91%) | 22 (100%) | |
|
| |||
| Booster (RTV or COBI) | 10 (43%) | 10 (45%) | >0.99 |
| Creatinine Clearance (ml/min) Cockroft Gault |
119 (36) | 96 (32) | 0.03 |
| Hematocrit (%) | 44 (2.9) | 44 (3.1) | 0.7 |
Factors associated with TFV concentrations in hair
Thirty-nine participants had sufficient hair (≥ 1 inch) to provide a sample. Nineteen were ≥60 years old, 6 had completely gray hair (all from the older cohort), 35 were male, 6 were black, and 10 were Hispanic. In univariate analysis, hair concentrations for older participants were 34.0% (95% CI: 1.5%, 76.9%; p=0.039) higher than those among younger participants. Similarly, in univariate analyses, gray hair was associated with a 46.1% (1.6%, 110.2%) increase in TFV concentration (p=0.041) and each 20 ml/min decrease in CrCl was associated with a 16.9% (9.1%, 25.3%) increase in hair TFV concentration (p=0.0001). In the multivariable model, older age and gray hair were no longer significantly associated with higher TFV hair concentrations [−1.4% (−26.7%, 32.7%); p=0.93] and [27.6% (−11.1%, 83.0%); p=0.18], respectively. In multivariate analysis, the association between renal function and TFV hair levels remained significant, with an increase of 15.9% (95% CI: 7.4%, 25.0%) in TFV for every 20 ml/min decrease in CrCl (p=0.0006).
Factors associated with TFV-DP concentrations in DBS
Forty-five participants provided at least one blood sample used in the DBS analysis. One DBS sample from Visit 1 was excluded due to elevated TFV-DP concentrations resulting from a drug interaction with ledipasvir/sofosbuvir for which interpretation benchmarks are available[20]. Unadjusted TFV-DP concentrations in DBS at baseline were not significantly higher in older versus younger participants [9.0% (−9.5%, 31.4%; p=0.36)]. As with hair, CrCl was significantly associated with TFV-DP in DBS in univariate analysis, with a 6.2% (0.6%, 12.1%) increase for every 20 ml/min decrease in CrCl (p=0.031). Hct was also a significant univariate predictor with a 3.3% (0.2%, 6.5%) TFV-DP increase (p=0.04) for every 1% increase in Hct. After adjustment for age and Hct in the multivariable model, the effect of CrCl on TFV-DP levels was attenuated, but still showed a trend towards the association of higher TFV-DP concentrations with decreased renal function: TFV-DP concentrations were 5.7% (−0.2%, 11.9) higher for every 20 ml/min decrease in CrCl (p=0.057). Older age was not predictive of higher concentrations in DBS [4.0% (−14.1%, 25.9%); p=0.68] after adjustment for CrCl and Hct. The Hct estimate (95% CI) did not change from the unadjusted model.
TFV in hair and TFV-DP in DBS
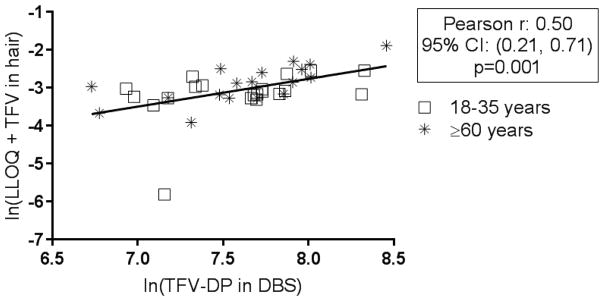
Thirty-nine participants provided both DBS and hair samples at Visit 1. As shown in Figure 1, concentrations of TFV-DP in DBS and TFV in hair were significantly, but moderately, correlated [Pearson correlation: 0.50, (95% CI: 0.21, 0.71); p=0.001].
Figure 1.
Hair and DBS Correlation at Visit 1
Correlation between TFV in hair and TFV-DP in DBS at Visit 1 (n=39 paired samples). Concentrations were natural log (ln) transformed prior to analysis. A constant was added to hair values to reduce skewness; the lower limit of quantification (LLOQ= 0.002 ng/ml) for the assay was chosen for the constant.
Discussion
This study evaluated TFV in hair and TFV-DP in DBS in a unique population of virologically-suppressed older (≥ 60 years) and younger (18–35 years) HIV-infected individuals. Using adjusted multivariable models, we found an inverse relationship between creatinine clearance and drug concentrations (TFV and TFV-DP) in hair and DBS, respectively. Furthermore, we found significant increases in TFV-DP in DBS with increasing Hct (p=0.04) in adjusted analyses. We did not find a relationship between age (or hair color) and drug concentrations in either matrix after adjusting for renal function. Given a small number of women (N=4), sex was not considered as a predictor and weight was collinear with other predictors. Lastly, we found a moderate correlation between TFV in hair and TFV-DP in DBS, suggesting similarities in the pharmacokinetics of these measures among younger and older individuals. Taken together, this data suggests that either hair or DBS measures could be used to measure exposure to TFV-based regimens in older individuals and that the association of renal function on these exposure measures requires study in a larger cohort.
The association between Hct among older individuals and TFV-DP concentrations in DBS was not unexpected. A similar association was observed in validation studies among HIV-uninfected volunteers [11, 14], where TFV-DP values for Hct values at the ends of the normal range (35% and 50%) were within 15% of TFV-DP values in the middle of the Hct range [11]. As baseline Hct was similar between age groups (Table 1), with similar estimates in the adjusted and unadjusted models, it does not appear to be a confounder in the relationship between age and TFV-DP concentration. In terms of hair color, our study included HIV-infected adults aged 60 years and older, 6 of whom had completely gray hair. Differences in hair color result from variable amounts and combinations of melanin in the hair cortex and hair graying is characterized by loss of pigment in the hair shaft [23]. In our study, however, hair color was not associated with TFV hair concentrations in adjusted analyses.
Our results indicate that slower renal clearance, regardless of age, was associated with higher concentrations of TFV in hair and a trend towards higher TFV-DP in DBS. Tenofovir is primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion[24]; higher plasma concentrations of drug are thereby expected with decreased kidney function[25]. In our study, all but three calculated CrCl values, including data from both study visits, were above 50 ml/min, suggesting that elevated drug concentrations may be occurring in individuals with relatively healthy kidneys, which has been previously observed [25]. While renal decline has been reported in individuals between the ages of 35 and 60 years old[9], our study did not include this age group so results may not characterize the impact of renal function for this demographic. Given that both TFV in hair and TFV-DP in DBS measure cumulative drug exposure, these drug concentrations may provide quantitative evidence of slower renal clearance in older and younger individuals. Of note, TFV exposure can also influence renal function, and one limitation of our analyses is that we could not determine the directionality of the association between drug concentrations and renal function. A larger longitudinal study is needed to determine the relationship between drug levels in hair and DBS and renal function among older individuals.
Finally, TFV in hair and TFV-DP in DBS have both been used to assess adherence to TDF-based regimens. In this study, both measures (as well as VAS and plasma drug detection) indicated near 100% adherence in both age groups based on prior directly observed therapy studies [4, 5]. Adherence to antiretroviral therapy continues to be the most significant predictor of clinical outcomes in prevention and treatment of HIV. This study indicates that both measures could be useful as adherence assessments in older HIV-infected individuals on treatment.
Acknowledgments
Sources of Funding: The lead author was funded by the Colorado HIV Research Training Program (Grant Number 5T32AI744723). The study physician (Castillo-Mancilla) received funding through a K23 award (Grant Number K23AI104315). The trial used resources provided by the Colorado Clinical and Translational Science Institute, which is supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
We would like to thank all study participants and research staff at the Clinical Translational Research Center for their time and cooperation as well as the members of the Colorado Antiviral Pharmacology Laboratory for their work on this project.
Footnotes
Data presentation: Preliminary results of this data were presented as a poster at the 18th International Workshop on Clinical Pharmacology of Antiviral Therapy held in Chicago, IL on June 14th–16th, 2017.
Contributor Information
Sharon M Seifert, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pharmaceutical Sciences, University of Colorado, Anschutz Medical Campus, USA.
Jose R Castillo-Mancilla, School of Medicine, Division of Infectious Diseases, University of Colorado, Anschutz Medical Campus, USA.
Kristine Erlandson, School of Medicine, Division of Infectious Diseases, University of Colorado, Anschutz Medical Campus, USA.
Mary Morrow, Colorado School of Public Health, Department of Biostatistics and Informatics, University of Colorado, Anschutz Medical Campus, USA.
Monica Gandhi, Department of Medicine, University of California San Francisco, USA.
Karen Kuncze, Department of Medicine, University of California San Francisco, USA.
Howard Horng, Department of Medicine, University of California San Francisco, USA.
Jia-Hua Zheng, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pharmaceutical Sciences, University of Colorado, Anschutz Medical Campus, USA.
Lane R Bushman, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pharmaceutical Sciences, University of Colorado, Anschutz Medical Campus, USA.
Jennifer J Kiser, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pharmaceutical Sciences, University of Colorado, Anschutz Medical Campus, USA.
Samantha MaWhinney, Colorado School of Public Health, Department of Biostatistics and Informatics, University of Colorado, Anschutz Medical Campus, USA.
Peter L Anderson, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pharmaceutical Sciences, University of Colorado, Anschutz Medical Campus, USA.
References
- 1.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017 doi: 10.1089/aid.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3(11):e521–e528. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Anderson PL, McMahan V, Liu A, Amico K, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, andHIV incidence in men and transgender women who have sexwith men: a cohort study. The Lancet Infectious Disease. 2014 doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu AY, Yang QY, Huang Y, Bacchetti P, Anderson PL, Jin CS, et al. Strong Relationship between Oral Dose and Tenofovir Hair Levels in a Randomized Trial: Hair as a Potential Adherence Measure for Pre-Exposure Prophylaxis (PrEP) Plos One. 2014;9(1):9. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy: the DOT-DBS Study. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P, et al. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis. 2015;212(9):1402–1406. doi: 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of Measures of Adherence to HIV Preexposure Prophylaxis among Adolescent and Young Men Who Have Sex with Men in the United States. Clin Infect Dis. 2017 doi: 10.1093/cid/cix755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi M, Murnane PM, Bacchetti P, Elion R, Kolber MA, Cohen SE, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. Aids. 2017;31(16):2245–2251. doi: 10.1097/QAD.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hommos MS, Glassock RJ, Rule AD. Structural and Functional Changes in Human Kidneys with Healthy Aging. 2017 doi: 10.1681/ASN.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal. 2016;122:16–20. doi: 10.1016/j.jpba.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(20–21):1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacchetti P. Logarithmic Transformation. 2013 http://www.ctspedia.org/do/view/CTSpedia/LogTransformation.
- 16.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68(1):13–20. doi: 10.1097/QAI.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivest L. Statistical properties of Winsorized means for skewed distributions. Biometricka. 1994:373–383. [Google Scholar]
- 18.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 19.Saville DJ. Multiple Comparison Procedures: The Practical Solution. The American Statistician. 1990:174–180. [Google Scholar]
- 20.MacBrayne C, Fierer DS, Marks KM, Anderson PL, Bushman LR, Hollabaugh KM, et al. LEDIPASVIR/SOFOSBUVIR RAISES TENOFOVIR DIPHOSPHATE CONCENTRATIONS IN RED CELLS. Conference on Retroviruses and Opportunistic Infections; Seattle. Feb 13–16 2017. [Google Scholar]
- 21.CDC. HIV Among People Aged 50 and Older. HIV/AIDS. 2017 [Google Scholar]
- 22.Liu A, Vittinghoff E, Anderson P, Cohen S, Doblecki-Lewis S, Bacon O, et al. Changes in Renal Function Associated with TDF/FTC PrEP Use in the US Demo Project. Conference of Retroviruses and Opportunistic Infections (CROI); Boston, MA. 2016. [Google Scholar]
- 23.Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004;150(3):435–443. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 24.Gilead Sciences. VIREAD® (Tenofovir disoproxil fumarate) 2016. [Google Scholar]
- 25.Calcagno A, Cusato J, Marinaro L, Simiele M, Lucchiari M, Alcantarini C, et al. Tenofovir clearance is reduced in HIV-positive patients with subclinical tubular impairment. Aids. 2016;30(6):915–920. doi: 10.1097/QAD.0000000000000995. [DOI] [PubMed] [Google Scholar]